Abstract
Purpose of Review
This review aims at outlining alpha-2-macroglobulin (A2M) injection, a novel non-operation strategy which could delay the process of osteoarthritis (OA). Meanwhile, some burning issues concerning “experimental” and “applied” are also indicated in this review.
Recent Findings
Many researchers have found that the alpha-2-macroglobulin, a sort of broad-spectrum proteinase inhibitor, presents remarkable inhibitive effect on intra-articular inflammation. Additionally, results of animal experiments prove that the A2M can postpone cartilage degeneration. Some treatments, such as hyaluronic acid (HA), which have been applied clinically for many years proved not to be as effective; thus, the advantage of A2M is presented.
Summary
A2M promises to be a new strategy of non-operative treatment of OA for its excellent anti-inflammation effect and biosafety. Better improved pharmaceutical preparations and treatment strategies shall be developed with the in-depth research.
Similar content being viewed by others
Introduction
Many researchers have been working to find an alternative to joint replacement in the treatment of osteoarthritis (OA), a chronic progressive disease with joint pain as a chief complaint, of which intra-articular inflammation and cartilage degeneration are the major pathological changes [1]. The degeneration of cartilage and joint pain are mainly due to the chronic intra-articular inflammation. And high levels of inflammatory mediators inducing cartilage loss, such as IL-1β, IL-6, IL-8, and TNFα, have been detected in the synovial fluid of OA patients [2]. The study of intra-articular inflammation reduce has been regarded as the main train of thought to develop a non-operative treatment. In this review, we hope to introduce a novel experimental strategy, the A2M treatment, as a treatment of OA, and comparing it with other kinds of newly developed and past methods.
alpha-2-Macroglobulin (A2M) is a broad-spectrum proteinase inhibitor detected in both serum and synovial fluid, which is capable of blocking almost all kinds of proteinases, benefiting from its special molecular structure, a bait region and four-arm structures. Proteinases that induce chronic inflammation can be captured by A2M, and the A2M-proteinase complex will be purged from the serum very soon. Thus, A2M plays a role in protecting the human body from endogenous and exogenous inflammatory injury [3]. However, the concentration of A2M in synovial fluid is much lower than that in serum, both in knee osteoarthritic (OA) patients and in normal people [4]. On the basis of the above, the curative effect of intra-articular injection of A2M on the knee OA has been studied in a large number of systematic researches. It has been proved that the supplement of A2M in knee joint could effectively delay the degeneration of articular cartilage [4,5,6]. Moreover, experiments have been conducted to test efficiency of A2M in animal models, and the protective effect has been verified by the results.
Researches around A2M particularly suggest that, being different from several mature and experimental treatments laterally, A2M therapy performs considerable efficiency and, at the same time, addresses the shortages of some treatments which used to be popular in clinics. In addition, autologous A2M enrichment technology and A2M-synthesized technique also provide the possibility for wider clinical application [6].
An Important Broad-Spectrum Proteinase Inhibitor in Plasma
alpha-2-Macroglobulin is widely existing in plasma of vertebrates, as a broad-spectrum proteinase inhibitor. Its anti-inflammatory property is structure-dependent, which can be regulated by the space structure. alpha-2-Macroglobulin is considered a coalition of two identical dimers. The molecule radius of each of two subunits is 179,000, and the radius of the whole alpha-2-macroglobulin molecule is 725,000 [3, 7].
In 1985, the model of A2M molecule had been developed for the first time. Each of dimeric subunits of A2M molecule has a bait region in the middle of the whole structure which can be cut by endogenous and exogenous proteinases easily [8]. The cleavage of the bait region leads to the conformational change in the structure of A2M molecule. Each dimer with four-arm structures would fold and capture the proteinase as soon as it cuts the bait region. This sort of trapping would not cause inactivation but separate the proteinase and its substrate [9].
The transformation of A2M molecular structure follows the cleavage of the bait region, which causes an increase in electrophoretic mobility [10] and sedimentation coefficient [11]. Deformed A2M has smaller radius of gyration [12], and its Stokes’ radius becomes much less [13]. This deformed molecular structure was called “fast” form. The a2M–proteinase complexes in the fast form are easily recognized by cell surface receptors and endocytosed. Thus, the complexes can be removed rapidly from plasma [14, 15].
The function of the A2M is determined by its unique structural characteristics which shall inhibit a large variety of proteinase, including serine, carboxyl, thiol, and metalloprotease, especially proteinase released from by granulocytes and other inflammation-related cells. In this circumstance, A2M plays a protecting role in a great deal of chronic autoimmune complaints; thus, the A2M is expected to slow down the degenerative processes which is generated from chronic inflammation (Fig. 1).
Can A2M Protect Articular Cartilage After Injury?
The pathologic changes of OA include the degeneration of articular cartilage and intra-articular local inflammation [16]. It is confirmed and proved that the increase of A2M level shall be simultaneous with the occurrence of intra-articular inflammation. Results from western blot analysis, protein mass spectrometry, ELISA, and immunohistochemistry (IHC) indicate that A2M is a component of synovial fluid. Compared with serum, however, the concentration of A2M in synovial fluid is much lower, even in patients with knee osteoarthritis. Previous studies also demonstrated that the A2M is not present in adequate concentrations to inhibit the catabolic proteinases and factors [4], probably because of its high molecular weight of 720 kDa [17]. Based on the fact that the mechanism of inhibitive effect of A2M is the transformation of molecular structure, capturing one or two molecules of proteinase will cost one molecule of A2M [18]. Thus, the insufficiency of A2M concentration in synovial fluid curbs its inhibitory effect of intra-articular inflammation.
As shown in previous researches, A2M used to be studied in-depth as a major inhibitor in serum. However, a new aspect of OA’s treatment has been provided for us due to the finding of the existence of A2M. Based on the characteristics of molecular structure of A2M and its mechanism of inflammation-inhibiting function, the knee osteoarthritis inflammation can be limited by supplying extra A2M within our expectation, and cartilage inflammatory degeneration might be slowed down too. Thus, A2M can be used as a supplemental intra-articular therapy to delay the degeneration in early stage of OA.
A2M Injection, Anesthetics, and Anti-inflammatory Drugs: Which Is the Best?
Prior treatment of knee osteoarthritis aims to achieve pain relief and mobility improvement [19]. To accomplish these goals, calcium channel–blocking anesthetics and intra-articular corticosteroid injection (IACI) are used in clinic treatment in earlier period.
The most direct approach to stop the joint pain is to use the anesthetics. But using calcium channel–blocking anesthetics even in joint topically might cause allergic reactions, perioral anesthesia, convulsions, and hemodynamic instability [20]. Besides, the calcium channel–blocking anesthetics further damage the injured cartilage, resulting in chondrocyte apoptosis and necrosis. Anesthetics also relate to caspase activation in chondrocytes and the increase of osteoarthritic cells [21]. Additionally, known from previous studies, this easing of osteoarthritis symptoms is temporary, only for about a few hours [22].
The US FDA–authenticated corticosteroids, like triamcinolone, are used as an effective treatment for osteoarthritis. It is believed that the analgesic effect of corticosteroids is better than the placebo [23]. However, the effect of corticosteroids is restricted in pain relief, lasting 1–2 weeks after injection. Probably because of its short half-life, the long-term effect of corticosteroid gets little supportive evidence [24,25,26,27]. Furthermore, a study suggested that the corticosteroid even produces a negative effect in the process of cartilage degeneration. Intra-articular injection of corticosteroid leads to more cartilage loss than the intra-articular saline [28]. Besides, the corticosteroid produces an unpleasant side effect, such as tendinopathy, skin atrophy, and systemic hyperglycemia [29].
Compared with simple pain control and dephlogisticating, there is a pressing requirement of a regenerative medicine which contains both cartilage protection and degeneration postponement. It is fortunate that we have found that the A2M is a very promising therapeutic injection which is capable to satisfy the needs.
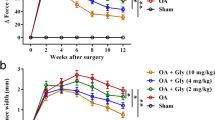
In human cartilage explants and chondrocyte (chondrocyte cell line C-28/12), MMP13 activity was inhibited by A2M. Meanwhile, IL-1β-induced catabolic cytokines and enzymes, such as IL-1β, IL-8, TNFα, and GM-CSF, shall be decreased with the supplement of A2M. This outcome urged researchers on further experiments on in vivo animal models. OA rat models induced by ACLT with intra-articular A2M injection showed better results, compared with those in the control group treated with saline. Intuitively, in histology, the A2M-treated group showed stronger Safranin O staining, which means slighter cartilage injury and less fibrillation, tallying with the Indian-ink-staining outcomes. RT-PCR results demonstrated that the mRNA level of Mmp13, Runx2, MMP-3, MMP-9, MMP-13, and Col10a1 decreased, while the level of Col2a1 and ACAN increased in the A2M group. Western blot outcomes showed a fall of expression of MMP13 and type X collagen and a rise of type II collagen. These western blot results were verified by the immunohistochemical staining [4].
The A2M preforming an anti-inflammation effect on the ankle joint in collagen II–induced arthritis mice model (CIA mice model) is contained in Li’s research released in 2018. Meanwhile, the study confirmed the efficiency of A2M against intra-articular inflammation from another side. Mice in groups treated with A2M suffered milder redness and swelling than groups treated with PBS. Besides, the score of the A2M groups is low in terms of radiography, and the degree of erosions and space narrowing of joint shall be reflected by the score given. It could be observed from fluorescence molecular tomography (FMT) that the A2M therapy significantly reduced the level of inflammatory in inflamed joints. Same as the results mentioned before, OARSI scores gotten from Safranin O staining suggested that the A2M can protect cartilage from inflammatory cytokines and enzymes [30].
It shall be sufficiently attested by means of the studies that the A2M can downregulate damage index of cartilage catabolic process (MMP13- and X-type collagen), and can surely protect the cartilage of knee joint morphologically. Hence, what can be indicated is that it is feasible to select the A2M as an intra-articular injection in OA patients. Furthermore, the merit of A2M is not only feasibility but also superiority. Therefore, the degeneration of knee joint cartilage shall be delayed significantly, and the defected of other intra-articular injection treatment shall be avoided simultaneously by adopting A2M as a new intra-articular therapy.
Introduction of Several Other Regenerative Medicines and Their Merits and Demerits
A2M is not the only chondroprotective medicine, and there are still various existing and examining therapies like platelet-rich plasma (PRP), autologous conditioned serum (ACS), mesenchymal stem/stromal cells (MSC), and hyaluronic acid (HA) [31].
In recent year, PRP has been gradually receiving the attention of scholars and clinician. Therapeutic effects of PRP have been confirmed by theory, including protection of joint cartilage against inflammation factors and pain control. The pain control effect of PRP therapy is fair, and it begins at about 2 months after injection and lasts for 6 months to 1 year [32]. But the existing major problem of PRP is its uncertain formulations. PRP is extracted from patient’s own serum, avoiding autoimmune response. After that, however, individual diversities of sources lead to various formulations of PRP therapy. Thus, the therapeutic effect cannot be assessed and standardized. Only a few of clinical trials have described details about the recipe of PRP, including concentrations of red blood cell, white blood cell, and platelet [33].
Same as PRP, autologous conditioned serum (ACS) is another sort of blood-derived products. ACS was reported valid in OA, because ACS contains a large amount of IL-1-Ra, inhibitor of IL-1 receptor (140 times to normal). IL-1 is the key factor of joint degenerative process, destroying joint cartilage by upregulating matrix metalloproteinases. With a high level of IL-1-Ra, ACS can block against IL-1 and protect cartilage from metalloproteinases [34]. Up to now, there are not many clinical trials testing the efficiency of ACS. Dr Baltzer and his colleagues tested 376 patients in Germany and obtained a positive result proving ACS is helpful to knee OA [35]. But another trial held by Auw Yang KG did not get a definitive result about the effectiveness of ACS. By far, tested studies found no statistically significant difference between curative effects of ACS and saline placebo [36]. It is probable that the indeterminacy of ACS’s component causes the heterogeneity. Moreover, a study indicated that in patient with high C reactive protein level, there is possibility ACS would aggravate the inflammatory reaction. The study assumed that the existing systemic inflammation could bring pro-inflammatory factor into ACS products [37].
Likewise, mesenchymal stem cell (MSC) is a promising direction for OA treatment. As a sort of autologous stem cell, MSC can avoid immune rejection. Furthermore, MSC has a remarkable anti-inflammatory effect. Its ability to inhibit local joint inflammation comes from expression of IL-1 Ra and reduces pro-inflammatory cytokines by muting resident macrophages in the joint [38, 39]. Meanwhile, culture and proliferation of MSC can be well-manipulated in vitro [40]. These features make the MSC therapy a possible way to be applied in clinics. Moreover, the safety of MSC has been confirmed by clinical trials [41]. Nevertheless, this new treatment still stays in experimental stage, and only a limited number of human trials have been performed [31].
Compared with treatments mentioned before, corticosteroids have been used for over 50 years as an intra-articular injection treatment [42]. The US FDA–authenticated corticosteroids, like Triamcinolone, are used as an effective treatment for osteoarthritis. It is believed that the analgesic effect of corticosteroids is better than placebo [23]. However, only the short-term effect of corticosteroids is confirmed, while its long-term is uncertain. Furthermore, the curative effect of corticosteroids is restricted in pain relief, and the relief lasts 1–2 weeks after injection. Probably because of its short half-life, the long-term effect of corticosteroid gets little supportive evidence [24,25,26,27]. Unfortunately, a study suggested that the corticosteroid even produces a negative effect in process of cartilage degeneration. Intra-articular corticosteroid leads to more cartilage loss than the intra-articular saline [28]. Besides, the corticosteroid produces an unpleasant side effect, such as tendinopathy, skin atrophy, and systemic hyperglycemia [29].
Synovial fluid contains HA, providing the synovial fluid with elasticity and viscosity. As an intrinsic component, HA would be flowing out during the process of OA [43]. Accordingly, it seems reasonable that the supplement of HA by intra-articular injection can ameliorate the intra-articular environment. However, substantial amount of reviews, clinical trial, and meta-analysis suggest that HA has no significant efficiency in clinical practices. Grace H Lo and Jasmin Arrich presented their meta-analysis in 2003 and 2005 respectively, coming to the similar conclusion that the HA did not have evident advantage on relief of symptom, compared with placebo [44, 45]. A meta-analysis published several years later, presented by Anne WS Rutjes, concluded that the HA treatment is associated with a slight clinical effect, confirming results of Grace and Jasmin mentioned before [46]. Hence, it is obvious that established clinical trials cannot lead to a definite conclusion on HA efficacy. Besides, disagreements and different views might result from the heterogeneity of various HA products and the discrepancy between laboratory situation and clinical practice [47]. The American Academy of Orthopedic Surgery (AAOS), the National Institute for Health and Care Excellence (NICE), and the American College of Rheumatology (ACR) all take a negative attitude about clinical application of HA in knee OA [48, 49]. Osteoarthritis Research Society International (OARSI) did not give a certain recommendation about HA treatment in OA.
Intra-articular injection treatment for knee OA promises to be a better way than systemic pharmacological therapy and surgery. More direct effect and fewer invasions are the advantages of intra-articular injection. In contrast to oral NSAIDs and surgical management, what is proved to be unfortunate is that the intra-articular injection is relatively not widely used in clinics, due to all those limitations. A2M, as a novel therapeutic drug for knee OA, has a pretty good prospect in solving the shortcomings mentioned above. Compared with other drugs above, A2M is an autologous proteinase inhibitor so that it has no autoimmune rejection. Additionally, A2M injection contains only one kind of active ingredient, but inhibits various inflammatory factors and degenerative proteinase, because of broad-spectrum anti-inflammation effect of A2M. Therefore, A2M injection eliminates the abovementioned limitations. As a result, the safety is guaranteed and risk is controllable.
Clinical Application of A2M and Outlook
In clinics, according to Dr Jason M. Cuéllar’s report, A2M has found a utilization in several orthopedic painful diseases, such as subacromial bursitis, lateral epicondylitis, and Achilles tendonitis, and the curative effect is gratifying and encouraging [50]. And the curative effect of A2M on discogenic back pain and enthesopathies, a degenerative disease which is similar to knee OA, has also been reported.
The specific method of A2M therapy is to concentrate A2M molecule from autologous plasma, and inject it into the affected area. As previous studies did, A2M-rich preparation was injected into the knee joint of experimental animal, to compensate for the low level of A2M in the synovial fluid. Mature relevant technology makes A2M concentrating a reality. There is a recently developed system that provides a new method to concentrate A2M from plasma. The tangential flow filter technology utilized by this concentration system can enrich certain large protein like A2M and filter out small molecules. This system prepares A2M-rich concentration available for injection in less than 1 h [50].
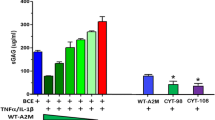
Although a rather mature method of A2M concentration technique has been formed, researchers are still working on developing a more economic and convenient way. According to A2M’s molecular properties, more than 100 targeted variants of A2M have been synthesized, two of which with the best inhibitory effect are variants CYT-98 and CYT-108. Experiments have been conducted in order to assay the effectiveness of these two synthesized variants. In BCE culture, CYT-108 A2M performed the finest inhibition cartilage catabolism induced by TNFα and IL-1β. And the inhibitory effect of CYT-108 A2M far exceeded wild-type A2M. Then the expression level of metalloproteinases in vivo was demonstrated by fluorescence molecular tomography (FMT). Wild-type A2M and both two variants successfully downregulated MMPs and cathepsin, whereas the PBS-treated group did not. IHC staining indicated that the increasing of expression level of MMP13, type X collagen, and type II collagen degraded products in PBS group and decreasing of those indices in groups treated by A2M and its variants. Additionally, results of histological analysis, assessed by OARSI score, were consistent with the data before—groups treated by wild-type A2M and two variants suffer slighter cartilage injury. Moreover, hematoxylin/eosin staining showed that the treated group has mild hyperplasia compared with the PBS group. That means wild-type A2M and synthesized A2M variants inhibited inflammation [6].
As per statistics, it is very exciting to find the synthesized targeted A2M variants performed outstandingly in protecting joint cartilage from inflammatory injury, even better than the wild-type A2M. Further clinical test shall be expected to be performed to verify its safety in the human body and the suitability of mass production shall be promised.
Conclusion
A general understanding of the A2M shall be obtained from what has been mentioned above. As an auto-transplanted proteinase inhibitor, large portion of side effects or adverse reactions of other existing drugs shall be avoided by injecting A2M, such as corticosteroids and anesthetics. Furthermore, the method appreciates more precise curative effect compared with other experimental regenerative medical methods. In the future, individualized treatment based on autologous A2M enrichment technology will be put into clinical application. OA patients can use A2M filtered from their own plasma to downregulate the level of inflammation in knee joints. And perhaps, molecular engineering technique and biological engineering will provide us high-efficacy A2M variants with great quality and quantity, like the synthetic insulin.
However, some defects and insufficiencies have been found in the course of research. As mentioned before, the major mechanism of inhibitive effect of A2M is the transform of its molecular structure. Thus, the activation of A2M molecules is determined by its molecular conformation. A2M preparation gotten by autologous enrichment is comparatively heterogeneous for the existing technology is not advanced enough to examine the proportion of molecules with active conformation. In the future, we expect to mark the active site of A2M with a fluorescent probe, so that valuing the activity of A2M preparation is possible. As a broad-spectrum proteinase inhibitor, the levels of A2M in SF may change in the development process of OA. A new direction of early diagnosis of OA shall be provided if the combination of A2M active site and fluorescent probe is proved to be successful.
It is already known that the A2M-proteinase complex will be endocytosed by macrophages. And the important role of macrophages in tissue homeostasis and repair makes that macrophages can influence the immune microenvironment. It still needs to be explored that whether and how the macrophage-related metabolic process of A2M alters the intra-articular immune microenvironment of OA. Therefore, intervention on macrophage by artificial modification of A2M molecules might offer more possibilities for research of OA treatments.
References
Mobasheri A, Batt M. An update on the pathophysiology of osteoarthritis. Ann Phys Rehabil Med. 2016;59(5–6):333–9. https://doi.org/10.1016/j.rehab.2016.07.004.
Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7(1):33–42. https://doi.org/10.1038/nrrheum.2010.196.
Rehman AA, Ahsan H, Khan FH. alpha-2-Macroglobulin: a physiological guardian. J Cell Physiol. 2013;228(8):1665–75. https://doi.org/10.1002/jcp.24266.
Wang S, Wei X, Zhou J, Zhang J, Li K, Chen Q, et al. Identification of alpha2-macroglobulin as a master inhibitor of cartilage-degrading factors that attenuates the progression of posttraumatic osteoarthritis. Arthritis Rheum. 2014;66(7):1843–53. https://doi.org/10.1002/art.38576.
Li S, Xiang C, Wei X, Sun X, Li R, Li P, et al. Early supplemental alpha2-macroglobulin attenuates cartilage and bone damage by inhibiting inflammation in collagen II-induced arthritis model. Int J Rheum Dis. 2019;22(4):654–65. https://doi.org/10.1111/1756-185X.13457.
Zhang Y, Wei X, Browning S, Scuderi G, Hanna LS, Wei L. Targeted designed variants of alpha-2-macroglobulin (A2M) attenuate cartilage degeneration in a rat model of osteoarthritis induced by anterior cruciate ligament transection. Arthritis Res Ther. 2017;19(1):175. https://doi.org/10.1186/s13075-017-1363-4.
Garcia-Ferrer I, Marrero A, Gomis-Ruth FX, Goulas T. alpha2-macroglobulins: structure and function. Subcell Biochem. 2017;83:149–83. https://doi.org/10.1007/978-3-319-46503-6_6.
Bahhady R, Kim KJ, Borok Z, Crandall ED, Shen WC. Characterization of protein factor(s) in rat bronchoalveolar lavage fluid that enhance insulin transport via transcytosis across primary rat alveolar epithelial cell monolayers. Eur J Pharm Biopharm. 2008;69(3):808–16. https://doi.org/10.1016/j.ejpb.2008.01.028.
Feldman SR, Gonias SL, Pizzo SV. Model of alpha 2-macroglobulin structure and function. Proc Natl Acad Sci U S A. 1985;82(17):5700–4. https://doi.org/10.1073/pnas.82.17.5700.
Shi Y, Yamauchi T, Gaultier A, Takimoto S, Campana WM, Gonias SL. Regulation of cytokine expression by Schwann cells in response to alpha2-macroglobulin binding to LRP1. J Neurosci Res. 2011;89(4):544–51. https://doi.org/10.1002/jnr.22576.
Habtemichael EN, Brewer PD, Romenskaia I, Mastick CC. Kinetic evidence that Glut4 follows different endocytic pathways than the receptors for transferrin and alpha2-macroglobulin. J Biol Chem. 2011;286(12):10115–25. https://doi.org/10.1074/jbc.M111.217935.
Osterberg R, Pap S. Structure of alpha 2-macroglobulin in solution and its interaction with proteases: an X-ray scattering study using the contrast variation method. Ann N Y Acad Sci. 1983;421:98–111. https://doi.org/10.1111/j.1749-6632.1983.tb18096.x.
Akashi S, Nagakura S, Yamamoto S, Okuda M, Ohkuma Y, Nishimura Y. Structural characterization of human general transcription factor TFIIF in solution. Protein Sci. 2008;17(3):389–400. https://doi.org/10.1110/ps.073258108.
Imber MJ, Pizzo SV. Clearance and binding of two electrophoretic “fast” forms of human alpha 2-macroglobulin. J Biol Chem. 1981;256(15):8134–9.
Wang X, Schmidt DR, Joyce EJ, Kao WJ. Application of MS-based proteomics to study serum protein adsorption/absorption and complement C3 activation on poly(ethylene glycol) hydrogels. J Biomater Sci Polym Ed. 2011;22(10):1343–62. https://doi.org/10.1163/092050610X508400.
Pap T, Korb-Pap A. Cartilage damage in osteoarthritis and rheumatoid arthritis--two unequal siblings. Nat Rev Rheumatol. 2015;11(10):606–15. https://doi.org/10.1038/nrrheum.2015.95.
Demirag B, Sarisozen B, Durak K, Bilgen OF, Ozturk C. The effect of alpha-2 macroglobulin on the healing of ruptured anterior cruciate ligament in rabbits. Connect Tissue Res. 2004;45(1):23–7. https://doi.org/10.1080/03008200490278115.
Arandjelovic S, Dragojlovic N, Li X, Myers RR, Campana WM, Gonias SL. A derivative of the plasma protease inhibitor alpha(2)-macroglobulin regulates the response to peripheral nerve injury. J Neurochem. 2007;103(2):694–705. https://doi.org/10.1111/j.1471-4159.2007.04800.x.
Bert JM, Bert TM. Nonoperative treatment of unicompartmental arthritis: from bracing to injection. Clin Sports Med. 2014;33(1):1–10. https://doi.org/10.1016/j.csm.2013.08.002.
Calmet J, Esteve C, Boada S, Gine J. Analgesic effect of intra-articular ketorolac in knee arthroscopy: comparison of morphine and bupivacaine. Knee Surg Sports Traumatol Arthrosc. 2004;12(6):552–5. https://doi.org/10.1007/s00167-003-0483-3.
Breu A, Rosenmeier K, Kujat R, Angele P, Zink W. The cytotoxicity of bupivacaine, ropivacaine, and mepivacaine on human chondrocytes and cartilage. Anesth Analg. 2013;117(2):514–22. https://doi.org/10.1213/ANE.0b013e31829481ed.
Ng HP, Nordstrom U, Axelsson K, Perniola AD, Gustav E, Ryttberg L, et al. Efficacy of intra-articular bupivacaine, ropivacaine, or a combination of ropivacaine, morphine, and ketorolac on postoperative pain relief after ambulatory arthroscopic knee surgery: a randomized double-blind study. Reg Anesth Pain Med. 2006;31(1):26–33. https://doi.org/10.1016/j.rapm.2005.09.009.
Evans CH, Kraus VB, Setton LA. Progress in intra-articular therapy. Nat Rev Rheumatol. 2014;10(1):11–22. https://doi.org/10.1038/nrrheum.2013.159.
Cheng OT, Souzdalnitski D, Vrooman B, Cheng J. Evidence-based knee injections for the management of arthritis. Pain Med. 2012;13(6):740–53. https://doi.org/10.1111/j.1526-4637.2012.01394.x.
Arroll B, Goodyear-Smith F. Corticosteroid injections for osteoarthritis of the knee: meta-analysis. BMJ. 2004;328(7444):869. https://doi.org/10.1136/bmj.38039.573970.7C.
Godwin M, Dawes M. Intra-articular steroid injections for painful knees. Systematic review with meta-analysis. Can Fam Physician. 2004;50:241–8.
Bannuru RR, Natov NS, Obadan IE, Price LL, Schmid CH, McAlindon TE. Therapeutic trajectory of hyaluronic acid versus corticosteroids in the treatment of knee osteoarthritis: a systematic review and meta-analysis. Arthritis Rheum. 2009;61(12):1704–11. https://doi.org/10.1002/art.24925.
McAlindon TE, LaValley MP, Harvey WF, Price LL, Driban JB, Zhang M, et al. Effect of intra-articular triamcinolone vs saline on knee cartilage volume and pain in patients with knee osteoarthritis: a randomized clinical trial. JAMA. 2017;317(19):1967–75. https://doi.org/10.1001/jama.2017.5283.
McGarry JG, Daruwalla ZJ. The efficacy, accuracy and complications of corticosteroid injections of the knee joint. Knee Surg Sports Traumatol Arthrosc. 2011;19(10):1649–54. https://doi.org/10.1007/s00167-010-1380-1.
Li S, Xiang C, Wei X, Sun X, Li R, Li P, et al. Early supplemental alpha2-macroglobulin attenuates cartilage and bone damage by inhibiting inflammation in collagen II-induced arthritis model. Int J Rheum Dis. 2019;22:654–65. https://doi.org/10.1111/1756-185X.13457.
Richards MM, Maxwell JS, Weng L, Angelos MG, Golzarian J. Intra-articular treatment of knee osteoarthritis: from anti-inflammatories to products of regenerative medicine. Phys Sportsmed. 2016;44(2):101–8. https://doi.org/10.1080/00913847.2016.1168272.
Tietze DC, Geissler K, Borchers J. The effects of platelet-rich plasma in the treatment of large-joint osteoarthritis: a systematic review. Phys Sportsmed. 2014;42(2):27–37. https://doi.org/10.3810/psm.2014.05.2055.
Braun HJ, Kim HJ, Chu CR, Dragoo JL. The effect of platelet-rich plasma formulations and blood products on human synoviocytes: implications for intra-articular injury and therapy. Am J Sports Med. 2014;42(5):1204–10. https://doi.org/10.1177/0363546514525593.
Frizziero A, Giannotti E, Oliva F, Masiero S, Maffulli N. Autologous conditioned serum for the treatment of osteoarthritis and other possible applications in musculoskeletal disorders. Br Med Bull. 2013;105:169–84. https://doi.org/10.1093/bmb/lds016.
Baltzer AW, Moser C, Jansen SA, Krauspe R. Autologous conditioned serum (Orthokine) is an effective treatment for knee osteoarthritis. Osteoarthr Cartil. 2009;17(2):152–60. https://doi.org/10.1016/j.joca.2008.06.014.
Auw Yang KG, Raijmakers NJ, van Arkel ER, Caron JJ, Rijk PC, Willems WJ, et al. Autologous interleukin-1 receptor antagonist improves function and symptoms in osteoarthritis when compared to placebo in a prospective randomized controlled trial. Osteoarthr Cartil. 2008;16(4):498–505. https://doi.org/10.1016/j.joca.2007.07.008.
Crnogaca K, Bicanic G, Delimar D. Elevated CRP level could herald less efficient autologous conditioned serum (ACS) treatment. Med Hypotheses. 2016;86:135–7. https://doi.org/10.1016/j.mehy.2015.11.001.
da Silva ML, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008;26(9):2287–99. https://doi.org/10.1634/stemcells.2007-1122.
Najar M, Raicevic G, Crompot E, Fayyad-Kazan H, Bron D, Toungouz M, et al. The immunomodulatory potential of mesenchymal stromal cells: a story of a regulatory network. J Immunother. 2016;39(2):45–59. https://doi.org/10.1097/CJI.0000000000000108.
Wuchter P, Vetter M, Saffrich R, Diehlmann A, Bieback K, Ho AD, et al. Evaluation of GMP-compliant culture media for in vitro expansion of human bone marrow mesenchymal stromal cells. Exp Hematol. 2016;44(6):508–18. https://doi.org/10.1016/j.exphem.2016.02.004.
Squillaro T, Peluso G, Galderisi U. Clinical trials with mesenchymal stem cells: an update. Cell Transplant. 2016;25(5):829–48. https://doi.org/10.3727/096368915X689622.
Wehling P, Evans C, Wehling J, Maixner W. Effectiveness of intra-articular therapies in osteoarthritis: a literature review. Ther Adv Musculoskelet Dis. 2017;9(8):183–96. https://doi.org/10.1177/1759720X17712695.
Altman RD, Manjoo A, Fierlinger A, Niazi F, Nicholls M. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: a systematic review. BMC Musculoskelet Disord. 2015;16:321. https://doi.org/10.1186/s12891-015-0775-z.
Lo GH, LaValley M, McAlindon T, Felson DT. Intra-articular hyaluronic acid in treatment of knee osteoarthritis: a meta-analysis. JAMA. 2003;290(23):3115–21. https://doi.org/10.1001/jama.290.23.3115.
Arrich J, Piribauer F, Mad P, Schmid D, Klaushofer K, Mullner M. Intra-articular hyaluronic acid for the treatment of osteoarthritis of the knee: systematic review and meta-analysis. CMAJ. 2005;172(8):1039–43. https://doi.org/10.1503/cmaj.1041203.
Rutjes AW, Juni P, da Costa BR, Trelle S, Nuesch E, Reichenbach S. Viscosupplementation for osteoarthritis of the knee: a systematic review and meta-analysis. Ann Intern Med. 2012;157(3):180–91. https://doi.org/10.7326/0003-4819-157-3-201208070-00473.
Printz JO, Lee JJ, Knesek M, Urquhart AG. Conflict of interest in the assessment of hyaluronic acid injections for osteoarthritis of the knee: an updated systematic review. J Arthroplast. 2013;28(8 Suppl):30–3 e1. https://doi.org/10.1016/j.arth.2013.05.034.
Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2012;64(4):465–74. https://doi.org/10.1002/acr.21596.
Jevsevar DS. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;21(9):571–6. https://doi.org/10.5435/JAAOS-21-09-571.
Cuellar JM, Cuellar VG, Scuderi GJ. alpha2-Macroglobulin: autologous protease inhibition technology. Phys Med Rehabil Clin N Am. 2016;27(4):909–18. https://doi.org/10.1016/j.pmr.2016.06.008.
Funding
The present project has been supported by the National Natural Science Foundation of China (Grant Nos. 81572207, 81201435) and the Cultivate Scientific Research Excellence Programs of Higher Education Institutions in Shanxi (CSREP), China.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Molecular Biology of Cell Death and Aging
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhu, M., Zhao, B., Wei, L. et al. alpha-2-Macroglobulin, a Native and Powerful Proteinase Inhibitor, Prevents Cartilage Degeneration Disease by Inhibiting Majority of Catabolic Enzymes and Cytokines. Curr Mol Bio Rep 7, 1–7 (2021). https://doi.org/10.1007/s40610-020-00142-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40610-020-00142-z