Abstract
Purpose
The proportion of older populations living with cancer is on the increase. Maintaining or improving their quality of life (QoL) has become an important goal in the treatment of cancer and has become an endpoint in clinical trials. Melatonin regulates a wide variety of physiological functions and is involved in the initiation of sleep and the improvement of QoL. With age, the secretion of melatonin decreases and could lead to a deterioration in QoL.
Methods
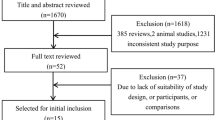
Literature searches were conducted using the PubMed database. The search terms and derivatives of “metastatic cancer”, “older patients”, “quality of life” and “melatonin” were used. Titles and abstracts were screened to identify whether studies were relevant for full-text screening.
Results
There is major concern about the symptoms older cancer patients encounter during treatment because they can impact their QoL. Melatonin supplementation presents several benefits for older patients: improvement in survival, decrease in symptoms induced by cancer and cancer treatment, and also improvements in quality of life.
Conclusion
It therefore seems appropriate to study the impact of melatonin supplementation during cytotoxic therapy on QoL among elderly patients with metastatic cancer. The use of melatonin as a therapeutic strategy seems particularly suitable for elderly patients, a population known to secrete significantly less melatonin. However, to date, no studies have been conducted in this population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
In 2018, 18.1 million new cancers were recorded worldwide and 9.6 million deaths were attributed to cancer [1]. Europe accounts for 9% of the world’s population, but for 23.4% of all cancer cases and for 20.3% of cancer deaths [1]. For most types of cancer, incidence rates increase with age. In the USA, an increase in cancer incidence from 2010 to 2030 of 67% has been estimated for people of 65 years or older, compared to an increase of only 11% among patients younger than 65 years [2]. The management of cancer among elderly patients is a public health problem.
The age of 70 is a threshold commonly used in clinical trials in oncology, but some authors use 65 as a reference age [3]. The European Medicines Agency (EMA) considers 65 years as a cut-off for the definition of old age (E7 Studies in Support of Special Populations: geriatrics). Despite the high frequency of cancer in the elderly population, these patients are under-represented in clinical trials [4,5,6]. Among studies by the National Cancer Institute on early stage breast cancer, only 18% of patients were over 65 years, while this population accounts for 49% of eligible patients [7]. Thus, we note here the importance of taking into account this population in clinical trials to improve current therapeutic treatment.Cancers among older patients are often diagnosed late and they often remain undertreated [8]. Late diagnoses can affect survival in this subpopulation [9]. The main reasons for the late diagnosis of cancer are comorbidities, the lack of a supportive social environment, difficulties in accessing public transport and deteriorating cognitive function [10]. A detailed study of the literature data has shown that compared to younger patients, older patients are subjected to a higher percentage of certain types of cancer at advanced stages [8], and also a higher percentage of non-staged cancers and a higher percentage of non-histological or non-cytological confirmations of cancer [11]. In fact, the study by De Rijke et al., performed on a cohort of 6911 patients aged 50 or over showed that, overall, 16% of patients were not treated, with the percentage rising to 22% for those over 70 years of age [11].
Elderly patients present particular characteristics that make the choice of the appropriate treatment more difficult to determine. The elderly population is very heterogeneous and cancer treatment for these patients requires knowledge not only of the disease but also of the physiological and pathophysiological features associated with aging. In oncology, as all anti-cancer drugs have side effects, it is important to consider the benefits and the risks associated with these treatments, especially in the elderly population. They are indeed more vulnerable to treatment toxicities and are more likely to have side effects [12]. Indeed, age is associated with several physiological changes in organ function that could alter drug pharmacokinetics and have an impact on cytotoxic chemotherapy tolerability and toxicity [12]. For example, we can mention the decline in renal function [13], diminishing bone marrow reserves [14], anaemia [15], poor nutrition [16] or even changes within the gastrointestinal system [17]. Geriatric syndromes (e.g., impaired vision and hearing, incontinence, poor nutrition) engender additional considerable difficulties for the treatment of the elderly [18].
In addition, comorbidities have an impact on the choice of treatment (e.g., anthracyclin, increasing the risk of cardio-toxicity such as heart failure, cisplatin, increasing the risk of renal failure) and they could lead to poly-medication, which contributes to increased chemotherapy toxicity among older patients [12].
As described above, the older cancer population is heterogeneous and complex (comorbidities, general health, drug interactions, dosage adjustment) and maintaining quality of life (QoL) is a major challenge in the care of these patients [19, 20]. In advanced disease, treatment is palliative, where the aim is to control the disease and pain, limit toxicities and maintain an overall level of QoL. However, with age, the pineal gland produces less melatonin. Melatonin regulates a wide variety of physiological functions and is involved in the initiation of sleep and the improvement of QoL. Thus melatonin has potential value in cancer pathologies in addition to the standard treatment to prevent and/or reduce the symptoms associated with cancer and its management, such as asthenia, depressive syndromes, sleep disorders, cognitive decline or even performance status, these dimensions being constitutive of QoL. The absence of any particular toxicity [21,22,23] in various clinical trials and the possible anti-tumor efficacy of melatonin strengthen its beneficial roles. Finally, the use of melatonin as a therapeutic strategy seems particularly suitable for older patients, a population known to secrete significantly less melatonin. However, to date no studies have been conducted in this population. This article aims to review the interest of melatonin for older patients with cancer to improve their quality of life during treatment.
Methods
A literature search was conducted on PubMed database. The search terms and derivatives of “metastatic cancer”, “older patients”, “quality of life” and “melatonin” were used. Titles and abstracts were screened to identify whether studies were relevant for full-text screening. Only English-language studies were included. Articles were selected for full-text screening if the title or abstract mentioned one or more search terms.
Older patients with cancer and quality of life
In 1993, the World Health Organization (WHO) defined QoL as “an individual’s perception of their position in life in the context of the culture and the value systems in which they live and in relation to their goals, expectations, standards and concerns” [24]. Health-related QoL (HRQoL) is a multidimensional concept including the physical, mental and social fields and the symptoms related to the disease and treatment [25]. Several studies have highlighted the prognostic value of data from HRQoL measures for cancer patients, especially in the advanced stages [26,27,28,29].
Two measurement tools are primarily used in oncology: the Functional Assessment of Cancer Therapy (FACT) [30] and the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30) [31], appropriate for self-evaluation and applicable across a wide range of settings. The core questionnaire is supplemented by disease-specific modules, e.g. breast, lung, head and neck cancer, palliative care, and a specific module for the elderly (QLQ ELD14).
There is major concern about the symptoms older cancer patients have to cope with during treatment because they can impact their QoL. Particularly at the metastatic stage, the aim of therapeutic management is to control the main symptoms. Thus QoL research incorporates these dimensions and is particularly relevant for decisional management for older patients with metastatic cancer. It is important to underline that the cancer treatment itself influences QoL (e.g., chemo-induced anaemia, asthenia, anorexia, reduced mobility, or even depressive symptoms) [32]. Pain, fatigue, insomnia and mood disturbance are the four most common symptoms and the most distressful that were reported by elderly patients with cancer during the illness and treatment [33]. The interrelations in this cluster of symptoms could affect QoL [34].
A study on 120 patients (> 65 years), receiving cancer therapy showed that the patients had numerous symptoms, with a mean number of 5 ± 3 symptoms per patient. Mood disturbance was the most prevalent (87%). The high-symptom group obtained a significantly lower mean Karnofsky performance score and FACT-G sub-scale and total scores (p < 0.01) (Cheng and Yeung, 2013). A recent study on 74 older patients with solid tumours showed a significant increase in excessive daytime sleepiness (EDS) after chemotherapy [from 6 patients (8.1%) exhibiting EDS at baseline to 16 patients (21.6%) after chemotherapy; p < 0.01]. The authors concluded that this impact of chemotherapy on daytime sleepiness could affect quality of life [35].
Furthermore, the management of cancer, and chemotherapy in particular, can induce a disruption of the circadian system, which can impact quality of life negatively. Mormont et al., in a study on 200 metastatic colorectal cancer patients, demonstrated a link between the rest-activity rhythm and QoL, showing a positive correlation between 24 h rhythm indicators and the global QLQ-C30 global score [36]. Innominato et al., in a study on 96 patients with metastatic colorectal cancer also showed that disruption of the circadian system was associated with lower QoL, global and in various domains, including the social environment [37]. The same team conducted another study on 237 metastatic colorectal cancer patients. They highlighted that fatigue and anorexia were more marked among patients with circadian disruption, and that QoL was better for patients with a robust circadian rhythm. The authors concluded that circadian disruption was associated with cancer symptoms and had an impact on physical and social functioning. Furthermore, they showed that the circadian system was important in the level of HRQoL for patients with cancer [38].
Several studies have shown a link between circadian rhythm disruption and survival among patients with cancer especially in metastatic colorectal cancer [39], advanced breast cancer [40], and lung cancer [41]. In the study by Innominato et al., circadian disruption among patients with metastatic colon cancer was a pejorative prognostic factor for overall survival, independently from other known prognostic factors [37]. The disruption of the circadian clock can also be caused by the chemotherapy itself. A study on 77 patients showed that chemotherapy-induced circadian disruption was also associated with significantly shorter survival [42]. Thus, these results justify the development of specific therapies aiming to restore the circadian system, which could potentially improve survival among cancer patients undergoing treatment.
Interrelation between melatonin, older patients with cancer and quality of life
Melatonin is a compound synthesized by the pineal gland in the human brain. It is considered as a hormone regulating the circadian day–night rhythm. Tryptophan and serotonin are the precursors of melatonin. The regulation of melatonin synthesis is controlled by the light–dark cycle, acting through neural activation of the anterior hypothalamus CNS, via the axons of the retinal ganglion cells running from the optic nerves and forming the retino-hypothalamic tract). The secretion of melatonin has a typical circadian rhythm, reaching a peak value (80–150 pg/ml) between midnight and 3 a.m.[43, 44].
Since its discovery, many in vitro studies and animal models have been developed to determine the specific roles of melatonin in the body: antioxidant and onco-static properties [45, 46]; an anti-angiogenic role [47]; control of the immune system [48]; its role in sleep (reduced sleep latency and induction of sleepiness and drowsiness) [49]; its role in disorders of the biological rhythms [50].
Melatonin is metabolized in the liver and excreted in the urine as 6-sulfatoxymelatonin [51]. Due to the rapid absorption and short half-life of melatonin (40–50 min) after intake of an exogenous sustained-release formulation, a maximum plasma concentration occurring between 20 and 240 min is observed, followed by a decline after less than one and a half hours, depending on the dose [52]. Maintaining effective body concentrations of melatonin throughout the night thus requires either a high dose or an exogenous sustained-release formulation.
For example, Circadin© is an exogenous sustained-release formulation of melatonin, which overcomes the rapid clearance of the hormone in the intestine over an extended period of time, thus mimicking the physiological pattern of melatonin secretion. Thereby, the peak plasma concentration is reached 2.6 h after intake and lasts an additional three and a half hours before falling, covering the whole night-time duration. In 2007, Circadin® received its authorization on the European market as a monotherapy. It should be taken 2 h prior to bedtime and it can be used in the short-term treatment of primary insomnia among adults aged 55 and over. This authorization is based on results from two studies on patients (> 55 years) with primary insomnia [53, 54]. These studies and a meta-analysis [55] showed a significant benefit on sleep latency. This benefit is similar to that obtained from the already marketed hypnotics (sleep latency shortened by 25 min on average). In addition, these studies showed significant benefits in sleep quality, morning alertness and patient QoL. The aforementioned studies also showed that tolerance of the treatment is comparable to that of a placebo. After stopping the treatment for three nights, sleep quality deteriorates, but is still better among patients taking Circadin®, suggesting that there are not only no withdrawal symptoms and no risk in stopping treatment, but also that the treatment has a residual beneficial effect.
In these clinical trials, the most common side effects reported were headache, nasopharyngitis, back pain and arthralgia; they were observed in both the Circadin© and the placebo groups.
With its role in the control of the circadian rhythm, we can hypothesize that, an intake of melatonin could have a beneficial effect on QoL and systemic symptoms. It can be remarked that, in humans, the pineal gland becomes less functional with age and melatonin levels gradually decline through life [56]. The causes of the decrease in melatonin secretion are not fully understood at this stage, and several hypotheses have been suggested, such as CNS degeneration, calcification of the pineal gland, or abnormal transmission of light signals [57]. Another hypothesis suggested is a decrease in melatonin receptors (MT1) in the brain, which is age related [58].
Between 40 and 70% of people suffer from sleep disorders, potentially affecting their psychological, social and cognitive functioning, and thus affecting their QoL [59]. Melatonin secretion is inversely correlated with sleep disturbances during the aging process. One study also demonstrated a link between reductions in urinary melatonin and poor quality of sleep in the elderly, and delayed sleep phase, identified by comparison with a control group of the same age [49].
Benefits of melatonin supplementation for older cancer patients
Table 1 summarizes the main findings described in this section.
Combined effects of melatonin with chemotherapy: improving survival
The effect of melatonin coupled with chemotherapy has been analysed in different cancers. Several trials support the hypothesis that melatonin enhances the effect of chemotherapy, especially in colorectal carcinoma Cerea et al. evaluated the effect of simultaneous administration of melatonin and camptothecin (Irinotecan©) to 30 patients with metastatic colorectal carcinoma. They showed that melatonin with Irinotecan© was more effective in controlling the disease than Irinotecan© alone [60].
A recent meta-analysis [23] of eight clinical trials tested the effect of melatonin supplementation among 761 patients treated with chemotherapy or radiation therapy for solid tumors in metastatic setting. The tested melatonin dose was 20 mg/day, prescribed concurrently with chemotherapy and/or radiotherapy. Melatonin significantly improved complete and partial remission rates (16.5 vs. 32.6%; risk ratio RR = 1.95, 95% CI 1.49–2.54; p < 0.00001) as well as 1-year survival (28.4 vs. 52.2%; RR = 1.90; 95% CI 1.28–2.83; p = 0.001). However, it is important to remain cautious because this meta-analysis included six studies conducted in the same centre on a small study population. Hence, an analysis using double-blind placebo and multicentre studies with larger patient samples is needed to evaluate the efficacy of melatonin in the treatment of cancer.
Another meta-analysis [22] included a larger group of trials (21 trials). The trials combined adjuvant chemotherapy with melatonin. Melatonin decreased 1-year mortality (RR = 0.60, 95% CI 0.54–0.67) and contributed to improved results for complete response, partial response and stable disease, with RR of 2.53 (1.36–4.71), 1.70 (1.37–2:12), and 1.15 (1.00–1.33), respectively.
Improvement of symptoms induced by cancer and its treatment
A study [21] on 200 patients with chemotherapy-resistant metastatic cancer, comparing chemotherapy versus chemotherapy + melatonin at a dose of 20 mg/day for at least 2 months, showed a significant reduction in chemotherapy-induced toxicities such as asthenia, thrombocytopenia, neurotoxicity and stomatitis. Another study conducted by the same team [21] showed a significant reduction in certain cancer-induced symptoms by providing melatonin to 1440 patients: 718 patients treated with supportive care alone, and 722 treated with supportive care + melatonin at 20 mg/day. The symptoms that improved were cachexia, asthenia, anorexia, depressive syndromes and thrombocytopenia.
A recent study conducted on 32 metastatic breast cancer patients who took 5 mg/day of melatonin for two months at bedtime showed a reduction in sleep fragmentation (p = 0,0015) and an increase in sleep duration [61]. Similarly, a double-blind, placebo-controlled, randomized study among breast cancer patients comparing 6 mg/day of melatonin (n = 27) to placebo (n = 21), from three nights before surgery until one week post-surgery, showed an increase in sleep efficacy in the melatonin group (p = 0,007) and a reduction in waking-after-sleep onset [62]. Also according to the above-mentioned meta-analysis [22, 23], melatonin significantly reduces symptoms such as asthenia, leukopenia, nausea and vomiting, hypotension, and thrombocytopenia. As most of these symptoms are included in QoL measures, their improvement by melatonin could translate into patient QoL improvement.
Role on quality of life
In a randomized study [63], an improvement of anxiety and depressive symptoms was observed among patients treated for metastatic breast cancer with Tamoxifen© + melatonin. Another more recent study [64] showed a significantly lower risk of developing depressive syndrome after surgery for breast cancer among 54 patients receiving melatonin supplementation (6 mg/day, 3 months) versus placebo, RR 0.25 [95% CI 0.077–0.80].
An improvement of performance status (PS) was observed among patients with non-small cell lung cancer resistant to first-line chemotherapy with cisplatin, treated with supportive care + melatonin at 10 mg/day compared to patients treated with supportive care alone [65]. In addition, it is important to highlight that in many studies PS was correlated with QoL.
A double-blind trial [66] recently studied the effect of melatonin supplementation at 20 mg/day for 4 weeks on appetite in cachexia patients with advanced cancer. At the interim analysis of 48 patients, the trial was stopped early for futility, noting the absence of any advantage for the melatonin arm. In this study, QoL was also assessed, but no apparent difference between the two groups was observed. However, it is difficult to take these results into account, because the study was not designed to conclude on QoL, which was a secondary objective.
Only one double-blind trial [67], recently studied the effect of melatonin supplementation (10 or 20 mg/day) on QoL and reported a better meanadjusted QoL score (FACT-L) with a slightly significant better score (2.69 points, CI 0.01–5.38, p = 0.049) found for social wellbeing.
However, melatonin supplementation for older patients could entail a weakness. Indeed, this subpopulation, because of their numerous comorbidities, often has a lot of tablets to take; this would add yet one more tablet, which could be a limitation. It is also important to emphasize that sustained-release formulation of melatonin is not reimbursed by social security system in France (except for children with specific syndromes). So another weakness is the price of the supplementation: approximately 30 € for 30 tablets of Circadin® 2 mg. Furthermore, in the studies, the dosage of melatonin varies from 5 mg/day to 20 mg/day while standard dosage of sustained-release formulation is 2 mg/day (Table 1). The smaller dosages lead to good results with an improvement in quality of sleep and depressive symptoms [61, 62, 64]. A systematic review, conducted on 16 articles, concluded that optimal dosage for melatonin supplementation in older adults is the lowest possible dose because it is closest to physiological circadian rhythm of melatonin [68].
Conclusion
The use of melatonin as a therapeutic strategy seems particularly suitable for elderly patients, a population known to secrete significantly less melatonin. However, to date no studies have been conducted in this population. The elderly population is heterogeneous and complex (comorbidities, general health, drug interactions, dosage adjustment), and maintaining QoL is a major challenge in the care of elderly patients. In advanced disease, treatment is palliative and the aim is to control the disease and pain, limit toxicities and maintain an overall level of QoL. Thus, it seems appropriate to study the impact of melatonin supplementation during cytotoxic therapy on QoL among elderly metastatic cancer patients.
References
Bray F, Ferlay J, Soerjomataram I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424. https://doi.org/10.3322/caac.21492
Smith BD, Smith GL, Hurria A et al (2009) Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol 27:2758–2765. https://doi.org/10.1200/JCO.2008.20.8983
Balducci L (2000) Geriatric oncology: challenges for the new century. Eur J Cancer 36:1741–1754. https://doi.org/10.1016/s0959-8049(00)00169-6
Downing NS, Shah ND, Neiman JH et al (2016) Participation of the elderly, women, and minorities in pivotal trials supporting 2011–2013 US. Food and drug administration approvals. Trials 17:199. https://doi.org/10.1186/s13063-016-1322-4
Hurria A, Dale W, Mooney M et al (2014) Designing therapeutic clinical trials for older and frail adults with cancer: U13 conference recommendations. J Clin Oncol 32:2587–2594. https://doi.org/10.1200/JCO.2013.55.0418
Hutchins LF, Unger JM, Crowley JJ et al (1999) Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med 341:2061–2067. https://doi.org/10.1056/NEJM199912303412706
Lewis JH, Kilgore ML, Goldman DP et al (2003) Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol 21:1383–1389. https://doi.org/10.1200/JCO.2003.08.010
Freyer G, Braud A-C, Chaibi P et al (2006) Dealing with metastatic breast cancer in elderly women: results from a French study on a large cohort carried out by the “Observatory on Elderly Patients”. Ann Oncol 17:211–216. https://doi.org/10.1093/annonc/mdj043
Bouchardy C, Rapiti E, Fioretta G et al (2003) Undertreatment strongly decreases prognosis of breast cancer in elderly women. J Clin Oncol 21:3580–3587. https://doi.org/10.1200/JCO.2003.02.046
Goodwin JS, Hunt WC, Samet JM (1993) Determinants of cancer therapy in elderly patients. Cancer 72:594–601
de Rijke JM, Schouten LJ, Schouten HC et al (1996) Age-specific differences in the diagnostics and treatment of cancer patients aged 50 years and older in the province of Limburg The Netherlands. Ann Oncol 7:677–685
Popa MA, Wallace KJ, Brunello A et al (2014) Potential drug interactions and chemotoxicity in older patients with cancer receiving chemotherapy. J Geriatr Oncol 5:307–314. https://doi.org/10.1016/j.jgo.2014.04.002
Lichtman SM, Wildiers H, Launay-Vacher V et al (1990) (2007) International society of geriatric oncology (SIOG) recommendations for the adjustment of dosing in elderly cancer patients with renal insufficiency. Eur J Cancer Oxf Engl 43:14–34. https://doi.org/10.1016/j.ejca.2006.11.004
Dees EC, O’Reilly S, Goodman SN et al (2000) A prospective pharmacologic evaluation of age-related toxicity of adjuvant chemotherapy in women with breast cancer. Cancer Invest 18:521–529
Wu WC, Rathore SS, Wang Y et al (2001) Blood transfusion in elderly patients with acute myocardial infarction. N Engl J Med 345:1230–1236. https://doi.org/10.1056/NEJMoa010615
Landi F, Zuccalà G, Gambassi G et al (1999) Body mass index and mortality among older people living in the community. J Am Geriatr Soc 47:1072–1076
Baker SD, Grochow LB (1997) Pharmacology of cancer chemotherapy in the older person. Clin Geriatr Med 13:169–183
Kane RL, Ouslander JG, Abrase IB (1989) The is of geriatric syndromes. Essent Clin Geriatr Ed 2
Lavdaniti M, Zyga S, Vlachou E, Sapountzi-Krepia D (2017) Quality of life in elderly cancer patients undergoing chemotherapy. Adv Exp Med Biol 989:291–295. https://doi.org/10.1007/978-3-319-57348-9_27
Naeim A, Aapro M, Subbarao R, Balducci L (2014) Supportive care considerations for older adults with cancer. J Clin Oncol 32:2627–2634. https://doi.org/10.1200/JCO.2014.55.3065
Lissoni P (2002) Is there a role for melatonin in supportive care? Support Care Cancer 10:110–116. https://doi.org/10.1007/s005200100281
Seely D, Wu P, Fritz H et al (2012) Melatonin as adjuvant cancer care with and without chemotherapy: a systematic review and meta-analysis of randomized trials. Integr Cancer Ther 11:293–303. https://doi.org/10.1177/1534735411425484
Wang Y, Jin B, Ai F et al (2012) The efficacy and safety of melatonin in concurrent chemotherapy or radiotherapy for solid tumors: a meta-analysis of randomized controlled trials. Cancer Chemother Pharmacol 69:1213–1220. https://doi.org/10.1007/s00280-012-1828-8
WHO (1995) The WORLD HEALTH ORGANIZATION QUALITY OF LIFE assessment (WHOQOL): position paper from the World Health Organization. Soc Sci Med 1982(41):1403–1409. https://doi.org/10.1016/0277-9536(95)00112-k
Cella DF (1994) Quality of life: concepts and definition. J Pain Symptom Manage 9:186–192
Ediebah DE, Quinten C, Coens C et al (2018) Quality of life as a prognostic indicator of survival: a pooled analysis of individual patient data from Canadian cancer trials group clinical trials. Cancer 124:3409–3416. https://doi.org/10.1002/cncr.31556
Kypriotakis G, Vidrine DJ, Francis LE, Rose JH (2016) The longitudinal relationship between quality of life and survival in advanced stage cancer. Psychooncology 25:225–231. https://doi.org/10.1002/pon.3846
Park S, Eo W, Lee S (2018) The relationship between health-related quality of life and survival in metastatic colorectal cancer patients treated with Korean medicine. Integr Cancer Ther 17:65–72. https://doi.org/10.1177/1534735416684015
Quinten C, Martinelli F, Coens C et al (2014) A global analysis of multitrial data investigating quality of life and symptoms as prognostic factors for survival in different tumor sites. Cancer 120:302–311. https://doi.org/10.1002/cncr.28382
Cella DF, Tulsky DS, Gray G et al (1993) The functional assessment of cancer therapy scale: development and validation of the general measure. J Clin Oncol 11:570–579
Aaronson NK, Ahmedzai S, Bergman B et al (1993) The European organization for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85:365–376
Kirkhus L, Harneshaug M, Šaltytė Benth J et al (2019) Modifiable factors affecting older patients’ quality of life and physical function during cancer treatment. J Geriatr Oncol 10:904–912. https://doi.org/10.1016/j.jgo.2019.08.001
Van Lancker A, Velghe A, Van Hecke A et al (2014) Prevalence of symptoms in older cancer patients receiving palliative care: a systematic review and meta-analysis. J Pain Symptom Manage 47:90–104. https://doi.org/10.1016/j.jpainsymman.2013.02.016
Cheng KKF, Lee DTF (2011) Effects of pain, fatigue, insomnia, and mood disturbance on functional status and quality of life of elderly patients with cancer. Crit Rev Oncol Hematol 78:127–137. https://doi.org/10.1016/j.critrevonc.2010.03.002
Saberzadeh-Ardestani B, Khosravi B, Zebardast J, Sadighi S (2019) Chemotherapy effect on daytime sleepiness and contributing factors in older adults with cancer. J Geriatr Oncol 10:632–636. https://doi.org/10.1016/j.jgo.2018.10.003
Mormont M-C, Waterhouse J (2002) Contribution of the rest-activity circadian rhythm to quality of life in cancer patients. Chronobiol Int 19:313–323. https://doi.org/10.1081/cbi-120002606
Innominato PF, Focan C, Gorlia T et al (2009) Circadian rhythm in rest and activity: a biological correlate of quality of life and a predictor of survival in patients with metastatic colorectal cancer. Cancer Res 69:4700–4707. https://doi.org/10.1158/0008-5472.CAN-08-4747
Innominato PF, Komarzynski S, Palesh OG et al (2018) Circadian rest-activity rhythm as an objective biomarker of patient-reported outcomes in patients with advanced cancer. Cancer Med 7:4396–4405. https://doi.org/10.1002/cam4.1711
Mormont MC, Waterhouse J, Bleuzen P et al (2000) Marked 24-h rest/activity rhythms are associated with better quality of life, better response, and longer survival in patients with metastatic colorectal cancer and good performance status. Clin Cancer Res 6:3038–3045
Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D (2000) Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst 92:994–1000. https://doi.org/10.1093/jnci/92.12.994
Sephton SE, Lush E, Dedert EA et al (2013) Diurnal cortisol rhythm as a predictor of lung cancer survival. Brain Behav Immun 30(Suppl):S163–170. https://doi.org/10.1016/j.bbi.2012.07.019
Innominato PF, Giacchetti S, Bjarnason GA et al (2012) Prediction of overall survival through circadian rest-activity monitoring during chemotherapy for metastatic colorectal cancer. Int J Cancer 131:2684–2692. https://doi.org/10.1002/ijc.27574
Arendt J, Skene DJ (2005) Melatonin as a chronobiotic. Sleep Med Rev 9:25–39. https://doi.org/10.1016/j.smrv.2004.05.002
Claustrat B, Leston J (2015) Melatonin: physiological effects in humans. Neurochirurgie 61:77–84. https://doi.org/10.1016/j.neuchi.2015.03.002
Bonnefont-Rousselot D, Collin F (2010) Melatonin: action as antioxidant and potential applications in human disease and aging. Toxicology 278:55–67. https://doi.org/10.1016/j.tox.2010.04.008
Mediavilla MD, Sanchez-Barcelo EJ, Tan DX et al (2010) Basic mechanisms involved in the anti-cancer effects of melatonin. Curr Med Chem 17:4462–4481
Jardim-Perassi BV, Arbab AS, Ferreira LC et al (2014) Effect of melatonin on tumor growth and angiogenesis in xenograft model of breast cancer. PLoS ONE 9:e85311. https://doi.org/10.1371/journal.pone.0085311
Carrillo-Vico A, Lardone PJ, Alvarez-Sanchez N et al (2013) Melatonin: buffering the immune system. Int J Mol Sci 14:8638–8683. https://doi.org/10.3390/ijms14048638
Haimov I, Laudon M, Zisapel N et al (1994) Sleep disorders and melatonin rhythms in elderly people. BMJ 309:167
Arendt J (1999) Jet-lag and shift work: (2). Therapeutic use of melatonin. J R Soc Med 92:402–405
Arendt J, Bojkowski C, Franey C et al (1985) Immunoassay of 6-hydroxymelatonin sulfate in human plasma and urine: abolition of the urinary 24-hour rhythm with atenolol. J Clin Endocrinol Metab 60:1166–1173. https://doi.org/10.1210/jcem-60-6-1166
Shirakawa S, Tsuchiya S, Tsutsumi Y et al (1998) Time course of saliva and serum melatonin levels after ingestion of melatonin. Psychiatry Clin Neurosci 52:266–267. https://doi.org/10.1111/j.1440-1819.1998.tb01067.x
Lemoine P, Nir T, Laudon M, Zisapel N (2007) Prolonged-release melatonin improves sleep quality and morning alertness in insomnia patients aged 55 years and older and has no withdrawal effects. J Sleep Res 16:372–380. https://doi.org/10.1111/j.1365-2869.2007.00613.x
Wade AG, Ford I, Crawford G et al (2007) Efficacy of prolonged release melatonin in insomnia patients aged 55–80 years: quality of sleep and next-day alertness outcomes. Curr Med Res Opin 23:2597–2605. https://doi.org/10.1185/030079907X233098
Ferracioli-Oda E, Qawasmi A, Bloch MH (2013) Meta-analysis: melatonin for the treatment of primary sleep disorders. PLoS ONE 8:e63773. https://doi.org/10.1371/journal.pone.0063773
Nogueira LM, Sampson JN, Chu LW et al (2013) Individual variations in serum melatonin levels through time: implications for epidemiologic studies. PLoS ONE 8:e83208. https://doi.org/10.1371/journal.pone.0083208
Skene DJ, Swaab DF (2003) Melatonin rhythmicity: effect of age and Alzheimer’s disease. Exp Gerontol 38:199–206
Wu Y-H, Zhou J-N, Van Heerikhuize J et al (2007) Decreased MT1 melatonin receptor expression in the suprachiasmatic nucleus in aging and Alzheimer’s disease. Neurobiol Aging 28:1239–1247. https://doi.org/10.1016/j.neurobiolaging.2006.06.002
Pandi-Perumal SR, Seils LK, Kayumov L et al (2002) Senescence, sleep, and circadian rhythms. Ageing Res Rev 1:559–604
Cerea G, Vaghi M, Ardizzoia A et al (2003) Biomodulation of cancer chemotherapy for metastatic colorectal cancer: a randomized study of weekly low-dose irinotecan alone versus irinotecan plus the oncostatic pineal hormone melatonin in metastatic colorectal cancer patients progressing on 5-fluorouracil-containing combinations. Anticancer Res 23:1951–1954
Innominato PF, Lim AS, Palesh O et al (2016) The effect of melatonin on sleep and quality of life in patients with advanced breast cancer. Support Care Cancer 24:1097–1105. https://doi.org/10.1007/s00520-015-2883-6
Madsen MT, Hansen MV, Andersen LT et al (2016) Effect of melatonin on sleep in the perioperative period after breast cancer surgery: a randomized, double-blind, placebo-controlled trial. J Clin Sleep Med 12:225–233. https://doi.org/10.5664/jcsm.5490
Lissoni P, Ardizzoia A, Barni S et al (1995) A randomized study of tamoxifen alone versus tamoxifen plus melatonin in estrogen receptor-negative heavily pretreated metastatic breast cancer patients. Oncol Rep 2:871–873
Hansen MV, Andersen LT, Madsen MT et al (2014) Effect of melatonin on depressive symptoms and anxiety in patients undergoing breast cancer surgery: a randomized, double-blind, placebo-controlled trial. Breast Cancer Res Treat 145:683–695. https://doi.org/10.1007/s10549-014-2962-2
Lissoni P, Barni S, Ardizzoia A et al (1992) Randomized study with the pineal hormone melatonin versus supportive care alone in advanced nonsmall cell lung cancer resistant to a first-line chemotherapy containing cisplatin. Oncology 49:336–339
Del Fabbro E, Dev R, Hui D et al (2013) Effects of melatonin on appetite and other symptoms in patients with advanced cancer and cachexia: a double-blind placebo-controlled trial. J Clin Oncol 31:1271–1276. https://doi.org/10.1200/JCO.2012.43.6766
Sookprasert A, Johns NP, Phunmanee A et al (2014) Melatonin in patients with cancer receiving chemotherapy: a randomized, double-blind, placebo-controlled trial. Anticancer Res 34:7327–7337
Vural EMS, Van Munster BC, de Rooij SE (2014) Optimal dosages for melatonin supplementation therapy in older adults: a systematic review of current literature. Drugs Aging 31:441–451. https://doi.org/10.1007/s40266-014-0178-0
Funding
None.
Author information
Authors and Affiliations
Contributions
SD, ET and XD has the idea for article; AG and SD performed the literature search, SD, ET, XD, AG drafted the work, MOH, FK, JP, JB, CA, MAMR critically revised the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ginzac, A., Dubois, S., Hager, MO. et al. Quality of life for older patients with cancer: a review of the evidence supporting melatonin use. Aging Clin Exp Res 32, 2459–2468 (2020). https://doi.org/10.1007/s40520-020-01532-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-020-01532-0