Abstract
Purpose of Review
This article reviews and summarises the recent findings on emerging seafood-borne parasites, Kudoa spp., that have received comparatively little attention in the literature.
Recent Findings
Currently, two species, namely, K. hexapunctata and K. septempunctata, have been identified as causing infections in humans. However, it is worth noting that the existing nomenclature and methods for identifying Kudoa spp. may undergo substantial changes as more effective identification techniques are developed. This implies the possibility of additional species capable of infecting humans. The symptoms induced by these parasites in humans, such as vomiting and diarrhoea, can easily be confused with similar symptoms caused by other pathogens like viruses or bacteria. Consequently, misdiagnosis or underdiagnosis is quite common. Moreover, new hosts and expanded distribution patterns are being discovered on a regular basis.
Summary
This review sheds light on the potential of Kudoa spp. to cause diseases in humans, emphasizing the importance of comprehensive seafood safety measures to ensure responsible seafood consumption. Further investigation into these lesser-known parasites is warranted to better understand their prevalence, distribution, and pathogenicity in seafood-related infections.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Seafood, like many other food items, offers a delectable array of flavours and nutritional benefits to consumers. However, it is crucial to maintain vigilance in seafood safety to prevent potential health risks [1]. Seafood consumption has the potential to be a source of diseases for humans, especially when complacency in food safety practices arises [2]. Among the various concerns associated with seafood consumption, the presence of parasites in wild fish, both in marine and freshwater environments, stands out as a significant issue. These parasites are common and, in some cases, can cause diseases in humans. As such, understanding the risks posed by seafood-borne parasites is essential for promoting safe and responsible seafood consumption.
When investigating the range of seafood-borne parasitic diseases, certain parasites consistently appear in the literature. Among them, anisakid nematodes and diphylobothriid cestode parasites stand out as prominent and well-studied contributors to human infections, due to their prevalence in various marine and freshwater environments and their potential to cause diseases in humans who consume contaminated seafood [3,4,5,6]. Their impact on human health and the importance of vigilant seafood safety measures have been extensively explored and documented. However, it is important to note that there exists a wide range of other seafood-borne parasites that have received comparatively less attention in the scientific literature. These lesser-studied parasites can also pose potential health risks to humans when ingested through infected seafood and therefore warrant further investigation to better understand their prevalence, distribution, and pathogenicity in seafood-related infections. In this article, Kudoa spp., a lesser-known group of parasites in wild fish and their potential to cause disease in humans is reviewed, shedding light on the importance of seafood safety measures.
The Parasite
Kudoa is comprised of highly specialized metazoan parasites of fish hosts with a wide host range. The genus Kudoa, originally described by Meglitsch [7], currently comprises a total of 95 recognised species of myxosporean parasites [8,9,10,11,12,13,14,15,16]. The identification of Kudoa spp. used to be based primarily on the examination of spore morphology and morphometry. Eiras et al. [8] have provided essential morphological and morphometric characteristics for these species, along with relevant data such as the site of infection within the fish host. However, by combining morphology, molecular, and other approaches, researchers revealed novel diversity and demonstrated that strains and morphologically cryptic species characterise many taxa [17]. Application of new approaches to the existing taxa within the genus Kudoa may lead to further resolution of cryptic species, discovery of new species, and assessment of the taxonomic status of the current taxa.
Kudoa spp. are predominantly histozoic parasites, meaning they sporulate within the muscle tissue of marine teleosts [18]. In their research, Whipps et al. [19] emphasized the significance of the site of infection in understanding the relationships among certain Kudoa species. Atkinson et al. [17] and Molnár and Eszterbauer [20] found that tissue selection is a key taxonomic feature for histozoic myxozoans, as they develop mature spores in only one tissue type.
Other Names for Kudoa spp.
Similar to numerous other parasites, the taxonomy of Kudoa spp. is complex and confusing. When researching the literature concerning any aspects of these parasites, such as previous records of human cases, it becomes crucial to take into account the earlier designations used for the parasite and any taxonomic modifications to its scientific nomenclature. Certain names that were previously utilized for this genus are now considered unacceptable. Some of the previous names, now unacceptable, for the genus are Hexacapsula Arai and Matsumoto, 1953; Neochloromyxum Matsumoto, 1954; Pentacapsula Naidenova and Zaika, 1970; and Septemcapsula Hsieh and Chen, 1984. For a comprehensive list, readers are referred to taxonomic platforms, such as the Word Register of Marine Species.
Fish Hosts
The presence of Kudoa infections in fish hosts poses concerns for both aquaculture and commercial fisheries, due to leading to necrosis of the flesh of the fish, followed by rapid postmortem deterioration and liquefaction [21, 22]. Species of Kudoa have the ability to infect a wide range of wild and farmed fish, including marine, estuarine, and anadromous species [8]. These parasites have been documented in various popular edible fish, including Atlantic Spanish mackerel (Scomberomorus maculatus), Atlantic herring (Clupea harengus), flathead grey mullet (Mugil japonicus), chub mackerel (Scomber japonicus), yellowtail amberjack (Seriola lalandi), Atlantic mackerel (Scomber scombrus), Japanese amberjack (Seriola quinqueradiata), Pacific hake (Merluccius productus), mahi mahi (Coryphaena hippurus), Atlantic salmon (Salmo salar), flatmouth sea catfish (Tachysurus platystomus), Chinese sea bass (Lateolabrax sp.), and red sea bream (Pagrus major) [8, 18, 23, 24] in many geographical regions. It should be noted that the list of susceptible fish species is not limited to these examples. New hosts are continuously reported for Kudoa spp. [11, 23, 25,26,27].
Kudoa spp. predominantly inhabit edible parts of fish hosts, including the skeletal musculature, but can also be found in various other organs such as the ovary [28], central nervous system, eyes, swim bladder, pericardial cavity, gall bladder, urinary bladder, and kidney [29,30,31,32,33,34]. Certain species of Kudoa, for example, K. islandica, K. megacapsula, K. musculoliquefaciens, K. paniformis, and K. thyrsites, are responsible for postmortem liquefaction of host tissues [35, 36], colloquially known as “jelly meat”, which significantly diminishes the commercial value of the affected fish. They can also cause unsightly macroscopic cysts in the musculature leading to a reduction in the market value of products of infected fish.
Knowledge on the impacts of parasites on live fish is limited. They may have adverse effects by inducing changes in the fatty acid and amino acid composition in the flesh of fish [34], impacting the overall quality and nutritional profile of the fish meat. If fish eggs are infected with K. ovivora, then they are not viable [28].
Most species of Kudoa have been considered host-specific. For example, K. atropi and K. branchiata display a high degree of host specificity and have only been recovered from one host. However, broad host specificity (i.e., the ability to infect a wide range of fish species) is also common. Kudoa thyrsites infects approximately 39 species across different fish families worldwide (19 families and 10 orders) [8, 37,38,39,40]. Similarly, K. nova, K. iwatai, and K. thalassomi infect 20 fish species [37, 38, 41]. However, it is worth considering the possibility that some of these species might be a collection of morphologically indistinguishable species, each infecting different fish populations around the world. This emphasizes the need for further research, especially utilising molecular characterisation and investigation into the complexities of Kudoa species and their interactions with various host species across different geographic regions.
Geographic Distribution
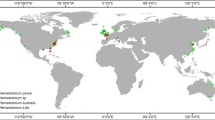
The genus Kudoa has been documented on all continents, except Antarctica, as depicted in Fig. 1 [8]. Kudoa spp. have been reported in various countries across South and North America (e.g., Brazil, Peru, Argentina, USA, Mexico, and Panama), Australia, Asia (e.g., Japan, India, Taiwan, China, Yemen, Philippine Islands, and South Korea), European countries (France, Ukraine, and Spain), and Africa (e.g., Tunisia, Senegal, Nigeria, and South Africa).
Life Cycle
The life cycle of Kudoa spp. remains largely obscure and poorly understood in the scientific literature. It is generally assumed, based on observations in other myxozoan parasites, that the life cycle involves both a fish host and an oligochaete or polychaete worm. It is presumed that the olive flounder is infected via ingestion of annelids. When it comes to K. septempunctata infection, it is probable that young, cultured flounder contract spores, which then undergo division and multiplication, eventually maturing as the flounder itself grows.
However, due to the diverse range of species within the genus and their varying preferences for specific locations within the fish’s body, it is likely that the life cycles differ among different Kudoa species. Apicomplexan protozoan parasites, for example, have also adapted and evolved unique life cycles suited to their specific needs, and, similar to Kudoa species, exhibit different life cycle patterns. For instance, intestinal species of Eimeria follow a direct life cycle through oral-faecal transmission, while blood-dwelling Apicomplexa typically undergo indirect life cycles with transmission via vector intermediate hosts. Similarly, it is plausible that various species of Kudoa have distinct life cycle strategies, given their diverse preferences for infecting different organs in various host species.
Additionally, some species of Kudoa show evidence of direct transmission from fish to fish. For instance, K. ovivora infects the eggs of the wrasse Thalassoma bifasciatum, and it may not require an intermediate host since fish-fed infected tissue developed more infections compared to unfed fish [28]. On the contrary, direct transmission through fresh myxospores does not occur in K. thyrsites. However, at some stage of its life cycle, K. thyrsites circulates throughout the blood, making it transmissible by injecting the blood of an infected salmon into the intraperitoneal cavity of a naïve salmon [18].
This variability in life cycle strategies within the genus Kudoa highlights the complexity and diversity of these parasites and underscores the need for further research to elucidate the specific life cycles of different Kudoa species and their interactions with various hosts. Understanding these life cycles is essential for developing effective strategies to control and manage Kudoa infections, particularly in aquaculture and commercial fisheries settings.
Human Infections
Until recently, it was believed that consuming fresh fish infected with Kudoa spp. does not cause any illness in humans. This was due to Kudoa islandica being common in Icelandic edible fish which have been part of the locals’ diet for centuries without any documented cases of food poisoning [42].
Studies dealing with the potential role of Kudoa spp. in causing disease in humans have been mainly conducted in Japan and Korea. The consumption of raw fish in the form of sashimi and sushi is deeply ingrained in the food cultures of these countries, making them at the forefront of knowledge and management of these parasitic infections. To the best of our knowledge, no other country reported human infection with Kudoa spp. Although it should be noted that in other parts of the world, the absence of such food-borne parasitic diseases may be attributed to misdiagnosis and/or lack of awareness [43].
Following an increase in reports of an unidentified food-borne illness in Japan, associated with the consumption of raw fish from 2003, it was found that 130 of the 158 reported cases were linked to the consumption of raw olive flounder (Paralichthys olivaceus) [44]. The causative agent has subsequently been identified as K. septempunctata [44]. Because this parasite was also identified in olive flounder imported from Korea [21], the Japanese Ministry of Health, Labour, and Welfare acknowledged K. septempunctata as a potential source of foodborne illness. Consequently, they increased scrutiny through testing, encompassing both domestically sourced and imported olive flounder from the Japanese [45]. Likewise, in Korea, when there were reports of K. septempunctata being found in farmed olive flounder, the Ministry of Food and Drug Safety initiated the development and dissemination of a method for identifying this parasite in olive flounder [21, 46, 47].
Currently, in Japan, there are approximately 40 cases of food poisoning due to K. septempunctata annually, affecting more than 450 patients [48]. Additionally, another species, K. hexapunctata was also found to infect humans in Japan [49,50,51]. Reported cases involving both species typically describe infections in groups of people after attending functions or events, such as 10 people falling ill after consuming Pacific blue tuna infected with K. hexapunctata, or similar incidents associated with K. septempunctata [52].
Misdiagnosis and underdiagnosis of these infections may be common due to the relatively new and emerging nature of the disease, as well as the presence of common food poisoning symptoms and self-recovery of infected people. Nevertheless, the implementation of control measures, such as periodic inspections of flounder hatcheries and farms, disinfection, and parasite elimination of rearing waters, has led to a decline in case numbers caused by farmed animals [48, 53]. However, new incidences have occurred in connection with wild and imported fish emphasizing the impact of parasite transmission between wild and farmed animals and the importance of utilising a holistic approach.
Causative Agents and Source of Infection
In Japan and Korea, it has been established that two Kudoa species, namely, K. septempunctata and K. hexapunctata, are the causative agents of human infections. Kudoa septempunctata, in particular, has been further classified into three distinct genotypes, labelled as ST1, ST2, and ST3, based on the combined analysis of two mitochondrial genes, namely, cytochrome c oxidase subunit 1 (cox1) and large subunit rRNA (rnl). Research findings indicate that K. septempunctata prevalent in Japan primarily displays either the ST1 or ST2 genotype, whereas the ST3 genotype is more commonly found in Korea [54,55,56].
The mechanism of pathogenicity of the disease caused by these Kudoa species is still largely unknown. Recently, Hong et al. [57] examined the gastroenteropathy of K. septempunctata in human colon adenocarcinoma cells as well as in experimental mice inoculated with K. septempunctata spores and concluded that Kudoa spores may induce diarrhoea and emesis by increasing intestinal permeability and serotonin secretion.
As mentioned earlier in this article, specific identification of the Kudoa spp. has been advancing, and therefore, the application of new approaches to the existing taxa within Kudoa spp. may lead to the discovery of a wider range of species being able to infect humans.
The parasite, K. septempunctata has been reported from a diverse range of fish, including olive flounder (family Paralichthyidae; order Pleuronectiformes) as well as fish belonging to the order Tetraodontiformes, including black scrapers (Thamnaconus modestus; family: Monacanthidae) and wild grass puffer (Takifugu alboplumbeus; family: Tetraodontidae) and Eupercaria fish, including Japanese whiting (Sillago japonica; family: Sillaginidae) [21, 38, 48]. This suggests that the source of human infections potentially could be across many commonly consumed species.
Moreover, an intriguing observation is that approximately 75% of Kudoa-related human infections occur during the late summer season [45]. Nevertheless, the underlying reasons for this distinctive seasonal pattern remain unclear, warranting further investigation.
Symptoms
The clinical signs associated with infection with K. septempunctata are transient and usually manifest as the common symptoms of vomiting, diarrhoea, nausea, and abdominal pain [58, 59], typically appearing after an incubation period of approximately 2 to 20 h following the consumption of a meal that includes raw fish [60]. The clinical signs associated with infection with K. hexapunctata are acute gastrointestinal symptoms 2–12 h after the meal, including diarrhoea and nausea being the most common symptoms, followed by abdominal pain and fever (37.0–37.8 °C) [51]. It is noteworthy that in some reports, patients were co-infected with other pathogens, such as Giardia lamblia, Escherichia coli, and Norovirus, alongside Kudoa [52], while in other cases, cultures of patients’ stools were negative for Staphylococcus aureus, Salmonella spp., Vibrio spp., Shigella spp., Campylobacter spp., verotoxin-producing Escherichia coli, and norovirus [61].
The pathogenicity of Kudoa in humans is under debate among researchers. According to experiments carried out by Kawai et al. [44] involving animals, it was suggested that a considerable spore count exceeding 106 Kudoa spores per mouse was necessary to trigger any illness. As a result of these findings, Suzuki et al. [49] concluded that K. hexapunctata typically does not lead to gastrointestinal inflammatory symptoms unless it is present in significant abundance within the raw fish that is consumed.
Diagnosis
Typically, the diagnosis of acute gastroenteritis relies on a patient’s medical history and physical examination, with culture or blood tests being infrequently conducted. This can elevate the risk of either underdiagnosis or misdiagnosis of acute gastroenteritis, particularly when caused by less-studied parasites like Kudoa spp. in countries where awareness about seafood-borne parasites is not high, as it may be more commonly attributed to acute viral gastroenteritis. The symptoms of a Kudoa infection closely resemble those of other water and foodborne illnesses caused by pathogens such as Staphylococcus aureus or Bacillus cereus, which can further complicate the diagnostic process [52]. In humans, it is essential to detect Kudoa DNA in faecal or vomit samples from individuals experiencing food poisoning. Reports from Japan and Korea indicate that attempts have been made to detect DNA and morphological evidence of the parasite in the food consumed by affected individuals [52, 59]. In Japan, the Kudoa ( +) detection rate in faeces was 69.2% of cases with the detection rate in faeces decreasing rapidly between 25.5 and 28.5 h of the time interval from food intake to the epidemiologic survey. In Korea, the detection rate of faecal samples of symptomatic patients and raw fish consumed by them was 100% positive using molecular tools, whereas rectal swab samples yielded 25% positive results. Both studies concluded that obtaining stool samples/rectal swabs from patients with Kudoa food poisoning promptly following symptom onset is crucial.
The issue with using PCR for disease diagnosis, particularly in cases involving parasites like Kudoa, lies in its limitation that allows for the identification of the parasite’s genetic material in various samples such as gut samples (faeces, vomit, and saliva). However, the mere detection of the parasite’s genetic material in these samples does not definitively indicate that the parasite has infected the patient or is actively replicating within their body. To ascertain an infection and evaluate its severity, additional steps such as blood samples or tissue biopsy may be necessary. These samples can offer more direct evidence of the parasite’s presence in the body and its activity, such as replication or undergoing its developmental cycle within the human host. Therefore, PCR results should be interpreted cautiously and in conjunction with other clinical data to ensure an accurate diagnosis.
Additionally, the Illumina Genome Analyzer II has been successfully employed to detect Kudoa DNA in leftover portions of fish consumed by patients. However, it also should be noted that parasites may be present in some fish for a long time [62].
Despite persistent suspicions of Kudoa-related foodborne outbreaks, the rate of identifying K. septempunctata in Japan and Korea remains very low. One contributing factor is the challenge in obtaining stool and vomit samples for diagnosing Kudoa infection [59].
Tachibana and Watari [51] emphasized the importance of obtaining information about the source of fish used in the preparation of dishes like sashimi and sushi (raw flounder and adult or juvenile PBT) from patients to ascertain if their gastrointestinal symptoms, such as vomiting and diarrhoea, are linked to a Kudoa infection. This underscores the significance of understanding the origin of fish, especially in regions where awareness in this regard may be lacking [63, 64]. Diagnosing infections in fish can be challenging due to the intricate developmental cycle of these parasites. In fish hosts, the parasites undergo migrations from invasion sites to target tissues, leading to significant morphological changes that complicate morphological identification. Antibodies (monoclonal and polyclonal) against several myxozoans, including Kudoa spp., have been developed [65]. Additionally, lectin-based assays have proven successful [66]. However, both lectins and antibodies have inherent limitations for clinical diagnosis due to stage-specific affinities and cross-reactivity with host tissues and other parasites.
Japan is renowned for being at the forefront of seafood safety. Given the complexity and expertise involved in this process, it is reasonable to expect that other countries may face a more extended timeline in implementing similar measures. Nonetheless, by learning from Japan’s experiences and collaborating on global food safety initiatives, progress can be made towards enhancing seafood safety worldwide.
Treatment and Prevention
This illness is generally self-limiting, leading to a good prognosis in all reported cases. This means that the symptoms resolve on their own over time without the need for specific medical interventions. However, due to the potential severity of gastrointestinal symptoms and the uncertainty surrounding the causative agent, it is crucial for healthcare professionals to be vigilant in recognizing and managing cases of this illness. In the case of patients with severe symptoms, supportive care (infusion fluids, antiemetics, and antifebrile treatment) may be required [58].
Kudoa septempunctata infects the muscles of olive flounder and loses virulence by freezing at − 20 °C for 4 h or more or heating to a core temperature of 75 °C for 5 min or more [44]. In aquaculture systems (olive flounder hatcheries), double treatments of a water supply with sand filtration and UV irradiation have been effective in prevention [53].
Conclusion
Kudoa is recognised as a seafood-borne pathogen primarily in Japan and Korea, with no reported cases in humans from other countries to date. Currently, two species, K. septempunctata and K. hexapunctata, are identified as the causative agents of disease in humans, although the specific identification of Kudoa spp. may undergo significant revision given recent advances in this area. Symptoms primarily manifest as gastrointestinal discomfort and are typically self-limiting. The precise mechanism of pathogenicity induced by these parasites remains largely unknown, leading to debates among researchers outside Japan and Korea regarding whether Kudoa constitutes a seafood-borne pathogen. While some argue that further medical research is required to ascertain the role, if any, of Kudoa in causing human disease due to insufficient evidence presented to date, others emphasise the global dissemination of Japanese and Korean cuisines, particularly the consumption of raw fish dishes, which underscores the public health significance of identifying food poisoning attributed to Kudoa spp. The detection of K. septempunctata in imported fish from Korea to Japan suggests the potential for the international spread of this disease through the global trade of farmed fish. Consequently, meticulous risk assessment and management of fish harbouring Kudoa parasites are imperative to mitigate associated risks linked to the consumption of raw or undercooked fish worldwide.
References
FAO. FAO identifies top 10 foodborne parasites. Vet Rec. 2014;175(3):58-. https://doi.org/10.1136/vr.g4607.
Shamsi S. Seafood-borne parasitic diseases: a “one-health” approach is needed. Fishes. 2019;4(1):9. https://doi.org/10.3390/fishes4010009.
Rahmati AR, Kiani B, Afshari A, Moghaddas E, Williams M, Shamsi S. World-wide prevalence of Anisakis larvae in fish and its relationship to human allergic anisakiasis: a systematic review. Parasitol Res. 2020;119:3585–94. https://doi.org/10.1007/s00436-020-06892-0.
Baird FJ, Morishima Y, Sugiyama H. Anisakis, allergy and the globalization of food. In: Lopata AL, editor. Food Allergy. CRC Press. 2017. pp. 155–75.
Scholz T, Garcia HH, Kuchta R, Wicht B. Update on the human broad tapeworm genus Diphyllobothrium), including clinical relevance. Clin Microbiol Rev. 2009;22(1):146-+. https://doi.org/10.1128/cmr.00033-08.
Chai J-Y, Darwin Murrell K, Lymbery AJ. Fish-borne parasitic zoonoses: status and issues. Int J Parasitol. 2005;35(11–12):1233–54. https://doi.org/10.1016/j.ijpara.2005.07.013.
Meglitsch PA. Studies on Myxosporidia from the beaufort region. II. Observations on Kudoa clupeidae (Hahn), gen. nov. J Parasitol. 1947;33(3):271–7.
Eiras JC, Saraiva A, Cruz C. Synopsis of the species of Kudoa Meglitsch, 1947 (Myxozoa: Myxosporea: Multivalvulida). Syst Parasitol. 2014;87(2):153–80. https://doi.org/10.1007/s11230-013-9461-4.
Yurakhno VM, Slynko EE, Chinh NN, Ha VT, Whipps CM. Multivalvulidan myxosporeans from marine fishes in Nha Trang Bay, Vietnam, with descriptions of Kudoa igori n. sp. and Kudoa borimiri n. sp. from mullets. Parasitol Res. 2022;121(10):2927–43.
Iglesias R, Rangel LF, Fernández-Vázquez F, Santos MJ, García-Estévez JM. Morphometric and molecular characterization of Kudoa encrasicoli n. sp. (Myxozoa: Myxosporea) from the European anchovy, Engraulis encrasicolus (L.)(Clupeiformes: Engraulidae). Syst Parasitol. 2022;99(5):621–36.
Li Y-C, Inoue K, Zhang J-Y, Sato H. Descriptions of three new species and new host or distribution records of five species of the genus Kudoa (Myxozoa: Myxosporea: Multivalvulida) in commercial fishes collected from South China Sea. Acta Parasitol. 2022;67(2):976–96.
Velasco M, Eduard J, Neto JLS, Dias LdNS, Matos E, Gonçalves EC. Kudoa rousseauxii n. sp. (Cnidaria: Multivalvulida) infects the skeletal muscles of the freshwater fish Brachyplatystoma rousseauxii in the Amazon River. Acta Parasitologica. 2022;67(2):962–9.
da Silva DT, da Silva MF, Lima AM, Matos PS, de Carvalho Sanches O, Matos ER, et al. Utrastructure and molecular phylogeny of the myxozoan Kudoa ocellatus n. sp. (Myxozoa: Kudoidae), a parasite of the Oscar, Astronotus ocellatus (Agassiz, 1831; Teleostei: Cichlidae), a fish from northern Brazil. Parasitol Int. 2022;86:102472.
Li Y-C, Inoue K, Tanaka S, Zhang J-Y, Sato H. Identification of four new Kudoa spp. (Myxozoa: Myxosporea: Multivalvulida) in commercial fishes collected from South China Sea, Atlantic Ocean, and Bering Sea by integrated taxonomic approach. Parasitol Res. 2020;119:2113–28.
Cardim J, Araújo-Neto J, da Silva DT, Hamoy I, Matos E, Abrunhosa F. Kudoa yasai n. sp. (Multivalvulida: Kudoidae) from the skeletal muscle of Macrodon ancylodon (Sciaenidae) on the northern Atlantic coast, Brazil. Parasitol Res. 2020;119:1743–52.
Neto JPA, Cardim J, Silva D, Hamoy I, Matos E, Abrunhosa F. Kudoa ajurutellus n. sp. (Multivalvulida: Kudoidae), a parasite of the skeletal musculature of the Bressou sea catfish, Aspistor quadriscutis, in northeastern of the State of Para. Zootaxa. 2020;4718(3):371–80.
Atkinson SD, Bartošová-Sojková P, Whipps CM, Bartholomew JL. Approaches for characterising myxozoan species. In: Okamura B et al., editors. Myxozoan Evol Ecol Dev. 2015:111–23. Springer International Publishing Switzerland 2015. https://doi.org/10.1007/978-3-319-14753-6_16.
Moran JDW, Whitaker DJ, Kent ML. A review of the myxosporean genus Kudoa Meglitsch, 1947, and its impact on the international aquaculture industry and commercial fisheries. Aquaculture. 1999;172(1–2):163–96. https://doi.org/10.1016/s0044-8486(98)00437-2.
Whipps C, Grossel G, Adlard R, Yokoyama H, Bryant M, Munday B, et al. Phylogeny of the Multivalvulidae (Myxozoa: Myxosporea) based on comparative ribosomal DNA sequence analysis. J Parasitol. 2004;90(3):618–22.
Molnár K, Eszterbauer E. Specificity of infection sites in vertebrate hosts. In: Okamura B et al., editors. Myxozoan Evol Ecol Dev. 2015:295–313. Springer International Publishing Switzerland.
Matsukane Y, Sato H, Tanaka S, Kamata Y, Sugita-Konishi Y. Kudoa septempunctata n. sp (Myxosporea: Multivalvulida) from an aquacultured olive flounder (Paralichthys olivaceus) imported from Korea. Parasitol Res. 2010;107(4):865–72. https://doi.org/10.1007/s00436-010-1941-8.
Bolin JA, Cummins SF, Mitu SA, Schoeman DS, Evans KJ, Scales KL. First report of Kudoa thunni and Kudoa musculoliquefaciens affecting the quality of commercially harvested yellowfin tuna and broadbill swordfish in Eastern Australia. Parasitol Res. 2021;120(7):2493–503. https://doi.org/10.1007/s00436-021-07206-8.
Whipps CM, Adlard RD, Bryant MS, Lester RJG, Findlay V, Kent ML. First report of three Kudoa species from Eastern Australia: Kudoa thyrsites from mahi mahi (Coryphaena hippurus), Kudoa amamiensis and Kudoa minithyrsites n. sp from sweeper (Pempheris ypsilychnus). J Eukaryot Microbiol. 2003;50(3):215–9. https://doi.org/10.1111/j.1550-7408.2003.tb00120.x.
Yokoyama H, Grabner D, Shirakashi S. Transmission biology of the Myxozoa. In: Carvalho D, Silva G, David and Silva RJ, editors. Health and environment in aquaculture 2012. IntechOpen
Inoue K, Kasai A, Argamjav E, Sato H. Four carangid fish species as new host records for Kudoa trachuri Matsukane, Sato, Tanaka, Kamata et Sugita-Konishi, 2011 (Myxozoa: Multivalvulida), and description of a new species, Kudoa longichorda sp. n., forming pseudocysts in the muscle of Decapterus tabl Berry. Folia Parasitologica. 2022;69:1–13.
Li Y-C, Inoue K, Zhang J-Y, Sato H. New records of three commercial fish hosts for two Unicapsula spp. and Kudoa megacapsula (Myxozoa: Myxosporea: Multivalvulida). Parasitol Res. 2022;121(11):3133–45. https://doi.org/10.1007/s00436-022-07584-7.
Hoai TD, Nhinh DT, Giang NTH, Senapin S, Dong HT. Detection and characterization of Kudoa thunni from uncooked yellowfin tuna (Thunnus albacares) in Southeast Asia. Parasitol Int. 2022;87:102536.
Swearer SE, Robertson DR. Life history, pathology, and description of Kudoa ovivora n. sp (Myxozoa, Myxosporea): an ovarian parasite of Caribbean labroid fishes. J Parasitol. 1999;85(2):337–53. https://doi.org/10.2307/3285645.
Burger MAA, Cribb TH, Adlard RD. Patterns of relatedness in the Kudoidae with descriptions of Kudoa chaetodoni n. sp and K. lethrini n. sp (Myxosporea: Multivalvulida). Parasitology. 2007;134:669–81. https://doi.org/10.1017/s0031182006001995.
Grossel GW, Dykova I, Handlinger J, Munday BL. Pentacapsula neurophila sp. n. (Multivalvulida) from the central nervous system of striped trumpeter, Latris lineata (Forster). J Fish Dis. 2003;26(6):315–20. https://doi.org/10.1046/j.1365-2761.2003.00459.x.
Whipps CM, Grossel G, Adlard RD, Yokoyama H, Bryant MS, Munday BL, et al. Phylogeny of the multivalvulidae (Myxozoa : Myxosporea) based on comparative ribosomal DNA sequence analysis. J Parasitol. 2004;90(3):618–22. https://doi.org/10.1645/ge-153r.
Heiniger H, Adlard RD. Host specificity and local infection dynamics of Kudoa leptacanthae n. sp (Multivalvulida: Kudoidae) from the pericardial cavity of two Zoramia spp. (Perciformes: Apogonidae) at Lizard Island lagoon, Queensland, Australia. Parasitol Int. 2012;61(4):697–706. https://doi.org/10.1016/j.parint.2012.08.001.
Wang P-C, Huang J-p, Tsai M-A, Cheng S-Y, Tsai S-S, Chen S-D, et al. Systemic infection of Kudoa lutjanus n. sp. (Myxozoa: Myxosporea) in red snapper Lutjanus erythropterus from Taiwan. Dis Aquat Organ. 2005;67(1–2):115–24.
Özer A, Kaya Y, Okkay S, Kocatepe D. Infection of Kudoa anatolica (Cnidaria: Myxozoa): impact on the nutritional composition of Atherina hepsetus (Atherinidae) flesh. J Aquat Food Prod Technol. 2023;32(4):462–74. https://doi.org/10.1080/10498850.2023.2229820.
Langdon JS. Myoliquefaction postmortem (milky flesh) due to Kudoa thyrsites (Gilchrist) (Myxosporea, Multivalvulida) in mahi mahi, Coryphaena hippurus. J Fish Dis. 1991;14(1):45–54. https://doi.org/10.1111/j.1365-2761.1991.tb00575.x.
Yokoyama H, Whipps CM, Kent ML, Mizuno K, Kawakami H. Kudoa thyrsites from Japanese flounder and Kudoa lateolabracis n. sp from Chinese sea bass: causative myxozoans of post-mortem myoliquefaction. Fish Pathol. 2004;39(2):79–85. https://doi.org/10.3147/jsfp.39.79.
Burger MAA, Adlard RD. Low host specificity in the Kudoidae (Myxosporea: Multivalvulida) including seventeen new host records for Kudoa thalassomi. Folia Parasitol. 2011;58(1):1–16. https://doi.org/10.14411/fp.2011.001.
Kasai A, Li Y-C, Mafie E, Sato H. New host records of monacanthid fish for three Kudoa spp. (K. septempunctata, K. thyrsites, and K. shiomitsui) prevalent in the olive flounder (Paralichthys olivaceus), with the description of K. parathyrsites n. sp. from a black scraper (Thamnaconus modestus). Parasitol Res. 2016;115(7):2741–55.
Whipps CM, Kent ML. Phylogeography of the cosmopolitan marine parasite Kudoa thyrsites (Myxozoa : Myxosporea). J Eukaryot Microbiol. 2006;53(5):364–73. https://doi.org/10.1111/j.1550-7408.2006.00114.x.
Cavaleiro B, Serrão J, Nogueira S, Ribeiro L, Hermida M, Cruz C, et al. Survey of Kudoa spp. (Myxozoa, Cnidaria) in fishes from the Madeira Archipelago and the Portuguese mainland coast: detection of Kudoa thyrsites in new hosts Scomber colias and Micromesistius poutassou. Folia Parasitologica. 2021;98(1):1–7.
Shirakashi S, Yamane K, Ishitani H, Yanagida T, Yokoyama H. First report of Kudoa species in the somatic muscle of the Japanese parrotfish Calotomus japonicus (Scaridae) and a description of Kudoa igami, n. sp. (Myxozoa: Multivalvulida). Parasitol Res. 2014;113:2515–24.
Kristmundsson Á, Freeman MA. Negative effects of Kudoa islandica n. sp. (Myxosporea: Kudoidae) on aquaculture and wild fisheries in Iceland. Int J Parasitol Parasites Wildl. 2014;3(2):135–46. https://doi.org/10.1016/j.ijppaw.2014.06.001.
Shamsi S, Sheorey H. Seafood-borne parasitic diseases in Australia: are they rare or underdiagnosed? Intern Med J. 2018;48(5):591–6.
Kawai T, Sekizuka T, Yahata Y, Kuroda M, Kumeda Y, Iijima Y, et al. Identification of Kudoa septempunctata as the causative agent of novel food poisoning outbreaks in Japan by consumption of Paralichthys olivaceus in raw fish. Clin Infect Dis. 2012;54(8):1046–52. https://doi.org/10.1093/cid/cir1040.
Sugita-Konishi Y, Sato H, Ohnishi T. Novel foodborne disease associated with consumption of raw fish, olive flounder (Paralichthys olivaceus). Food Safety. 2014;2(4):141–50.
Song J-Y, Choi J-H, Choi H-S, Jung SH, Park M. Monitoring of Kudoa septempunctata in cultured olive flounder and wild fish in Jeju Island during 2012. J Fish Pathol. 2013;26(3):129–37.
Kim M-J, Choi H-S, Jung SH. Monitoring Kudoa septempunctata in cultured olive flounder Paralichthys olivaceus in different regions of Korea in 2013. Korean J Fish Aquat Sci. 2014;47(5):611–21.
Shirakashi S, Shin SP, Mekata T, Kiryu I. Infections of Kudoa septempunctata (Myxozoa: Multivalvulida) in wild grass puffer Takifugu alboplumbeus and Japanese whiting Sillago japonica. Fish Pathol. 2021;56(3):140–8. https://doi.org/10.3147/jsfp.56.140.
Suzuki J, Murata R, Yokoyama H, Sadamasu K, Kai A. Detection rate of diarrhoea-causing Kudoa hexapunctata in Pacific bluefin tuna Thunnus orientalis from Japanese waters. Int J Food Microbiol. 2015;194:1–6.
Yoshihito T, Tsuyoshi S, Naoki O, Eiki M. A rare case of food poisoning by Kudoa hexapunctata. BMJ Case Rep. 2021;14(9):e246111. https://doi.org/10.1136/bcr-2021-246111.
Tachibana T, Watari T. A novel case of food poisoning caused by the consumption of Pacific bluefin tuna infected with Kudoa hexapunctata. Clin Case Rep. 2021;9(6). https://doi.org/10.1002/ccr3.4222.
Kim JJ, Ryu S, Lee H. Foodborne illness outbreaks in Gyeonggi province, Korea, following seafood consumption potentially caused by Kudoa septempunctata between 2015 and 2016. Osong Public Health Res Perspect. 2018;9(2):66–72.
Nishioka T, Satoh J, Mekata T, Mori K-i, Ohta K, Morioka T, et al. Efficacy of sand filtration and ultraviolet irradiation as seawater treatment to prevent Kudoa septempunctata (Myxozoa: Multivalvulida) infection in olive flounder Paralichthys olivaceus. Fish Pathol. 2016;51(1):23–7.
Jang Y, Ahn M, Bang H, Kang B. Effects of Kudoa septempunctata genotype ST3 isolate from Korea on ddY suckling mice. Parasite (Paris). 2016;23:18–18.
Ahn M, Woo H, Kang B, Jang Y, Shin T. Effect of oral administration of Kudoa septempunctata genotype ST3 in adult BALB/c mice. Parasite (Paris). 2015;22:35–35.
Takeuchi F, Ogasawara Y, Kato K, Sekizuka T, Nozaki T, Sugita-Konishi Y, et al. Genetic variants of Kudoa septempunctata (Myxozoa: Multivalvulida), a flounder parasite causing foodborne disease. J Fish Dis. 2016;39(6):667–72. https://doi.org/10.1111/jfd.12395.
Hong S-H, Kwon J-Y, Lee S-O, Lee H-I, Hong S-J, Ju J-W. Kudoa septempunctata spores cause acute gastroenteric symptoms in mouse and musk shrew models as evidenced in vitro in human colon cells. Pathogens. 2023;12(5):739.
Tachibana T, Watari T. Kudoa septempunctata infection: an underdiagnosed pathogen of acute gastrointestinal symptoms. QJM Int J Med. 2020;113(1):43–4. https://doi.org/10.1093/qjmed/hcz227.
Sung G-H, Park I-J, Koo HS, Park E-H, Lee M-O. Molecular detection and genotype analysis of Kudoa septempunctata from food poisoning outbreaks in Korea. Parasites Hosts Dis. 2023;61(1):15–23. https://doi.org/10.3347/phd.22034.
Yahata Y, Sugita-Konishi Y, Ohnishi T, Toyokawa T, Nakamura N, Taniguchi K, et al. Kudoa septempunctata-induced gastroenteritis in humans after flounder consumption in Japan: a case-controlled study. Jpn J Infect Dis. 2015;68(2):119–23. https://doi.org/10.7883/yoken.JJID.2014.027.
Iwashita Y, Kamijo Y, Nakahashi S, Shindo A, Yokoyama K, Yamamoto A, et al. Food poisoning associated with Kudoa septempunctata. J Emerg Med. 2013;44(5):943–5. https://doi.org/10.1016/j.jemermed.2012.11.026.
Marshall WL, Sitjà-Bobadilla A, Brown HM, MacWilliam T, Richmond Z, Lamson H, et al. Long-term epidemiological survey of Kudoa thyrsites (Myxozoa) in Atlantic salmon (Salmo salar L.) from commercial aquaculture farms. J Fish Dis. 2016;39(8):929–46.
Williams M, Hernandez-Jover M, Shamsi S. Fish substitutions which may increase human health risks from zoonotic seafood borne parasites: a review. Food Control. 2020;118:107429.
Williams M, Hernandez-Jover M, Shamsi S. Illegal, unreported, and unregulated fishing: a risk scoring method for prioritizing inspection of fish imported to Australia for zoonotic parasites. J Biosaf Biosecur. 2020;2(2):81–90. https://doi.org/10.1016/j.jobb.2020.11.002.
Jinnai M, Kawai T, Harada T, Nishiyama Y, Yokoyama H, Shirakashi S, et al. Production of a novel monoclonal antibody applicable for an immunochromatographic assay for Kudoa septempunctata spores contaminating the raw olive flounder (Paralichthys olivaceus). Int J Food Microbiol. 2017;259:59–67. https://doi.org/10.1016/j.ijfoodmicro.2017.08.003.
Shin J-H, Yang J-P, Seo S-H, Kim S-G, Kim E-M, Ham D-W, et al. Immune-triggering effect of the foodborne parasite Kudoa septempunctata through the C-type lectin Mincle in HT29 cells. BMB Rep. 2020;53(9):478–83. https://doi.org/10.5483/BMBRep.2020.53.9.079.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions The authors did not receive support from any organization for the submitted work. Open access has been granted through CSU’s contract with publisher.
Author information
Authors and Affiliations
Contributions
SS: First draft of the manuscript, literature review
DB: Writing of the manuscript, literature review
Corresponding author
Ethics declarations
Conflict of Interest
SS is on the editorial board of the journal.
Human and Animal Rights and Informed Consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shamsi, S., Barton, D.P. Exploring the Potential Role of the Genus Kudoa (Myxosporea: Kudoidae) as an Emerging Seafood-Borne Parasite in Humans. Curr Clin Micro Rpt 11, 107–114 (2024). https://doi.org/10.1007/s40588-024-00220-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40588-024-00220-1