Abstract
Purpose of Review
The goal of this paper is to review drug allergy alert systems (DAAS), to summarise their key components, and to overview potential benefits and challenges associated with these tools. Methods for validation of their effects on patient safety, alternative uses, and strategies to streamline DAAS’ functions and reduce system fatigue are discussed.
Recent Findings
DAAS are clinical decision support systems (CDSS) that focus on preventing drug adverse events within healthcare settings. The advent of electronic medical records has facilitated the development of digital DAAS. Existing versions use different methods to document diagnosed allergies, and rely on distinct rules and matching strategies for the generation of real-time alerts. DAAS promote the automation of several processes, facilitate prompt patient referral, and may be customised. Information overload, alert overrides by clinicians, and the development of “alert fatigue” may interfere with their usefulness. The newest strategies to streamline the function of DAAS include the use of artificial intelligence (AI) and other predictive techniques.
Summary
The rising prevalence of drug allergies underscores the importance of effective DAAS. Further research is needed to evaluate their usefulness, to optimise their performance, to explore different algorithms and data sources, and to enhance the standardised integration of these systems into clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hospitalized patients and those receiving outpatient medical care are often exposed to adverse events related to such care. Adverse drug events represent the most frequent type of healthcare-associated adverse events [1, 2]. Adverse drug events can be categorised into those caused by an inappropriate use of the drug and those that occur despite a proper use of it, with the latter being referred to as adverse reactions. According to the World Health Organization (WHO), an adverse drug reaction is a “harmful reaction that occurs unintentionally after administering the usual dose of a drug in humans” [3]. These are classified as type A reactions, related to the known mechanism of action of the drug, and type B reactions, which are unrelated to it. Allergic reactions fall within this last category [4]. They can occur when a patient receives a drug that has been previously identified as one to which the patient is allergic, and are therefore considered preventable adverse events. In addition to the harmful effect on the patient, they lead to relevant healthcare costs that can be avoided.
Drug allergy alert systems (DAAS) are clinical decision support systems (CDSS) aimed to prevent these errors and to reduce said costs for individuals and institutions. Before the existence of information technology, allergy information was collected manually on paper in patients’ medical records. The first CDSS were developed in the 1960s [5]. Patient information was supplied to the program, which calculated the probabilities of different diagnoses [6]. The common use of CDSS became widespread in the 1980s, integrating various functions, including the recording of patients’ pharmacological allergies, among many others. With the incorporation of electronic health records into medical practice, drug allergy information is now recorded in a computerised format, giving rise to the digital DAAS we know today [7•]. These systems leverage technology and coordinate with electronic health records to provide real-time alerts and clinical decision support to healthcare professionals. By effectively identifying potential medication allergies, providers can make informed decisions, mitigate risks, and optimise patient outcomes.
The importance of DAAS seems to be growing with the increasing number of drug-allergic patients. Currently, up to 10% of parents report that their child has an allergy to some type of medication [8]. DAAS, therefore, depend on an efficient storage and processing of large quantities of information, which accumulates exponentially. Sometimes, however, absolute certainty of the accuracy of this data is lacking. Questions may arise regarding the actual usefulness of alert systems. Do they truly contribute to greater patient well-being, or do they pose obstacles for healthcare professionals by causing an overload of unnecessary information?
In this article, we explore DAAS and their role in improving patient safety. We delve into their key components, including methods for allergy documentation, integration with EHRs, and real-time alert mechanisms. Additionally, we discuss the importance of accurate and comprehensive allergy information, as well as the role of clinical judgement in conjunction with these systems.
Furthermore, we explore the potential benefits and challenges associated with DAAS. The risks of “system fatigue”, a phenomenon by which users become desensitised to safety alerts, and therefore incur in “overrides” [9], are discussed. Finally, we highlight ongoing research and future directions in the field of DAAS, aiming to enhance the effectiveness and usability of these systems, including the potential for the use of artificial intelligence-based approaches that can complement DAAS.
Existing components of drug allergy systems today
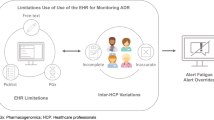
Currently, different kinds of DAAS are available for use in healthcare. Each healthcare centre may choose whether they prefer using a commercial system, modifying a commercial one, or developing one of their own custom-design [10••]. The central component of the DAAS consists of alerts, which are generated by medical personnel who registers a known adverse event to a drug based upon details of the clinical history, or a confirmed diagnosis provided by an allergist, within the hospital, or from another centre. Most of the systems available today use a multiple-choice method of registration to document drug allergies. Even though previous versions included an option that allowed data registration using free text, it is a practice that is being relegated, mainly because it does not streamline the process of alert generation when prescribing drugs [11]. In general, most systems allow both nurses and doctors to enter alerts, after. In some cases, physician’s assistants can also generate alerts. For safety and documentation, DAAS usually record the person who generated the alert and the date and time of said input. Alerts can also have degrees of complexity in terms of the quality of the information entered. Alerts can be confirmed only when a specialist has conducted a study and validated the allergy, and probable, when an official study has not been conducted.
The drug allergy questionnaire, which should be completed when admitting a patient to the hospital, is a key component of many DAAS. In fact, in some systems it is compulsory to fill this information to access the drug’s prescription. Appropriate alert generation depends on the proper completion of said questionnaire, as well as consistent updates of the questions asked. Regarding which items should be recorded, there is no consensus on which information is needed [10••]. Besides recording the culprit drug, some systems record the date of the reaction, its severity, and the symptoms observed during the reaction (e.g., mucocutaneous, respiratory, anaphylaxis), as well as the knowledge level of the individual who suspects the allergy (suspected by the patients or by the physician, assessed by the allergologist) [12]. It is also interesting to discuss which healthcare professionals should be in charge of completing the drug allergy questionnaire, and who can edit it. Depending on the hospital and the system used, the questionnaire might be filled out by a physician and/or a nurse. A controversial discussion point is whether the ability to edit the drug allergy questionnaire might be limited to allergologists only. Focusing this task on one specialist not only may guarantee quality of the information included, but may also reduce the opportunities for registration of allergy information.
Drug alerts have different characteristics that enable them to interfere with erroneous prescription. Some DAAS include interruptive alerts, which consist of a pop-up message that appears in the middle of the prescription workflow, therefore prompting the prescriber to change the initial therapy to another [10••]; and non-interruptive alerts, which do not generate such a pause, and could be used, for example, in situations where a change in prescription may be less critical, as in the case of intolerances or duplicated alerts [10••].
On the other hand, pharmacogenomics is a subject of growing importance in the development of DAAS. It is well-known that some polymorphisms and HLA genotypes are risk factors to develop an allergy to some drugs; for instance, HLA-B*57:01 is a risk factor to suffer a drug reaction with eosinophilia and systemic symptoms (DRESS) while taking abacavir [13]. Whether to include this new item in existing DAAS is still up for discussion. We believe including this kind of information within DAAS records might be confusing for healthcare professionals. In the future, when knowledge about the genetic predisposition to suffer allergic reactions to certain drugs has been expanded, this information could be included in DAAS, as it would help prevent future allergic reactions in patients with specific polymorphisms. Solid research and data based on large cohorts of patients is needed to further amplify the usefulness of integrating this tool in DAAS.
In terms of certain individuals’ predisposition to drug hypersensitivity, the management of patients with mast-cell activation syndromes is also up for discussion. Patients with mast-cell activation syndromes receive specific instructions in terms of medication use and anaesthetic procedures. In this case, we believe that interruptive alerts should be used for drugs with which patients have had confirmed hypersensitivity reactions. Non-interruptive alerts and prompts to consult with the evaluating allergist at the time of interventions or use of other medications can be used to complement risk management in individuals with this complex diagnosis.
Another point that should be taken into account is whether to implement the differentiation between intolerances (or, “adverse effects”') and allergies. Some systems include an item denominated “no drug allergy” or “drug intolerances”, where the physician can register drug intolerances [9, 14••]. In our experience, it is preferable to include only those reactions that can be clinically defined by the term “allergy” as “allergic reactions” within a DAAS. A special alert-triggering option should be created in the case of hypersensitivity reactions to NSAIDs that are considered “non-immunological”, as they imply a similar risk to other immediate hypersensitivity reactions. However, we believe that information on dubious adverse reactions where the mechanisms are unclear, or when symptoms have been attributed to the drug without a clear causal relationship and by the patient themselves, should not be included in active alerts. For this reason, at our centre, drug allergies are registered using a selection of multiple-choice options, while adverse effects are recorded manually, using a free-text option. This information is not analysed by DAAS, since it can potentially trigger a high number of false alerts, causing an increased frequency of overrides, and greater system fatigue, which is deleterious to its preventative effectiveness as a whole.
The possibility to delete a drug allergy from the medical record is another interesting aspect in the functioning of DAAS. Some systems only allow users to inactivate a drug allergy that has been previously filled out, and justify the reason for this change, but not to delete it [15]. This feature allows teams to conserve the history of this drug allergy questionnaire, permitting other physicians to see when and why the alert was inactivated, preventing cyclic attempts to reactivate the alert incorrectly. This aspect of DAAS highlights the fundamental role a proper anamnesis has to allow a correct functioning of the DAAS. In our centre, the drug allergy questionnaire registers when an alert activated or inactivated and by whom, allowing the physician to find more information when opening the respective report. Moreover, the system provides the option of adding an observation, where the physician can register more details once a patient has been tested by an allergy team. For example, recommendations on whether other drugs of the same family can be prescribed, or whether premedication is needed before the administration of iodinated contrast, can be included.
One of the biggest differences between existing DAAS is the matching strategy they use to generate drug alerts. There are a wide variety of rule bases to generate alerts, some more accurate and time-consuming than others. While prescribing, a warning can appear if the prescription involved the exact same drug previously reported as a cause of allergy, if the new drug belongs to the same pharmacological family, if there is a probability of cross-reactivity between both drugs, or if their chemical structures, excipient, or base active ingredients match [10••]. Different matching strategies used for drug allergy alert generation are summarised in Table 1. Constant updates on these operating systems are essential to maintaining the performance on DAAS. Databases that are reliant on “cross-reactivity” data between drugs require more constant and frequent updating, and therefore more resources, while those reliant on other methods of classification demand less costs.
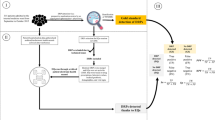
Figure 1 summarises the key steps involved in the effects of a standard DAAS as it would function to date. In each step, possible opportunities for errors related to each phase are listed. Initially, healthcare professionals promptly notify the occurrence of a drug allergy in the patient’s medical record. This step is crucial, as incorrectly labelling the patient allergic to a drug may limit therapeutic options in the future. Examples of this include registering a drug’s side effect as an allergy or mistaking a drug for another, which is spelled similarly (such as in LASA drugs, e.g., metamizole and methimazole) [16]. When another physician attempts to prescribe a medication that previously triggered an allergic reaction in the patient, the DAAS is activated. An automated alert promptly notifies the prescribing physician, drawing their attention to the potential risks associated with the intended prescription. Due to alert fatigue, medical practitioners may decide to inappropriately override the alert, making them oblivious to the patient’s allergies [17]. In response to the drug allergy alert, the physician considers alternative drugs that can be safely prescribed, taking into account the patient’s known allergic history. At this point, physicians may not find a suitable alternative and may choose to consult an allergy specialist to consider other options. The physician transmits relevant information regarding the patient’s drug allergy to the personnel responsible for administering the medications. This exchange ensures that the assigned healthcare providers are aware of the allergy and can consequently avoid administering the allergenic drug to the patient. As a result of this well-coordinated process, the patient remains free from allergic reactions associated with the prescribed drug.
Advantages and disadvantages of drug allergy alert systems
The implementation of DAAS has many benefits [18]. Healthcare alert systems improve quality and patient safety by preventing errors and adverse events. They provide relevant information and evidence-based decision support, improving adherence to clinical guidelines and treatment protocols. They also save time and resources by automating the alerting process and reducing the need to manually review information. These systems can be customised to suit the individual needs of each patient and healthcare institution. Furthermore, they integrate with electronic medical record systems, facilitating better information management [19]. This integration allows them to enhance existing systems and optimise key functionalities, such as electronic problem lists, medication lists, allergy lists, and laboratory test results. Another advantage is that the electronic alarms generated in the DAAS from medical records optimise the speed of referral of patients requiring priority allergy testing.
Despite these advantages, however, the use of DAAS may present challenges. They generate irrelevant or clinically unimportant alerts [20], which can lead to alert fatigue and decreased attention [21]. Moreover, healthcare providers may override important alerts without careful assessment, reducing the effectiveness of the system [22]. Information overload makes it difficult to identify important alerts and can hinder decision-making [23]. On the other hand, some alerts may have unintended consequences, such as delays in treatment. In addition, they are perceived as interruptions in the medical workflow, creating additional workload and decreasing efficiency [24]. There are limitations in the ability to personalise alerts and there may be differences in approaches and vocabulary used in different healthcare systems. They also face potential technical failures, affecting their effectiveness and reliability. Implementing and maintaining these systems can be costly in time and resources [25]. Finally, as mentioned previously, DAAS are highly reliant on the accuracy of the allergy/adverse-event questionnaires. Unnecessary or erroneous alerts regarding frequently used drugs, such as beta-lactam antibiotics or non-steroidal anti-inflammatory drugs, can increase the expense and toxicity of treatment plans, often while reducing their effectiveness. Outdated questionnaires therefore produce unnecessary or irrelevant alerts. Potential advantages and disadvantages related to DAAS are listed in Table 2.
Evaluating the usefulness of drug allergy alert systems
The usefulness of DAAS lies mainly on their capacity to function as automated security tools which guarantee patient safety, while partially reducing the burden of monitorization from individuals. In contrast to other pharmacovigilance interventions, which require active involvement of personnel in their execution, drug allergy alerts need only be programmed and consistently updated. Once established, their task is performed, without much investment of new resources, which also results in a reduction of maintenance costs progressively over time. This also makes them profitable in the long term.
Furthermore, establishing DAAS may be useful when recollecting data for regulatory purposes. Mechanised electronic records of prescription “overrides” by clinicians allow scientists and monitors to locate potential errors by reviewing selected lists of instances of exceptions recorded by the system. Data for regulatory agencies can also be collected automatically using these types of alerts. On the other hand, the use of automated DAAS contributes to the reconciliation of electronic health records within a single entity, avoiding redundant documentation in various sections and loss of information [26••].
The aspects mentioned make the usefulness of DAAS seem intuitive and logical. Conducting proper validation studies to prove their efficiency, however, is necessary, and especially complex. The main objective, other than proving cost-effectiveness and feasibility, is to prove an objective reduction in the quantity and frequency of adverse drug reactions after implementation of this tool. On the other hand, systems should be able to exact changes in prescribing patterns for physicians [27].
How should such a study be designed? Page et al. conducted a systematic review of the effectiveness of interruptive medication prescribing alerts to modify behaviour in prescribers [27]. Out of the studies included in their review, five employed a prospective study design, out of which three conducted randomized control trials, one conducted an interrupted time-series design, and one conducted a one-group pretest–posttest design. Eighteen of the studies were retrospective: 14 of them used a one-group pretest–posttest design, two used an interrupted time-series design, one used a posttest only design, and one used a repeated-treatment design. In the majority of categories, only one category of alert was examined, and all studies investigated the effect of the alert on at least one prescriber behaviour. Regarding this last aspect, 53% reported a statistically significant impact on prescriber behaviour as a result of the intervention alert.
The ideal study for the validation of DAAS should (a) be prospective, conducting follow-up over a number of years, (b) evaluate both impacts on prescribing behaviour as well as patient outcomes, and (c) should evaluate and compare effects of more than one type of DAAS. Conducting such a study remains challenging. An ever more comprehensive validation study would examine whether the matching table used to establish alert-generation rules covers all known allergies, and fails to generate unnecessary alerts.
The continuous evaluation of DAAS may require further validation processes. Application of standardized quality control procedures and accreditation may be useful in maintaining these systems’ functionality and reliability. To ensure quality control in DAASs, for example, ISO/TS 22703:2021 [28], an international standard that establishes requirements for the implementation and use of medication alerts, was proposed. This standard can be applied to the use of DAAS in various organisations, such as drug suppliers, health services, and health authorities.
Drug alert overrides and system fatigue
One of the main issues interrupting the usefulness of DAAS is the excess of incorrect reports, which leads doctors to ignore the alerts. If this happens too frequently, it causes system fatigue and loss of effectiveness, as doctors become accustomed to override the alerts, increasing the risk of reactions. In fact, the override rate is at least 44%, and in some cases, it goes up to 97% [10••]. In a retrospective study conducted by our group, the rate of override alerts in our centre was 44.8% [29]. In clinical practice, it is not known which type of DAAS system yields better patient safety results [10••]. However, it is documented that the DAAS override rate was higher when using a commercial system compared to a system designed within the hospital [30]. The main documented motive for overrides was that the patient had previously tolerated the drug. Other reasons reported were that the physician agreed to monitor the patient whilst receiving the drug, that there was no reasonable alternative, that there was a low risk of cross-reactivity, that the patient reported no allergy, that the allergy might not be true or was questionable, and that the benefit of receiving the prescribed drug outweighed the risk [10••]. Considering these reasons, some of which explain “appropriate overrides”, it is of great importance for the maintenance of the alert system that healthcare personnel are able to correct the patient’s medical history so that, once tolerance has been verified, the alert does not reappear in the future.
There is limited reliable data on the clinical consequences of DAAS overrides, and the conclusions are sometimes contradictory. However, it appears that inappropriate overrides more frequently lead to allergic reactions than overrides considered appropriate. These data are influenced by the use of different definitions of allergic reactions. In the retrospective study conducted by our group on overrides at our centre, only 3.1% of the overrides caused an allergic reaction, which in all cases was mild. The rate of overrides was higher in inappropriate overrides (15.8%) than in appropriate ones (1.7%) [29].
Different proposals have been described with the aim of reducing the number of overrides. These include replacing interruptive alerts with non-interruptive ones in certain cases (mild reactions, duplicate alerts, intolerances); distinguishing between allergy alerts and alerts related to other types of adverse reactions (drug interactions); or presenting the alert based on the severity of the reaction, or even within the clinical context of the patient’s situation [29]. In the future, as will be discussed below, artificial intelligence (AI) can be a very useful tool to help keep the system updated and to reduce the number of unnecessary overrides.
Artificial intelligence and its applications in DAAS
Like any initiative involving patient safety, DAAS are tools whose function depends on the organised use of data. Not only must they organise and store new information about diagnosed allergies, but they also rely on the extraction of key information about drugs, their characteristics, and their relationship to each other from extensive databases. Currently, AI processes and machine learning approaches are increasingly being used to assess, organise, and transform data.
In the realm of patient safety, Choudhury et al. conducted a systematic review of 53 publications on the role of artificial intelligence on outcomes related to this objective, including clinical decision support systems, and adverse event detection [31]. Nine of the studies pertained to clinical alerts, and 23 related to drug safety. The study found heterogeneity in the way AI results were reported and an absence of a reference standard within AI models; however, it concluded that they have the potential to improve patient safety outcomes as long as they are subjected to evaluation and review today.
As found by this review, AI models have been extensively used in initiatives related to drug safety. Recently, Ania Syrowatka and her colleagues carried out a systematic review of 78 articles on the use of IA to lessen the frequency of adverse drug events, including the likelihood of adverse drug reactions, the occurrence of hypotension during anaesthesia, the risk of opioid overdose, and the clinical response to various pharmacological treatments [32•]. This review focuses on modern machine learning techniques and natural language processing, a tool that allows a comprehensive analysis of the human language which enables extraction of medical information from patient reports, electronic medical records, and other platforms. Once again, the techniques employed are found to be variable, and most of the articles were published within the last 5 years. The authors therefore recognize this as an “emerging area of study”. The majority of the studies (86%) rely on techniques that predict reactions before they occur, whereas 14% rely on methods that detect occurring reactions early. Techniques detect several different types of adverse drug events, not only including allergic reactions.
AI approaches are also being used to improve the efficacy of DAAS. Poly et al., from Taiwan, specifically reviewed the application of five machine learning models to reduce the fatigue of drug-related clinical decision support systems [33•]. In their work, the following models were applied: (1) artificial neural networks (ANN) [34]; (2) random forests (RF) classification and interaction in random forests [35]; (3) Naive Bayes (NB) [36]; (4) gradient boosting [37]; and (5) support vector machine [38]. The study found that the results of machine learning prediction models have high sensitivity and specificity. Furthermore, the ANN model showed high-discrimination capabilities, showing an AUC value of 0.94 in the ROC curve performed, in addition to an accuracy of 85%, which is higher than the rest of the predictive models applied. This suggests that this model can be a useful tool to reduce fatigue of the clinical decision support systems and to correctly identify the individual acceptance rates. As limitations, they found that reasons for cancellation in the free-text format were not included in the study, nor data on physician characteristics (gender, age, work periods, etc.). These findings support the use of predictive models for the support of clinical decisions.
Alternative strategies for adverse event prevention
Despite the existence of DAAS, there are alternative approaches to the prevention of drug adverse events. Clinical pharmacists, for instance, have a very important role in the prevention of erroneous prescription of drugs. The European Society of Clinical Pharmacy recommends that all prescriptions should be reviewed and validated, as soon as possible, by a hospital pharmacist. This recommendation is yet another tool that is useful in preventing medication errors [39]. This “provider-based” approach may also have a role in deterring clinicians from overrides, since they experience yet another filter that is not entirely dependent on a computerised alert system.
Pre-screening and premedication strategies may be useful in certain situations [40]. Premedication strategies do not guarantee patient safety, and may instead provide a false sense of security [41]. Pre-screening strategies, on the other hand, may be more effective, but require mobilisation of large quantities of resources.
Over the years, other techniques, relying both on efficient systems and human review, have been developed for safety improvement regarding prescriptions. Indications-based prescribing, for example, is a method by which indications are incorporated systematically into the prescription process, thereby facilitating patient empowerment, error reporting, and medication reconciliation [42].
In order to maintain their validity, advantages from digital DAAS should outweigh the benefits and reduce the costs associated with all other drug adverse event prevention strategies. They should also integrate as flawlessly as possible with other accompanying tools that are essential to patient safety, such as reporting systems, dedicated to the documentation of medication errors, once they occur [43].
Conclusion
The rising prevalence of drug allergies underscores the importance of effective DAAS. Their integration into the electronic health system makes them one of the most relevant automated clinical-decision support tools which guarantee patient safety. Their continued validity relies on continued evaluation and adaptation to health needs, as well as combinations with new technologies that may streamline the processes involved. Education on the use of alerts and guidance regarding multidisciplinary collaboration to guarantee their effectiveness should be required for all professionals in the healthcare system. Further research is needed to continue to evaluate the usefulness, to optimise the performance, and to enhance the standardised use of DAAS in clinical practice.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Bates DW, Cullen DJ, Laird N, Petersen LA, Small SD, Servi D, et al. Incidence of adverse drug events and potential adverse drug events: implications for prevention. JAMA J Am Med Assoc. 1995;274(1):29–34.
Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta- analysis of prospective studies. J Am Med Assoc. 1998;279(15):1200–5.
WHO Meeting on International Drug Monitoring: The Role of National Centers & World Health Organization. International drug monitoring: the role of national centers, report of a WHO meeting (held in Geneva from 20 to 25 September 1971) [Internet]. World Health Organ Tech Rep Ser. 1972; 498: 1–25. [access July 22th, 2023]. Available in: https://apps.who.int/iris/handle/10665/40968.
Vervloet D, Thong B. Drug Allergies | World Allergy Organization [Internet]. World Allergy Organization Allergy Organization. 2021 [cited 2023 Jul 17]. Available from: https://www.worldallergy.org/education-and-programs/education/allergic-disease-resource-center/professionals/drug-allergies. Accessed 17 July 2023.
Anthony G, Gorry GOB. Experience with a model of sequential diagnosis. Comput Biomed Res. 1968;1(5):490–507.
Shortliffe EH, Buchanan BGA. model of inexact reasoning in medicine. Math Biosci. 1975;23:351–79.
• Sutton RT, Pincock D, Baumgart DC, Sadowski DC, Fedorak RN, Kroeker KI. An overview of clinical decision support systems: benefits, risks, and strategies for success. NPJ Digit Med. 2020;3(1):17. This review provides a complete overview of the use of computerised clinical decision support systems which are used to aid clinicians in their complex decision-making processes. The article not only discusses the increasing adoption of electronic medical records with advanced capabilities, but also points out the existing uncertainties regarding the impact of clinical decision support systems on providers, patient outcomes, and costs.
Gomes ER, Brockow K, Kuyucu S, Saretta F, Mori F, Blanca-Lopez N, et al. Drug hypersensitivity in children: report from the Pediatric Task Force of the EAACI Drug Allergy Interest Group. Allergy Eur J Allergy Clin Immunol. 2016;71(2):149–61.
Slight SP, Beeler PE, Seger DL, Amato MG, Her QL, Swerdloff M, et al. A cross-sectional observational study of high override rates of drug allergy alerts in inpatient and outpatient settings, and opportunities for improvement. BMJ Qual Saf. 2017;26(3):217–25.
•• Luri M, Leache L, Gastaminza G, Idoate A, Ortega A. A systematic review of drug allergy alert systems. Int J Med Inform. 2022;1(159): 104673. This study aims to identify, describe, and summarise the different DAAS used in hospitals. It analyses the characteristics of these systems and discusses the effectiveness of drug allergy alert systems in reducing preventable adverse drug events and improving patient safety in hospital settings.
Hsieh TC, Kuperman GJ, Jaggi T, Hojnowski-Diaz P, Fiskio J, Williams DH, et al. Characteristics and consequences of drug allergy alert overrides in a computerized physician order entry system. J Am Med Inf Assoc. 2004;11(6):482–91.
Swiderski SM, Pedersen CA, Schneider PJ, Miller AS. A study of the frequency and rationale for overriding allergy warnings in a computerized prescriber order entry system. J Patient Saf. 2007;3(2):91–6.
Cabañas R, Ramírez E, Sendagorta E, Alamar R, Barranco R, Blanca-López N, et al. Spanish guidelines for diagnosis, management, treatment, and prevention of DRESS syndrome. J Investig Allergol Clin Immunol. 2020;30(4):229–53.
•• Foreman C, Smith WB, Caughey GE, Shakib S. Categorization of adverse drug reactions in electronic health records. Pharmacol Res Perspect. 2020 1;8(2). The paper assesses accuracy rates of allergic reaction information. They conclude that the electronic health record design and user interface facilitated the mislabelling of adverse drug reactions.
Comas B. La seguridad de los pacientes en los Servicios de Urgencias. [PowerPoint presentation]. Curso de Seguridad del Paciente. Govern de les Illes Balears [updated 2013 Nov 27; cited 2023 Jun 26]. https://www.caib.es/sites/avaluacioacreditacio/ca/curso_de_seguridad_de_pacientes-60995/. Accessed 26 June 2023.
Bryan R, Aronson JK, Williams A, Jordan S. The problem of look-alike, sound-alike name errors: drivers and solutions. Br J Clin Pharmacol. 2021;87(2):386–94.
Johnson KR, Hagadorn JI, Sink DW. Alarm safety and alarm fatigue. Clin Perinatol. 2017;44(3):713–28.
Topaz M, Medicine GI, Goss F, Blumenthal K, Practice M, Hospital MG, et al. Towards improved drug allergy alerts: multidisciplinary expert recommendations. Int J Med Inform. 2017;97:353–5.
Zenziper Straichman Y, Kurnik D, Matok I, Halkin H, Markovits N, Ziv A, et al. Prescriber response to computerized drug alerts for electronic prescriptions among hospitalized patients. Int J Med Inform. 2017;107(July):70–5.
Brodowy B, Nguyen D. Optimization of clinical decision support through minimization of excessive drug allergy alerts. Am J Heal Pharm. 2016;73(8):526–8.
Hussain MI, Reynolds TL, Zheng K. Medication safety alert fatigue may be reduced via interaction design and clinical role tailoring: a systematic review. J Am Med Informatics Assoc. 2019;26(10):1141–9.
Wong A, Amato MG, Seger DL, Rehr C, Wright A, Slight SP, et al. Prospective evaluation of medication-related clinical decision support over-rides in the intensive care unit. BMJ Qual Saf. 2018;27(9):718–24.
Ancker JS, Edwards A, Nosal S, Hauser D, Mauer E, Kaushal R. Effects of workload, work complexity, and repeated alerts on alert fatigue in a clinical decision support system. BMC Med Inform Decis Mak. 2017;17(1):1–9.
Knight AM, Maygers J, Foltz KA, John IS, Yeh HC, Brotman DJ. The effect of eliminating intermediate severity drug-drug interaction alerts on overall medication alert burden and acceptance rate. Appl Clin Inform. 2019;10(5):927–34.
Légat L, Van Laere S, Nyssen M, Steurbaut S, Dupont AG, Cornu P. Clinical decision support systems for drug allergy checking: systematic review. J Med Internet Res. 2018; 20(9):e258.
•• Vallamkonda S, Ortega CA, Lo YC, Blackley SV, Wang L, Seger DL, et al. Identifying and reconciling patients’ allergy information within the electronic health record. Stud Health Technol Inform. 2022;6(290):120–4. This paper is a study that focuses on allergy information in the electronic health record, which aimed to assess the prevalence of incompleteness, inaccuracy, and redundancy of allergy information.
Page N, Baysari MT, Westbrook JI. A systematic review of the effectiveness of interruptive medication prescribing alerts in hospital CPOE systems to change prescriber behavior and improve patient safety. Int J Med Inform. 2017;1(105):22–30.
Informática en salud — Requisitos para las alertas de seguridad de medicamentos. ISO/TS 22703:2021(es). [España; 2021]
Luri M, Gastaminza G, Idoate A, Ortega A. Allergic adverse drug events after alert overrides in hospitalized patients. J Patient Saf. 2022;18(6):630–6.
Wong A, Wright A, Seger DL, Amato MG, Fiskio JM, Bates D. Comparison of overridden medication-related clinical decision support in the intensive care unit between a commercial system and a legacy system. Appl Clin Inform. 2017;8(3):866–79.
Choudhury A, Asan O. Role of artificial intelligence in patient safety outcomes: systematic literature review. JMIR Med Informatics. 2020;8(7):e18599.
• Syrowatka A, Song W, Amato MG, Foer D, Edrees H, Co Z, et al. Key use cases for artificial intelligence to reduce the frequency of adverse drug events: a scoping review. Lancet Digit Heal. 2022;4(2):e137–48. This is a scoping review that explores the potential of artificial intelligence (AI) in reducing the frequency and consequences of adverse drug events (ADEs). Key uses included using AI for prediction to prevent ADEs and early detection to mitigate their effects.
• Poly TN, Islam MM, Muhtar MS, Yang HC, Nguyen PA, Li YC (2020) Machine learning approach to reduce alert fatigue using a disease medication–related clinical decision support system: model development and validation. JMIR Med Inform 2020;8(11): E19489. This article overviews the potential of AI approaches in the prevention of alert fatigue.
Zou J, Han Y, So SS. Overview of artificial neural networks. Methods Mol Biol. 2008;458:15–23.
Denisko D, Hoffman MM. Classification and interaction in random forests. Proc Natl Acad Sci U S A. 2018;115(8):1690–2.
Zhang Z. Naïve bayes classification in R. Ann Transl Med. 2016;4(12):1–5.
Zhang Z, Zhao Y, Canes A, Steinberg D, Lyashevska O. Predictive analytics with gradient boosting in clinical medicine. Ann Transl Med. 2019;7(7):152–152.
Yu W, Liu T, Valdez R, Gwinn M, Khoury MJ. Application of support vector machine modeling for prediction of common diseases: the case of diabetes and pre-diabetes. BMC Med Inform Decis Mak. 2010;10:16.
Pérez-Moreno MA, Rodríguez-Camacho JM, Calderón-Hernanz B, Comas-Díaz B, Tarradas-Torras J. Clinical relevance of pharmacist intervention in an emergency department. Emerg Med J. 2017;34:495–501.
Yu-Hor Thong B, Vultaggio A, Rerkpattanapipat T, Schrijvers R. Prevention of drug hypersensitivity reactions: prescreening and premedication. J Allergy Clin Immunol Pract. 2021;9(8):2958–66.
Rosado Ingelmo A, Doña Diaz I, Cabañas Moreno R, Moya Quesada MC, García-Avilés C, García Nuñez I, et al. Clinical practice guidelines for diagnosis and management of hypersensitivity reactions to contrast media. J Investig Allergol Clin Immunol. 2016;26(3):144–55.
Schiff GD, Seoane-Vazquez E, Wright A. Incorporating indications into medication ordering — time to enter the age of reason. N Engl J Med. 2016;375(4):306–9.
Mutair A Al, Alhumaid S, Shamsan A, Zaidi ARZ, Mohaini M Al, Al Mutairi A, et al. The effective strategies to avoid medication errors and improving reporting systems. Medicines. 2021 27;8(9):46.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Contributions
GG designed the concept and structure of the manuscript, with help from PLQ. PLQ, SSF, LPG, ACA and GG, all wrote sections of the manuscript text. LPG and SSF created tables 1 and 2, respectively. JMBS prepared the figure and wrote accompanying explanations in the text. AOE and ML conducted a critical revision of the manuscript. All authors reviewed the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Conflict of Interest
Paola Leonor Quan declares no competing interests. Sergio Sánchez-Fernández declares no competing interests. Lucía Parrado Gil declares no competing interests. Alfonso Calvo Alonso declares no competing interests. José Miguel Bodero Sánchez declares no competing interests. Ana Ortega Eslava declares no competing interests. Marta Luri declares no competing interests. Gabriel Gastaminza Lasarte declares no competing interests.
Human and Animal Rights and Informed Consent
The article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Quan, P.L., Sánchez-Fernández, S., Parrado Gil, L. et al. Usefulness of Drug Allergy Alert Systems: Present and Future. Curr Treat Options Allergy 10, 413–427 (2023). https://doi.org/10.1007/s40521-023-00351-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40521-023-00351-8