Abstract
Background
Pivotal trials, the clinical studies that inform U.S. Food and Drug Administration (FDA) approval decisions, provide the foundational evidence supporting the safety and efficacy of novel therapeutics. We determined the representation of the elderly, women, and patients from racial and ethnic minorities in pivotal trials and whether the FDA is making subgroup efficacy analyses among these subpopulations available to the public.
Methods
We conducted a cross-sectional study of novel therapeutics approved by the FDA between 2011 and 2013. Using publicly available FDA documents, we collected information on the demographic characteristics of pivotal trial participants (age ≥65 years, sex [male, female], race [white, black, Asian, other], and ethnicity [Hispanic, non-Hispanic]) and determined the availability of subgroup analyses by age, sex, race, and ethnicity.
Results
We identified 86 novel therapeutic that were approved by the FDA between 2011 and 2013 for 92 indications on the basis of 206 pivotal trials. The median age of pivotal trial patients was 53.1 years (interquartile range 40.6–60.6), and the mean proportion of patients ≥65 years of age was 28.9 % (95 % CI 23.5–34.4 %). Similar proportions of pivotal trial participants were male (mean 50.3 %, 95 % CI 45.3–55.2 %) and female (mean 49.7 %, 95 % CI 44.7–54.7 %). Most participants were white (mean 79.2 %, 95 % CI 75.9–82.6 %), while the mean proportion of black patients was 7.4 % (95 % CI 5.5–9.3 %), that of Asian patients was 7.4 % (95 % CI 5.2–9.7 %), and that of patients of other races was 5.9 % (95 % CI 4.4–7.5 %). Information about ethnicity was available for only 59.8 % of indications, and where such data were available, the mean proportion of Hispanic participants was 13.3 % (95 % CI 10.3–16.3 %). FDA reviewers performed and made available subgroup efficacy analyses by age, sex, and race for at least one of the pivotal trials used as the basis of approval for over 80 % of indications.
Conclusions
Although women are equally represented in pivotal trials supporting recent novel therapeutic approvals by the FDA, elderly patients and those from racial and ethnic minorities are underrepresented. FDA reviewers generally perform subgroup efficacy analyses by age, sex, and race and make these subgroup analyses available to the public.
Similar content being viewed by others
Background
Clinical trials provide the foundational evidence that supports U.S. Food and Drug Administration (FDA) approval and informs physicians and patients as they make decisions about the use of new therapies. For this evidence to inform both regulators and clinical practice, it is critical that the patients participating in these trials, wherever they are conducted, reflect the population of patients expected to use the novel therapeutic agent. This is particularly important for pivotal clinical trials, the late-stage clinical trials that are intended to demonstrate the safety and efficacy of novel therapeutics and upon which the FDA bases its approval decisions [1]. However, several groups of individuals, including the elderly, women, and persons from racial and ethnic minority groups, have historically been underrepresented in clinical research, limiting the ability of many studies to inform decisions about their care [2–8].
Over the years, the FDA has taken several measures aimed at improving and ensuring the representativeness of patients participating in pivotal clinical trials. First, in 1998, the FDA issued the “demographic rule,” requiring drug makers to report the age, sex, and race of clinical trial participants in their annual reports to the agency [9]. While the demographic rule ensured that this information was available to the FDA, it did not mandate its availability to the public. However, the 2012 FDA Safety and Innovation Act (FDASIA) urged the FDA to address this issue, and, in August 2013, the agency released a report describing the demographic characteristics of participants in trials of drugs and devices approved in 2011, as well as the availability of subgroup analyses for key age, sex, and race subgroups [10]. More recently, the FDA published an expanded investigation of the representativeness of clinical trials supporting drugs approved between 2010 and 2012 [11].
In this article, we describe the results of our research to characterize the demographic characteristics of patients participating in pivotal clinical trials for all novel therapeutics approved by the FDA between 2011 and 2013 and to determine the availability of subgroup efficacy analyses using publicly available data sources. Our research was similarly focused on participation in these trials by members of historically underrepresented populations: the elderly (defined as patients aged 65 years or older), women, persons of nonwhite race, and Hispanics. Although our study was initiated before the publication of the FDA’s initial report prompted by the FDASIA and subsequent research study [10, 11], there are three specific reasons that support the current dissemination of our work: (1) to validate the FDA’s findings of the age, sex, and race of clinical trial participants, exclusively using publicly available data sources, since the FDA’s study made use of internal documents submitted to the agency that are not available to the public; (2) to confirm the FDA’s findings among 2013 drug approvals, since the FDA’s study focused on 2010 through 2012 approvals; and (3) to provide a broader investigation of the issue by examining trial participation by ethnicity, since the FDA study focused only on age, sex, and race.
Methods
Study sample
Using the Drugs@FDA database, a publicly available index of FDA regulatory actions, we identified all novel therapeutics (i.e., new molecular entities—drugs—and novel biologics) that were approved by the FDA between 1 January 2011 and 31 December 2013 [12]. Generic drugs, reformulations, combinations of nonnovel therapeutic agents, and nontherapeutic agents (i.e., diagnostic agents) were excluded. For each of the novel therapeutics included in our sample, we determined the indications for which it was first approved for use by reviewing the FDA’s approval letter and the initial drug label. All indications were classified into one of eight therapeutic areas (cancer, cardiovascular [including diabetes mellitus and hyperlipidemia], infectious disease, autoimmune and musculoskeletal disease, neurology, dermatology, psychiatry, and other). Next, we determined whether these novel therapeutics had been granted orphan status, indicating that they are primarily intended for the treatment of a rare disease. For small molecules, orphan status was determined using the Drugs@FDA database [12]; for biologics, we searched the Orphan Drug Product Designation Database [13].
Pivotal trials
The FDA makes a series of documents available in the Drugs@FDA database that correspond to the key components of the agency’s review process, explain the basis of its approval decision, and summarize notable information presented in sponsors’ applications. The “medical review” discusses the safety and efficacy of novel therapeutics, describing the pivotal trials in detail. For each of the novel therapeutics included in our sample, we manually searched the corresponding medical review documents to identify the pivotal trials that supported their approval and recorded the intention-to-treat (ITT) population (i.e., all participants who received at least one dose of the study drug or its comparator). If the medical review did not clearly identify the pivotal trials used as the basis of approval, we reviewed other FDA documents to identify such trials, such as the summary review and office director memos, and if necessary manually classified trials as pivotal following an established approach [1].
Demographic characteristics of pivotal trial participants
Information describing the age, sex, race, and ethnicity of the participants in each of the identified pivotal trials was abstracted from the FDA’s medical review documents. In circumstances when the medical review did not contain all of this information, the agency’s statistical reviews were searched. To characterize the ages of pivotal trial participants, we recorded the mean as a summary statistic; if the mean was unavailable, the median was used. When FDA documents provided such statistics for individual trial arms, but not in the aggregate, we used a weighted-average approach to estimate the corresponding statistic for the entire study population. In addition, we determined the number of elderly clinical trial participants, defined as those aged 65 years or older. Next, we recorded the number of male and female participants in each study. To characterize clinical trial participants’ race and ethnicity, we counted the number of participants in four racial subgroups (white, black, Asian, and other) and two ethnic subgroups (Hispanic and non-Hispanic). Two reviewers (JHN and JAA) independently abstracted this information from FDA documents. A second reviewer (NSD) validated the initial abstraction by reabstracting all data, and all discrepancies were resolved through discussion with the senior investigator (JSR).
Availability of subgroup efficacy analyses
To determine whether FDA reviewers documented the efficacy of novel therapeutics for age, sex, and race as well as ethnic subgroups, we searched the medical and statistical reviews to identify the presence of subgroup analyses for each pivotal trial. Analyses of efficacy involving patients of any two age groups (e.g., <40 years vs. ≥40 years; <65 years vs. ≥65 years) were considered to represent a subgroup analysis by age. We considered an acknowledgement of insufficient sample size in certain subgroups to represent subgroup analysis because the agency made an attempt to produce this information.
Statistical analysis
For each pivotal trial, we computed the proportions of patients in various age (≥65 years), sex (male, female), race (white, black, Asian, other), and ethnicity (Hispanic, non-Hispanic) subgroups. Next, to reflect the overall demographic composition of the clinical development program for each novel therapeutic, we aggregated these data, which describe the characteristics of patients participating in individual pivotal trials, to the indication level. To do this, we weighted the contribution from each pivotal trial according to its ITT population. In cases where certain data fields were available for some but not all of the pivotal trials supporting an indication, we aggregated only data from trials for which the field was available; for fields that were not available for any pivotal trials within an indication, we noted that the data were missing.
After aggregating this information to the indication level, we characterized the demographic composition of pivotal trial patients using descriptive statistics, reporting the median and interquartile range (IQR) for age and computing the mean proportion of patients in the various age, sex, race, and ethnicity subgroups with the corresponding 95 % CIs. In addition, we performed a stratified analysis by therapeutic area and orphan status. To contextualize our results, we also computed summary statistics for the corresponding demographics of the U.S. population using data derived from 2010 U.S. Census [14]. Last, we counted the number of indications for which analyses of efficacy in age, sex, race, and ethnicity subgroups were documented in the FDA’s review of at least one of the associated pivotal trials.
Since the epidemiology of a disease may preclude recruitment of pivotal trial participants of differing ages, sexes, races, and ethnicities, we repeated all analyses after excluding those therapeutics indicated for diseases that are uncommon among historically underrepresented populations, including the elderly, females, and racial and ethnic minorities. For instance, a pivotal trial for a novel therapeutic used to treat prostate cancer would not be expected to recruit women. To identify such therapeutics, two investigators (NSD and JSR) independently reviewed the indications for which each novel therapeutic was initially approved and, based on their clinical experience, excluded those where the disease prevalence in the four historically underrepresented populations was approximately less than half the disease prevalence among the “majority” populations. As another example, ivacaftor, a drug for the treatment of cystic fibrosis, a disease that affects primarily young white individuals, was excluded from analyses examining age, race, and ethnicity subgroups, because it is unrealistic to expect that pivotal trials involving this drug would include elderly, nonwhite, or Hispanic patients. (For a complete list of therapeutic subgroup population exclusions, see Tables 5, 6 and 7 in Appendix). All data were collected and analyzed using Microsoft Excel software (Microsoft, Redmond, WA, USA). Statistical analysis was performed using JMP software (SAS Institute, Cary, NC, USA).
Results
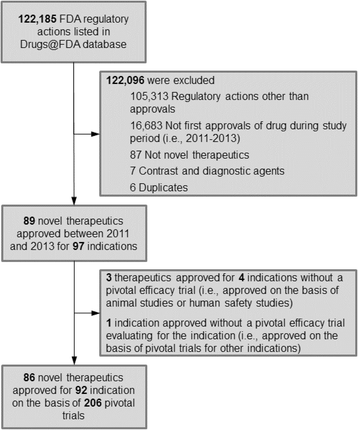
We identified 89 novel therapeutics that the FDA approved between 2011 and 2013 for use in 97 indications. (For details of sample construction, see Fig. 2 in the Appendix) After removing therapeutics and indications that were approved in the absence of a pivotal trial, our final sample consisted of 86 therapeutics approved for use in 92 indications. Of these 86 therapeutics, 27 (31.4 %) were approved in 2011, 35 (40.7 %) in 2012, and 24 (27.9 %) in 2013 (Table 1). Drugs approved for indications in cancer (30.4 %), cardiovascular disease (12.0 %), and infectious disease (9.8 %) accounted for over half of approvals, while 30 (32.6 %) indications received orphan status.
The 92 indications were approved on the basis of 206 pivotal trials that included a total of 174,855 patients. The number of pivotal trials supporting each approval varied: 41 (44.6 %) indications were approved on the basis of a single pivotal trial, 28 (30.4 %) on the basis of two pivotal trials, and 23 (25.0 %) on the basis of three or more pivotal trials. When aggregated to the indication level, the mean number of patients enrolled in the pivotal trials supporting each approved indication was 1900 (standard deviation 3156).
Demographic characteristics of pivotal trial participants
At the indication level, the median age of pivotal trial patients was 53.1 years (IQR 40.6–60.6) (Table 2). Subgroup categorization of participants’ age was available for at least one trial supporting 61 (66.3 %) of 92 indications, and among these indications, the mean proportion of patients aged ≥65 years was 28.9 % (95 % CI 23.5–34.4 %). For 9 indications (14.5 %), more than half of the patients were aged 65 years or older (Fig. 1). Of the 92 indications, 22 (23.9 %) are used to treat diseases that are uncommon in the elderly. When we limited our analyses to indications for which the inclusion of the elderly should be expected, we found fairly consistent results (Table 2).
Information about the sex of clinical trial participants was universally available, and equal proportions of clinical trial participants were male (50.3 %, 95 % CI 45.3–55.2 %) and female (49.7 %, 95 % CI 44.7–54.7 %), although there was variation: in 12.0 % of indications, over 80 % of patients were female, and in 9.8 % of indications, over 80 % were male. Of the 92 indications, 14 (15.2 %) are used to treat diseases that are uncommon in women. When we limited our analyses to indications for which the inclusion of women should be expected, we found fairly consistent results (Table 2).
Approximately 80 % of clinical trial participants were white (79.2 %, 95 % CI 75.9–82.6 %), while 7.4 % (95 % CI 5.5–9.3 %) were black, 7.4 % (95 % CI 5.2–9.7 %) were Asian, and 5.9 % (95 % CI 4.4–7.5 %) were of other races. When compared with the overall U.S. population, more patients participating in clinical trials included in our study were white (79.2 % vs. 75.2 %) and Asian (7.4 % vs. 5.4 %), while fewer patients were black (7.4 % vs. 13.2 %). Nonwhite patients comprised 30 % or more of the clinical trial participants for almost one-fourth of indications (22 of 92, or 23.9 %). In contrast to information about the age, sex, and race of clinical trial participants, which was almost always available, information about the ethnicity of clinical trial participants was available for only 55 (59.8 %) of 92 indications. Among indications where these data were reported, 13.3 % (95 % CI 10.3–16.3 %) were Hispanic, which is less than the 16.2 % of the overall U.S. population. Hispanic participants comprised 20 % or less of participants in clinical trials supporting the approval of 81.8 % (45 of 55) of indications where ethnicity information was reported. Of the 92 indications included in this analysis, 14 (15.2 %) are used to treat diseases that are uncommon in racial minorities. When we limited our analyses to indications for which the inclusion of patients from racial minorities should be expected, we found fairly consistent results (Table 2).
Pivotal trial participants by therapeutic area
There was significant variation in the characteristics of patients participating in clinical trials according to therapeutic area (Table 3). Pivotal trials involving therapies for cancer tended to involve higher proportions of elderly patients than those for other indications (36.6 % [95 % CI 27.6–45.6 %] vs. 23.9 % [95 % CI 17.3–30.6 %]), higher proportions of white patients (82.6 % [95 % CI 76.4–88.8 %] vs. 77.8 % [95 % CI 73.7–81.8 %]), and lower proportions of Hispanic patients (3.3 % [95 % CI 0.5–6.0 %] vs. 10.0 % [95 % CI 7.1–12.9 %]). Male patients were more common in pivotal trials of therapies for infectious diseases than for other indications (66.2 % [95 % CI 55.5–76.8 %] vs. 48.6 % [95 % CI 43.2–53.9 %]), and a far higher proportion of black patients also participated in such trials (16.7 % [95 % CI 8.7–24.7 %] vs. 9.5 % [95 % CI 4.5–8.3 %]).
Pivotal trial participants by orphan status
The age and sex of participants in clinical trials involving therapies receiving orphan status tended to be similar to those that did not receive orphan status. Although there were comparable proportions of white patients participating in clinical trials of drugs receiving orphan status, the proportion of black patients was lower (5.4 % [95 % CI 2.8–8.2 %] vs. 8.3 % [95 % CI 5.8–10.9 %]), while the proportion of Asian patients was higher (10.6 % [95 % CI 4.9–16.4 %] vs. 5.9 % [95 % CI 3.8–7.9 %]). Hispanic patients tended to be underrepresented in clinical trials involving therapies receiving orphan status. The mean proportions of Hispanic patients were 10.9 % (95 % CI 4.1–17.6 %) for therapies with orphan status compared with 14.1 % (95 % CI 10.6–17.5 %) for therapies without orphan status.
Availability of subgroup efficacy analyses
FDA reviewers generally performed subgroup efficacy analyses by age, sex, and race for at least one of the pivotal trials used as the basis of approval for most indications included in our sample (Table 4). Specifically, subgroup analyses by age were performed for 85 (92.4 %) of 92 indications, by sex for 80 (87.0 %) of 92 indications, and by race 74 (80.4 %) of 92 indications. In contrast, subgroup analyses by ethnicity were performed in 21 (22.8 %) of 92 indications.
After removing indications involving disease processes that are uncommon in the elderly, females, and racial minorities, the rates with which the FDA reviewer performed subgroup analyses were consistent by age, sex, and race, but rose slightly.
Discussion
Our characterization of the demographic characteristics of patients participating in pivotal trials for all novel therapeutics approved by the FDA between 2011 and 2013 demonstrates that female patients, and to a certain extent elderly patients, appear to be appropriately represented, while black and Hispanic patients remain underrepresented. Specifically, we found that elderly patients made up between one-fourth and one-third of all pivotal trial participants and that the proportion of participating male and female patients was equal. White patients accounted for almost 80 % of pivotal trial participants, a higher proportion than in the general population; in contrast, between 5 % and 10 % of patients participating in pivotal trials were black, far less than their representation in the general population, and similarly low rates of participation were observed for Hispanic patients. Last, we found that the FDA’s subgroup efficacy analyses by age, sex, and race were usually but not always provided within publicly available documents, while such analyses by ethnicity were seldom available.
Our results suggest that information describing the sex of pivotal trial participants is being made available within FDA documentation and that women are equally represented in more recently conducted pivotal clinical trials. These findings suggest that progress has been made since the early 2000s, when the participation of women in clinical research was limited [3, 4, 6–8]. For example, in our present study we found that among therapeutics approved for cardiovascular disease, almost 45 % of participants in pivotal trials were female; in contrast, women comprised only 30 % of the trials used to inform the American Heart Association’s 2007 guideline for the prevention of cardiovascular disease [15].
Similarly, it appears that more elderly patients are being recruited to participate in clinical trials than in the past; however, rates are still unlikely to approximate disease burden. For example, the proportion of elderly patients in pivotal trials involving novel therapeutics for cancer was approximately 36 %. While this represents an improvement over trials conducted between 1996 and 1998, in which elderly patients accounted for 25 % of participants [5], the age-adjusted incidence rate of cancer is 10 times higher among those aged 65 years or older [16].
In contrast, we observed that the proportion of black patients participating in pivotal trials was relatively low, below rates that would be expected given the general population and well below rates that would be expected given disparities in disease burden, as minority populations are more likely to be diagnosed with many known diseases, including cancer, heart disease, and diabetes [17]. This finding indicates that racial disparities in clinical research participation that have been noted in the 1990s and 2000s likely persist today [4, 5, 8]. The limited reporting of ethnicity information and the apparent underrepresentation of Hispanic patients may reflect the fact that only in 2005 did the FDA clarify its expectation that pivotal trial participant ethnicity be documented [18]. Nevertheless, little is known about the study of historically underrepresented minorities in clinical trials after FDA approval, suggesting that the FDA approval itself offers an important opportunity to ensure adequate representation of pivotal trial populations.
Our findings validate the FDA’s research that characterized the demographic characteristics of participants in pivotal trials of drugs approved between 2010 and 2012 [10, 11] and demonstrate that the observations made in the agency’s analyses extend to drugs approved in 2013. We found that the availability of subgroup analyses of efficacy by age, sex, and race was slightly lower in our study than the FDA’s and could be explained by the FDA’s use of nonpublic data sources. In the aggregate, our study and the FDA’s research indicate that it may be possible for the agency to do more to encourage the participation of the elderly and racial and ethnic minorities in pivotal trials. However, the inclusion of such patients in clinical trials is not enough; subgroup efficacy analyses ought to be made more easily available to the public, rather than just in the agency’s lengthy review documents. The FDA took an important step forward in August 2014 by releasing an action plan to address the issue of demographic subgroup participation and the availability of the associated data [19]. In addition, the launch of the FDA’s “Drug Trials Snapshot” website provides straightforward information about the demographic characteristics of pivotal trial participants and the results of subgroup analyses of efficacy; however, this useful tool is available for only a handful of drugs at the moment [20].
There are several important limitations of this study to consider. First, we describe the demographic characteristics of participants in pivotal trials only. While these trials represent the primary source of information characterizing the safety and efficacy of novel therapeutics, it is possible that the demographic composition of other trials, which were not considered to be as important to regulators, may be different and could potentially include higher proportions of elderly and female patients as well as those from racial and ethnic minorities. Second, our analysis was focused on the public availability of information about the demographic characteristics of patients participating in clinical trials. Since applications submitted to the FDA are not available to the public, the agency may have additional insight into the demographics of pivotal trial participants that are not described in its publicly available review documents, which were the basis of our study. Moreover, any information is made available for only therapeutics approved by the FDA; we were unable to examine the demographic characteristics of participants in pivotal trials of unapproved therapeutics. Third, we did not determine whether the FDA subgroup efficacy analysis identified differences among population subgroups, only whether the analysis was performed. Nor did we determine whether the FDA conducted subgroup safety analyses, which the FDA should consider as part of new drug evaluations as directed by the FDASIA.
Fourth, our assessments of the representativeness of patients participating in clinical trials were necessarily crude and relied on comparisons with the demographics of the overall U.S. population. Ideally, the characteristics of pivotal trial participants would be compared with the age, sex, race, and ethnicity of the population of patients affected by the disease for which each novel therapeutic was indicated; however, with the exception of cancer, such detailed epidemiological data are not systematically available. Fifth, the location of the sites where these clinical trials were conducted may influence the demographic composition of pivotal trial participants. Information on locations of clinical trial sites was not systematically available in the FDA documents, such that we were unable to characterize clinical trial participants on the basis of clinical trial site locations. While many consider drug development and evaluation to be a global enterprise, the demographics of the pivotal trial participants should be reasonably expected to reflect the U.S. patient population for whom the therapeutic is being approved for use. We cannot be certain whether our findings suggesting that more elderly patients are being recruited to participate in clinical trials than in the past, but that black and Hispanic patients remain underrepresented, are a consequence of greater trial recruitment from non-U.S. countries or other reasons. Last, in our present study, we relied on the demographic data reported in FDA documents. It is not known what methods were used to ascertain this demographic data, originally collected by each manufacturer, or whether the data collection process was standardized across manufacturers. However, the FDA does suggest that patients participating in clinical trials self-report their race and ethnicity [18].
Conclusions
Although women are equally represented in pivotal trials supporting recent novel therapeutic approvals by the FDA, elderly patients and those from racial and ethnic minorities are underrepresented. FDA reviewers generally perform subgroup analyses by age, sex, and race and make these subgroup analyses available to the public, but not in the most accessible formats. More can be done to ensure adequate representation of pivotal trial populations to inform clinical decision-making at the time of drug approval.
Abbreviations
- FDA:
-
Food and Drug Administration, an agency within the U.S. Department of Health and Human Services that is responsible for protecting the U.S. public health by assuring the safety, effectiveness, quality, and security of human and veterinary drugs, vaccines and other biological products, and medical devices, as well as the safety and security of most of the U.S. food supply, all cosmetics, dietary supplements and products that give off radiation
- FDASIA:
-
Food and Drug Administration Safety and Innovation Act, U.S. legislation signed into law on 9 July 2012 that expanded the FDA’s authorities and strengthened the agency's ability to safeguard and advance public health
- IQR:
-
interquartile range
- ITT:
-
intention-to-treat
- n/a:
-
not applicable
- WHO:
-
World Health Organization
- CI:
-
Confidence Interval
References
Downing NS, Aminawung JA, Shah ND, Krumholz HM, Ross JS. Clinical trial evidence supporting FDA approval of novel therapeutic agents, 2005–2012. JAMA. 2014;311(4):368–77.
Gifford AL, Cunningham WE, Heslin KC, Andersen RM, Nakazono T, Lieu DK, et al. Participation in research and access to experimental treatments by HIV-infected patients. N Engl J Med. 2002;346(18):1373–82.
Harris DJ, Douglas PS. Enrollment of women in cardiovascular clinical trials funded by the National Heart, Lung, and Blood Institute. N Engl J Med. 2000;343(7):475–80.
Heiat A, Gross CP, Krumholz HM. Representation of the elderly, women, and minorities in heart failure clinical trials. Arch Intern Med. 2002;162(15):1682–8.
Hutchins LF, Unger JM, Crowley JJ, Coltman Jr CA, Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341(27):2061–7.
Kim ESH, Carrigan TP, Menon V. Enrollment of women in National Heart, Lung, and Blood Institute-funded cardiovascular randomized controlled trials fails to meet current federal mandates for inclusion. J Am Coll Cardiol. 2008;52(8):672–3.
Lee PY, Alexander KP, Hammill BG, Pasquali SK, Peterson ED. Representation of elderly persons and women in published randomized trials of acute coronary syndromes. JAMA. 2001;286(6):708–13.
Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291(22):2720–6.
U.S. Food and Drug Administration. Investigational new drug applications and new drug applications. 21 CFR 312, 63 FR 6854. 11 Feb 1998. http://www.gpo.gov/fdsys/granule/FR-1998-02-11/98-3422. Accessed 6 Apr 2016.
U.S. Food and Drug Administration (FDA). Collection, analysis, and availability of demographic subgroup data for FDA-approved medical products. FDA report. Silver Spring, MD: FDA; August 2013. http://www.fda.gov/downloads/RegulatoryInformation/Legislation/FederalFoodDrugandCosmeticActFDCAct/SignificantAmendmentstotheFDCAct/FDASIA/UCM365544.pdf. Accessed 6 Apr 2016.
Eshera N, Itana H, Zhang L, Soon G, Fadiran EO. Demographics of clinical trials participants in pivotal clinical trials for new molecular entity drugs and biologics approved by FDA from 2010 to 2012. Am J Ther. 2015;22(6):435–55. doi:10.1097/MJT.0000000000000177.
U.S. Food and Drug Administration. Drugs@FDA. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/. Accessed 6 Apr 2016.
U.S. Food and Drug Administration. Search orphan drug designations and approvals. http://www.accessdata.fda.gov/scripts/opdlisting/oopd/. Accessed 6 Apr 2016.
U.S. Census Bureau. American FactFinder. DP-1-Geography-United States: profile of general population and housing characteristics: 2010. http://factfinder.census.gov/faces/nav/jsf/pages/index.xhtml. Accessed 6 Apr 2016.
Melloni C, Berger JS, Wang TY, Gunes F, Stebbins A, Pieper KS, et al. Representation of women in randomized clinical trials of cardiovascular disease prevention. Circ Cardiovasc Qual Outcomes. 2010;3(2):135–42.
National Cancer Institute, Surveillance, Epidemiology, and End Results Program (SEER). SEER cancer statistics review 1975–2012: Table 2.7. Incidence and mortality rates by age. http://seer.cancer.gov/csr/1975_2012/browse_csr.php?sectionSEL=2&pageSEL=sect_02_table.07.html. Accessed 6 Apr 2016.
Smedley BD, Stith AY, Nelson AR, editors. Unequal treatment: confronting racial and ethnic disparities in health care. Washington, DC: The National Academies Press; 2003.
U.S. Food and Drug Administration. Guidance for industry: collection of race and ethnicity data in clinical trials. Washington, DC: U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER), Center for Devices and Radiologic Health (CDRH), Office of the Commissioner (OC); September 2005. http://www.fda.gov/downloads/RegulatoryInformation/Guidances/ucm126396.pdf. Accessed 6 Apr 2016.
U.S. Food and Drug Administration (FDA). FDA action plan to enhance the collection and availability of demographic subgroup data. FDA report. Silver Spring, MD: FDA; August 2014. http://www.fda.gov/downloads/RegulatoryInformation/Legislation/FederalFoodDrugandCosmeticActFDCAct/SignificantAmendmentstotheFDCAct/FDASIA/UCM410474.pdf. Accessed 6 Apr 2016.
U.S. Food and Drug Administration. Drug trials snapshots. http://www.fda.gov/Drugs/InformationOnDrugs/ucm412998.htm. Accessed 6 Apr 2016.
Acknowledgements
This research was not supported by any external grants or funds. HMK is supported by grant U01 HL105270-05 (Center for Cardiovascular Outcomes Research at Yale University) from the National Heart, Lung, and Blood Institute. JSR is supported by grant K08 AG032886 from the National Institute on Aging and by the American Federation for Aging Research through the Paul B. Beeson Career Development Award Program. These funders were not involved in the design of the study, conduct of the work, or the development and submission of the work for publication.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
HMK and JSR are funded by research agreements through Yale University from Medtronic and from Johnson & Johnson (Janssen) to develop methods of clinical trial data sharing, by the Centers for Medicare & Medicaid Services to develop and maintain performance measures, and by the FDA to develop methods for postmarket surveillance of medical devices. HMK chairs a cardiac scientific advisory board for UnitedHealth. The other authors declare that they have no competing interests.
Authors’ contributions
NSD, NDS, and JSR conceived and initiated the collaborative project. NSD, JAA, and JHN were responsible for the acquisition of the data. NSD and JSR drafted the manuscript. All authors interpreted the data, revised the manuscript for important intellectual content, and reviewed and approved the submitted manuscript. JSR is the guarantor of the article and affirms that it is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Appendix
Appendix
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Downing, N.S., Shah, N.D., Neiman, J.H. et al. Participation of the elderly, women, and minorities in pivotal trials supporting 2011–2013 U.S. Food and Drug Administration approvals. Trials 17, 199 (2016). https://doi.org/10.1186/s13063-016-1322-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13063-016-1322-4