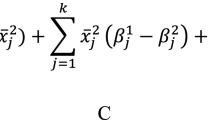Abstract
Background
In Chicago in 2018, the average life expectancy (ALE) for NH Blacks was 71.5 years, 9.1 fewer years than for NH Whites (80.6 years). Inasmuch as some causes of death are increasingly recognized products of structural racism, in urban areas, such causes may have potential for reducing racial inequities through public health intervention. Our purpose is to allocate racial inequities in ALE in Chicago to differentials in cause-specific mortality.
Methods
Using multiple decrement processes and decomposition analysis, we examine cause-specific mortality in Chicago to determine the causes of death that contribute to the gap in life expectancy between NH Blacks and NH Whites.
Results
Among females, the racial difference in ALE was 8.21 years; for males, it was 10.53 years. We find that cancer and heart disease mortality account for 3.03 years or 36% of the racial gap in average life expectancy among females. Differences in homicide and heart disease mortality rates comprised over 45% of the disparity among males.
Conclusions
Strategies for improving inequities in life expectancy should account for differences between males and females in cause-specific mortality rates. In urban areas with high levels of segregation, reducing inequities in ALE may be possible by dramatically reducing mortality rates from some causes.
Contribution
This paper illustrates the state of inequities in ALE between NH Blacks and NH Whites in Chicago for the period just prior to the onset of the COVID-19 pandemic, using a well-established method of decomposing mortality differentials for sub-populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fifty years after the passage of the Civil Rights Act of 1964, racial inequities in life expectancy remain a defining characteristic of public health in the United States (U.S.). In many ways, inequities in average life expectancy (ALE) are the critical indicator of systemic inequality, oppression, and marginalization. When the COVID-19 epidemic began in 2019, the difference in ALE between non-Hispanic (NH) Blacks and NH Whites was 3.6 years [1], where NH Blacks had an ALE of 75.3 years, whereas NH Whites’ ALE was 78.9 years.
Importantly, the U.S. public health system infrastructure is not a comprehensive, universal system working on behalf of residents to improve health, longevity, and quality of life. Rather, it is a patchwork of sub-national entities, organizations, and institutions with divergent goals, resources, and impact on health inequities [2]. Moreover, the largest U.S. cities have substantial and variable levels of racial health inequities related to structural racism. Often the magnitude of these health disparities are hidden when examined at the state and national level [3]. Therefore, examinations of racial health inequities in the U.S. are better undertaken at the sub-national level. These types of analyses have potential to influence sub-national health policy in ways that improve the health of disadvantaged groups in the U.S. [4]. Some of the racial gaps in ALE have persisted for decades in Chicago and are strongly related to economic hardship, disinvestment, and social conditions [4,5,6,7,8].
In an analysis of the 30 largest U.S. cities, Benjamins and De Maio [9] found that racial differences in ALE in Washington, DC, San Francisco, Los Angeles, and Chicago were double that of the U.S. Conversely, cities such as El Paso, Boston, and Jacksonville had much smaller racial inequities than the U.S. That research identified several causes of death for which mortality rates were higher in Chicago than the U.S., including HIV, homicide, and opioid overdose [9], but did not break down the magnitude of each cause’s impact on the life expectancy gap in Chicago or in any city. The current study expands upon existing research to identify which causes of death contribute the most to racial inequities in ALE in Chicago. Furthering our understanding of the causes that create inequities will allow stakeholders such as advocacy groups, community leaders, and policy makers to better develop programs and interventions that address racially unequal ALE in the city.
We utilize multiple decrement processes and life-table analysis to determine the contribution of differential cause-specific mortality rates to overall differences in ALE in Chicago in 2018–2019. The data used in these analyses represent the last “normal” pre-COVID-19 mortality levels in Chicago. These data were collected prior to the onset of the COVID-19 epidemic, which has produced overall declines in life expectancy in the U.S. [10] and shifts in cause-specific mortality structures.
Methods
Decomposing racial differences in ALE to differentials in cause-specific mortality by age is a well-established technique of population analysis. Multiple decrement processes (also known as competing risks) have been used to determine causes underlying group-level differences in life expectancy in a variety of contexts, time periods, and populations [10,11,12,13,14,15,16]. This methodology, popularized by Arriaga [17] “decomposes” group differentials in life expectancy to differences in cause and age-specific mortality rates.
Three main assumptions underpin these methods: (1) decomposition by age group that the sum of contributions from all age groups should equal the total life expectancy gap in years; (2) decomposition of cause-specific deaths within an age group, where the total contribution of one cause of death to the life expectancy gap is equal to the sum of cause-specific contributions across all age groups; and (3) when summed, differences in proportional contributions from all causes of death should equal the total life expectancy gap in years between NH Black and NH White residents.
We calculated abridged life tables with 5-year age intervals, using procedures originally proposed by Chiang [18] and explained in detail by Preston et al. [19]:
where:
- \({{}_n{}m}_x^i\left(2\right),{{}_n{}m}_x^i\left(1\right)=\) :
-
specific contribution of differences in mortality rates from cause 𝑖 between ages x and x + n in populations 1 and 2
- \({nR}_x^i\left(1\right)(2)=\) :
-
the proportion of deaths from cause 𝑖 between ages x and x + n in population 1 and 2
- \({n\Delta}_x^i=\) :
-
contribution of all-cause mortality differences in age group x to x+n to differences in \(e_0^0\)
Much of the literature in this area has demonstrated substantial sex-based differences in all-cause mortality rates, cause-specific mortality rates, and therefore life expectancy. Gender differentials in ALE have been documented previously [20, 21]; therefore, the decomposition analysis presented here is stratified by sex.
Data for these analyses come from the 2018 and 2019 Multiple Cause of Death data files collected by the National Vital Statistics System. Death records where the city of Chicago was recorded as the place of residence were included in the analysis. We extracted underlying deaths by sex, race, and age. We followed definitions used in previous studies [22, 23] to classify deaths using the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) codes into thirteen code groupings, as shown in Supplemental Table [1].
Deaths were categorized into standard 5-year age groups for all analysis. Additionally, deaths among the youngest residents were allocated to either 0–1 year of age or 1–4 years of age. Race-, sex-, and age-specific population denominators were obtained from the US Census Bureau’s 2010 Decennial Census.
Because our interest is the racial disparity in ALE in a hyper-segregated city, our analysis does not consider ethnicity in our calculations of ALE. While there are important considerations for ethnicity in examinations of ALE, we do not consider it here. This is because the intersection of race and ethnicity is a complicated one for Black individuals and the complexity [24] is not always reflected in vital data. For example, official census data for Chicago reports race and ethnicity separately for NH White, NH Black, and Hispanic/Latino residents, but does not parse data into Hispanic Black and White residents [25]. The analysis we present here focuses on non-Hispanic (NH) Black and NH White residents.
Results
Our analysis shows that the racial inequity in ALE was larger for males than for females. NH Black males have the shortest life expectancy (67.19 years) of the groups we examined, 10.53 fewer years than NH White males. Figure 1 shows the contribution of underlying causes of death to racial inequities in ALE in Chicago by sex.
During the period 2018–2019, NH White females had the longest life expectancy (84.39 years), 17.20 years longer than for NH Black males (67.19 years), who had the shortest. The disadvantage in life expectancy for NH Blacks was 8.21 years for females and 10.53 years for males. Among NH Whites, there was a gap of 6.67 years in ALE between females and males. The gap was larger for NH Blacks, where female life expectancy was almost 9 years longer than male life expectancy.
Over a fifth of the racial inequity in ALE among females was attributable to differences in heart disease mortality, where NH Black females had 1.75 fewer years than NH White females. Additionally, differences in age-specific cancer mortality rates among females contributed over 15% of the differential; NH Black females had 1.29 fewer years of life expectancy. Differences in influenza, pneumonia, HIV, and other infectious disease mortality between NH Black and NH White females explained only about 4.9% of the 8.21-year gap in life expectancy (0.40 years). About 3% (0.24 years) of racial inequities in ALE for females was due to perinatal conditions. Remaining causes of death, which included diabetes, kidney disease, stroke, opioid overdose, accidents, chronic lower respiratory disease, and influenza and pneumonia, comprised the 39% (3.19 years) of the difference in ALE between NH Black and NH White females over this period.
The ALE among NH Black males was 67.19 years, 10.53 years less than NH White males. Over 25% of this inequity was due to race differentials in age-specific homicide mortality rates (2.87 years). Excess heart disease mortality among NH Black males contributed to 18.6% of the racial gap in ALE, and differences in age-specific cancer mortality rates accounted for 1-year gap in ALE among males in Chicago (9.7% of the total difference). Differences in age-specific opioid overdose mortality rates comprised 7.4% of the difference in ALE between NH Black and NH White males (0.78 years). The contribution of differences in age-specific infectious disease mortality rates (HIV, infectious disease, influenza, and pneumonia) was similar for males as it was for females (4.9% or 0.51 years). The remaining causes of death accounted for 1.98 years (or 19%) of the racial disparity in ALE between NH Black and NH White males.
Discussion
Using life-table analysis of multiple decrement processes, we find that the large differences in ALE between NH Black and NH White residents in Chicago are attributable to substantial racial differences in age-adjusted cause-specific mortality rates (10.52 years for males and 8.21 years for females). Racial differences in heart disease and cancer mortality rates contribute substantially to inequities in ALE between NH Black and NH White residents in Chicago. These findings align with other research showing growing racial inequities in life expectancy in U.S. cities. In a study of the 30 most populous US cities, Benjamins et al. (2021) found that Chicago was one of the six cities that experienced a significant increase in racial inequities in all-cause mortality during the period 2009–2018.
The analysis included here provides evidence for the utility of examining inequity in cause-specific mortality rates at the sub-national level. While much of the funding and priority setting for public health in the U.S. is done at the national and state level, we show that within Chicago, there are dramatic race and sex-based differences in ALE. These results support the contributing role of heart disease and cancer to these inequities. We reveal that, of the ten leading causes of death, heart disease and cancer contribute the most excess Black deaths in Chicago, a finding reflected overall in the U.S. [9] and at the state and county level [23, 26,27,28]. The contribution of homicide mortality to disadvantages in life expectancy has been documented previously, both in the U.S. overall and in Chicago [29].
Notwithstanding individual experiences of racism leading to poor health outcomes [30], focusing on structural racism compels us to move from individual-level health dynamics to broader macro-ecological factors that continue to reproduce social stratification and patterns of racial and ethnic health inequities [31]. Multiple dimensions of structural racism impact the health and mortality of minority populations in the U.S. broadly [32].
For example, community areas with large concentrations of Black residents are also those in the closest proximity to environmental pollution [33]. Hyper-segregated cities with histories of redlining (discriminatory practices that shaped racial composition of residential areas in U.S. cities) are likely characterized by vastly different housing stock for Black and NH White residents, particularly those living in gentrified neighborhoods. Because gentrification tends to occur in more racially integrated neighborhoods [34], it may be that the neighborhoods with large concentrations of Black residents are also the neighborhoods with older housing stock in need of renovation to remove toxic substances (like leaded paint). Other municipal improvements (like replacing leaded water pipes) that require extraordinary financial resources are slow to happen in these areas, resulting in high lead exposure in the drinking water supply. For example, in Chicago, majority of Black and Hispanic neighborhoods comprise nine of the top 10 zip codes with the highest drinking water lead exposure [35]. Since lead poisoning has been linked to hypertension, heart disease, stroke, and chronic kidney disease [36], it is possible that city-level differentials in ALE are driven at least partially by the historical legacy of structurally racist policies (like redlining).
Indirectly, the historical remnants of redlining, including lower levels of social investment and municipal development, have produced neighborhoods characterized by higher levels of “social disorganization.” Racial segregation exacerbates these and combined with greater law enforcement presence impacts mental health, substance use, and sexual risk factors [37]. Essentially, the cumulative effects of structural racism are complex and persistent, operating at multiple levels of influence.
Methodological Considerations
This analysis was limited to comparisons between NH Black and NH White populations. We acknowledge the importance of including other racial and ethnic groups in mortality analyses related to ALE; however, we made the decision to highlight inequities affecting the Black community because the experience of this group is historically unique within Chicago. Determining the causes that contribute to shorter life expectancy for Blacks has the potential to inform the development of interventions that address this most fundamental inequality in Chicago.
It is also important to acknowledge the absence of other key variables in our analysis, such as socioeconomic status, that might help explain Black/White disparities in ALE [38]. Another limitation is our reliance on ICD-10 classification of cause of death. Some causes require knowledge about contributing circumstances that may not be captured on death certificates, which could influence official causes in vital data.
There are other methodological limitations that should be considered here. Our assumptions that (1) the sum of contributions from all age groups should equal the total gap in ALE, (2) the total contribution of one cause of death to the gap in ALE is equal to the sum of those cause-specific contributions across all age groups; and (3) when summed, differences in proportional contributions from all causes of death should equal to the total gap in ALE between NH Black and NH White residents may be considered flawed somewhat, because they are not verifiable using these data. These assumptions rely on well-known and previously established processes and procedures to collect, refine, and publish vital registration data, and it is possible that deviations from those procedures have resulted in a reality that is not reflected in the findings here. Because these assumptions rely so heavily on vital data, inaccurate or incomplete registration of key variables on the death certificate could lead to poor estimates of age-specific mortality rates and distortions in estimates of ALE.
These analyses may be limited somewhat by the available data for this period. We utilize 2010 census data for these analyses because we wanted to examine racial differences in perinatal causes that occur during the first year and require the age groups 0–1. Utilizing the ACS would have meant we were limited to using 5-year age groups, which would have restricted our capacity to examine these causes.
Finally, more recent work in this area of sub-national estimates of ALE utilizes Bayes regression models [39]. These models were considered for the present analysis, given the short period of investigation and the sub-national nature of our data. However, without evidence of incomplete vital registration, we eschewed these models in favor of life-table analysis, a traditional method of investigation in this area.
We believe our use of a well-developed methodology to parse racial differences in ALE to specific causes of mortality to be a strength. Additionally, examining the causes underlying the dramatic differences in ALE in Chicago is a useful exercise as there are other U.S. cities with similar historical patterns of redlining and levels of segregation, such as Detroit, Newark, Milwaukee, and Cleveland [40]. These similarities may underscore the need for similar examinations in these cities and to prioritize the health of residents in these cities.
Additionally, these analyses utilize the last cause-specific mortality data unaffected by the COVID-19 pandemic. Furthermore, an assessment of life expectancy prior to COVID-19 provides a point of comparison for life expectancy estimates and trends post COVID-19 as cities recover and mortality structures return to “normal.”
Racial inequities in health have existed in Chicago for some time, long before the COVID-19 epidemic. At their core, the inequities we show here originate in disparate social conditions and structural racism. When the COVID-19 epidemic is over, these inequities will persist. Understanding how Black/White differences in the distribution of causes of death contribute to inequities in life expectancy is essential for targeted interventions at the city level.
Interventions to reduce racial disparities in ALE need to address differences in cause-specific mortality between Black and White populations in Chicago. The city’s level of racial segregation means that city-led intervention efforts can be targeted in specific community areas to decrease race-based differences in ALE. Homicide alone contributes nearly one-third of the 10.53-year age difference in ALE for males. City and community area-level interventions [41,42,43,44,45,46] that reduce homicide mortality rates have the potential to dramatically reduce the inequity between Black and White males.
Conclusion
We utilize a well-developed method of population analysis to examine racial inequities in average life expectancy in Chicago in 2018–2019. The disadvantage in ALE for NH Blacks was 8.21 years for females and 10.53 years for males. We find dramatic racial differences in ALE related to cause-specific mortality structures, where causes comprising the largest differentials varied by gender.
References
Life expectancy in the U.S. increased between 2000–2019, but widespread gaps among racial and ethnic groups exist [Internet]. Natl. Inst. Health NIH. 2022 [cited 2022 Oct 19]. Available from: https://www.nih.gov/news-events/news-releases/life-expectancy-us-increased-between-2000-2019-widespread-gaps-among-racial-ethnic-groups-exist. Accessed 19 Oct 2022.
Ansell DA, Oliver -Hightower Darlene, Goodman LJ, Lateef OB, Johnson TJ. Health equity as a system strategy: the Rush University Medical Center Framework. NEJM Catal [Internet]. Massachusetts Medical Society; 2021. Available from: https://catalyst.nejm.org/doi/full/10.1056/CAT.20.0674. Accessed 17 Dec 2021.
Roesch PT, Saiyed NS, Laflamme E, De Maio FG, Benjamins MR. Life Expectancy Gaps Among Black and White Persons and Contributing Causes of Death in 3 Large US Cities, 2018-2019. JAMA Netw Open. 2023;6:e233146.
Hunt BR, Tran G, Whitman S. Life expectancy varies in local communities in Chicago: racial and spatial disparities and correlates. J Racial Ethn Health Disparities. 2015;2:425–33.
Barber S, Hickson DA, Kawachi I, Subramanian SV. Double-jeopardy: the joint impact of neighborhood disadvantage and low social cohesion on cumulative risk of disease among African American men and women in the Jackson Heart Study. Soc Sci Med. 2016;153:107–15.
Gee GC, Walsemann KM, Brondolo E. A life course perspective on how racism may be related to health inequities. Am J Public Health. 2012;102:967–74.
Lange-Maia BS, De Maio F, Avery EF, Lynch EB, Laflamme EM, Ansell DA, Shah, RC. Association of community-level inequities and premature mortality: Chicago, 2011–2015. J Epidemiol Community Health. 2018;72:1–5.
Subramanian SV, Chen JT, Rehkopf DH, Waterman PD, Krieger N. Racial disparities in context: a multilevel analysis of neighborhood variations in poverty and excess mortality among black populations in Massachusetts. Am J Public Health. 2005;92:260–5.
Benjamins MR, De Maio FG. Unequal cities. Baltimore: Johns Hopkins University Press; 2021.
Aburto JM, Kristensen FF, Sharp P. Black-white disparities during an epidemic: life expectancy and lifespan disparity in the US, 1980–2000. Econ Hum Biol. 2021;40:100937.
Andreev EM, Shkolnikov VM, Begun AZ. Algorithm for decomposition of differences between aggregate demographic measures and its application to life expectancies, healthy life expectancies, parity-progression ratios and total fertility rates. Demogr Res. Max-Planck-Gesellschaft zur Foerderung der Wissenschaften; 2002;7:499–522.
Elo IT, Beltrán-Sánchez H, Macinko J. The contribution of health care and other interventions to Black-White disparities in life expectancy, 1980–2007. Popul Res Policy Rev. 2014;33:97–126.
Monnat SM. Trends in U.S. Working-age non-Hispanic White mortality rural–urban and within-rural differences. Popul Res Policy Rev. 2020;39:805–34.
Remund A, Camarda CG, Riffe T. A cause-of-death decomposition of young adult excess mortality. Demography. 2018;55:957–78.
Sagna AO, Kemp MLS, DiNitto DM, Choi NG. Impact of suicide mortality on life expectancy in the United States, 2011 and 2015: age and sex decomposition. Public Health. 2020;179:76–83.
Singh GK, Camarda CG, Riffe T. Widening disparities in infant mortality and life expectancy between Appalachia and the rest of the United States, 1990–2013. Health Aff (Millwood). 2017;36:1423–32.
Arriaga EE. Measuring and explaining the change in life expectancies. Demography. 1984;21:83–96.
Chiang CL. An introduction to stochastic processes in biostatistics. New York: Wiley; 1968.
Preston SH, Heuveline P, Guillot M. Multiple decrement processes. Demogr Meas Model Popul Process. Malden, MA: Blackwell Publishers; 2002. p. 71–97.
Singh GK, Siahpush M. Widening socioeconomic inequalities in US life expectancy, 1980–2000. Int J Epidemiol. 2006;35:969–79.
Acciai F, Firebaugh G. Why did life expectancy decline in the United States in 2015? A gender-specific analysis. Soc Sci Med. 2017;190:174–80.
Kochanek KD, Arias E, Anderson R. Leading causes of death contributing to decrease in life expectancy gap between black and white populations: United States 1999-2013 [Internet]. Report No.: 218. Hyattsville, MD: National Center for Health Statistics; 2015. Available from: https://www.cdc.gov/nchs/data/databriefs/db218.pdf. Accessed 27 Feb 2023.
Kaufman JS, Riddell CA, Harper S. Black and White differences in life expectancy in 4 US states, 1969–2013. Public Health Rep. 2019;134:634–42.
LaVeist-Ramos TA, Galarraga J, Thorpe RJ, Bell CN, Austin CJ. Are black Hispanics black or Hispanic? Exploring disparities at the intersection of race and ethnicity. J Epidemiol Community Health. BMJ Publishing Group Ltd; 2012;66:e21–e21.
U.S. Census Bureau QuickFacts: Chicago city, Illinois [Internet]. Available from: https://www.census.gov/quickfacts/chicagocityillinois. Accessed 13 Feb 2023.
Dwyer-Lindgren L, Bertozzi-Villa A, Stubbs RW, Morozoff C, Mackenbach JP, van Lenthe FJ, Mokdad, AH, Murray CJ. Inequalities in life expectancy among US counties, 1980 to 2014: temporal trends and key drivers. JAMA Intern Med. 2017;177:1003–11.
Riddell CA, Morrison KT, Kaufman JS, Harper S. Trends in the contribution of major causes of death to the black-white life expectancy gap by US state. Health Place. 2018;52:85–100.
Roberts MT, Reither EN, Lim S. Contributors to Wisconsin’s persistent black-white gap in life expectancy. BMC Public Health. 2019;19.
Schober DJ, Hunt BR, Benjamins MR, Saiyed NS, Silva A, De Maio FG, Homan SM. Homicide mortality inequities in the 30 biggest cities in the US. Am J Prev Med. 2021;60(3):327–34.
Williams DR, Lawrence JA, Davis BA, Vu C. Understanding how discrimination can affect health. Health Serv Res. 2019;54(Suppl 2):1374–88.
Powell J. Structural racism: building upon the insights of John Calmore. N C Law Rev. 2008;86:791.
Gee GC, Ford CL. Structural racism and health inequities: old issues. New Directions Bois Rev. 2011;8:115–32.
Chase B, Judge P. Interactive map: pollution hits Chicago’s west, south sides hardest [Internet]. Chicago, IL: Better Government Association; 2018. Available from: https://www.bettergov.org/news/interactive-map-pollution-hits-chicagos-west-south-sides-hardest/. Accessed 4 Mar 2021.
Hwang J, McDaniel TW. Racialized reshuffling: urban change and the persistence of segregation in the twenty-first century. Annu Rev Sociol Annual Reviews. 2022;48:397–419.
McCormick E, Uteuova A, Moore T, Davis TM with photographs by JK. Revealed: the ‘shocking’ levels of toxic lead in Chicago tap water. The Guardian [Internet]. 2022. Available from: https://www.theguardian.com/us-news/2022/sep/21/lead-contamination-chicago-tap-water-revealed. Accessed 21 Oct 2022.
Landrigan PJ. Lead and the heart: an ancient metal’s contribution to modern disease. Lancet Public Health. Elsevier; 2018;3:e156–7.
Jindal M, Mistry KB, Trent M, McRae A, Thornton RLJ. Police exposures and the health and well-being of Black youth in the US: a systematic review. JAMA Pediatr. 2022;176:78–88.
Kawachi I, Daniels N, Robinson D. Health disparities by race and class: why both matter. Health Aff (Millwood). 2005;24:343–52.
Schmertmann CP, Gonzaga MR. Bayesian estimation of age-specific mortality and life expectancy for small areas with defective vital records. Demography. 2018;55:1363–88.
Most to Least segregated cities | Othering & Belonging Institute [Internet]. Available from: https://belonging.berkeley.edu/most-least-segregated-cities. Accessed 13 Feb 2023.
City of Chicago. Our City, Our safety: a comprehensive plan to reduce violence in Chicago [Internet]. Chicago; 2020. p. 108. Available from: https://www.chicago.gov/content/dam/city/sites/public-safety-and-violenc-reduction/pdfs/OurCityOurSafety.pdf. Accessed 1 Mar 2023.
Aiming to reduce gun violence in Chicago [Internet]. Chic. CRED. Available from: https://www.chicagocred.org/about/. Accessed 1 Mar 2023.
READI [Internet]. Heartl. Alliance. Available from: https://www.heartlandalliance.org/readi/. Accessed 2 Mar 2023.
Violence intervention & prevention services [Internet]. UCAN. Available from: https://ucanchicago.org/violence-intervention-prevention-services/. Accessed 1 Mar 2023.
Green E. Improving community safety in Chicago through pre-violence intervention [Internet]. Big Cities Health Coalit. 2022 Available from: https://www.bigcitieshealth.org/chicago-previolence-intervention/. Accessed 1 Mar 2023.
Healthy Chicago 2025: closing our life expectancy gap 2020–2025. Chicago: Chicago Department of Health; p. 40. Report No.: HC2025.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Statement
The authors certify that the material in this manuscript has not been published and is not considered for publication elsewhere.
Competing Interests
The authors declare no competing interests.
Animal Studies
No animal or human sties were carried out by the authors for this article.
Disclaimer
The ideas in this article are those of the authors and do not necessarily represent policy of the American Medical Association.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bishop-Royse, J., Saiyed, N.S., Schober, D.J. et al. Cause-Specific Mortality and Racial Differentials in Life Expectancy, Chicago 2018–2019. J. Racial and Ethnic Health Disparities 11, 846–852 (2024). https://doi.org/10.1007/s40615-023-01566-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40615-023-01566-w