Abstract
Purpose
Obesity is a significant risk factor for cardiovascular disease; however, the impact of weight loss on cardiovascular health (CVH) in individuals with specific obesity patterns remains incompletely understood. The objective of our study was to investigate the relationship weight loss percentage and CVH scores across individuals with various obesity patterns.
Methods
Our study utilized data from the National Health and Nutrition Examination Survey conducted between 2007 and 2018, involving a total of 12,835 participants aged 16 years or older, to conduct a cross-sectional analysis. Multiple linear regression and multinomial logistic regression methods were used to assess the correlation between the weight loss percentage and the CVH scores. Additionally, restricted cubic spline analysis was employed to examine the nonlinear relationship between the two variables.
Results
Compared to individuals with a weight loss percentage < 0%, participants with weight loss percentages of 0–5% and 5.1–10% showed improved CVH scores, with β values of 2.85 (95% CI 2.32–3.38) and 2.55 (95% CI 1.69–3.4), respectively. Regarding different obesity patterns, compared to participants with a weight loss percentage < 0%, participants with a weight loss percentage of 0–5% showed an increase in CVH scores in the normal weight and overweight/general obesity (OGO) groups, with β values of 1.45 (95% CI 0.7–2.19) and 1.22 (95% CI 0.46–1.97), respectively. Restricted cubic spline analysis revealed a significant inverted U-shaped relationship between the weight loss percentage and the CVH scores (with optimal CVH scores at 3%).
Conclusions
There was an inverted U-shaped relationship between weight loss percentage and CVH scores, with moderate weight loss (0–10%, optimal value of 3%) being associated with improved CVH scores, especially among individuals with OGO.
Level V
Opinions of respected authorities, based on descriptive studies, narrative reviews, clinical experience, or reports of expert committees.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity and its association with cardiovascular diseases (CVDs) present a considerable challenge in the field of global public health [1,2,3,4,5,6]. In 2015, approximately 604 million adults worldwide were affected by obesity [7]. Moreover, CVD is one of the leading causes of mortality globally, resulting in approximately 18 million deaths each year [8]. Obesity has long been recognized as a crucial risk factor for CVD and is closely associated with various metabolic abnormalities, such as hypertension, dyslipidemia, hyperglycemia, inflammation, and endothelial dysfunction [9,10,11,12,13]. These factors contribute to the accelerated development and progression of atherosclerosis. Moreover, obesity impacts cardiac structure and function, leading to complications such as myocardial hypertrophy, dilated cardiac chambers, heart failure, and arrhythmias [14]. As a modifiable risk factor for CVD, weight loss is particularly important for improving cardiovascular outcomes and reducing mortality rates associated with CVD [15,16,17].
In 2010, the American Heart Association (AHA) introduced the “Life’s Simple 7” (LS7) scoring system. This scoring system encompasses seven indicators, including diet, smoking, body mass index (BMI), physical activity, total cholesterol, blood pressure, and blood glucose [18]. The LS7 scoring system has been widely utilized in cardiovascular disease research as a means to assess the cardiovascular health (CVH) status of populations. Previous studies consistently found that improving cardiovascular health (CVH) indicators can reduce the incidence of CVD complications and mortality rates [19,20,21,22,23,24,25]. In 2022, the AHA updated and improved this scoring system by including “sleep health” as a factor, thus allowing a more comprehensive assessment of an individual’s CVH [26]. Since the introduction of “Life’s Essential 8” (LE8), studies have confirmed the association of these scores with cardiovascular outcomes and all-cause mortality [27]. However, to our knowledge, research on the relationship between weight loss and overall CVH remains scarce. Currently, clinically significant weight loss generally refers to a reduction of 5% or more relative to baseline weight [28, 29]. A study has demonstrated that a weight loss of ≥ 5% from baseline can improve lipid levels, glycemic control, and blood pressure management; however, these benefits are mainly observed in individuals with cardiovascular risk factors [30]. Therefore, based on extensive data from the National Health and Nutrition Examination Survey (NHANES), we aimed to investigate the correlation between weight loss and CVH scores. Additionally, we compare the outcomes among individuals with different patterns of obesity to achieve a more precise evaluation of the impact of weight loss on cardiovascular health, thus providing robust evidence for clinical practice and informing the development of public health policies.
Materials and methods
Study population
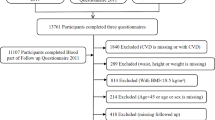
The NHANES is a crucial health and nutrition surveillance program in the United States overseen by the National Center for Health Statistics (NCHS). The NHANES aims to gather data on population characteristics, health status, and nutritional intake from a nationally representative sample, with the goal of supporting the development of public health policies and facilitating health research. The survey employs various data collection methods, including face-to-face interviews, physical measurements, laboratory tests, and dietary assessments. These data are released on a biennial basis, with a two-year cycle. In the present study, we collected a total of 59,842 participants using 6 continuous NHANES cycles from 2007 to 2018. Because the interviewees for certain variables (sleep duration, medication usage) were aged 16 and above, individuals younger than 16 (n = 21,280) were excluded from this study, as well as those with missing information pertaining to the components of the CVH scores, namely, diet, physical activity, sleep health, body mass index (BMI), blood lipids, blood glucose, and blood pressure (n = 23,191). Additionally, participants with missing waist circumference (WC) measurements (n = 169), self-reported weight from one year prior (n = 173), and self-reported attempts at weight loss (n = 2194) were omitted from the study. Ultimately, the analysis incorporated data from a total of 12,835 participants (Fig. 1). The survey procedures were authorized by the Centers for Disease Control and Prevention (CDC) and the Ethical Review Committee of the NCHS. All participants provided signed informed consent.
Measurements and variables
In the present study, the main independent variable was the percentage of weight loss. Specifically, participants reported the difference between their weight one year ago and their current weight, which was then divided by their weight one year ago. This number was then multiplied by 100 to obtain the weight loss percentage, and participants were categorized into the following groups: weight loss percentages of less than 0% (reference group), 0–5%, 5.1–10%, 10.1–15%, 15.1–20%, and greater than 20% (Supplementary Table S3). Additionally, height and WC were extracted from the database. The NHANES website provides the detailed procedures used to collect anthropometric measurements. The selection of these covariates is based on their relevance and frequent inclusion in studies regarding obesity and CVD. Covariate information, including sex, age, race and ethnicity, family educational level (based on the family reference person), family income and the poverty-to-income (PIR) ratio (categorized as ≥ 1.30 or < 1.30), alanine aminotransferase levels, aspartate aminotransferase levels, uric acid levels, self-reported weight one year ago, and history of attempted weight loss and weight loss methods, as well as health history/medical conditions (history of heart disease; history of diabetes; current pregnancy status; and current use of antihypertensive drugs, lipid-lowering drugs, and oral hypoglycemic agents), were obtained through baseline survey questionnaires and laboratory test data. Due to the different definitions of obesity in adults and children, we categorized the participants into four groups based on their BMI [31, 32]: > 18 years old, underweight (< 18.5 kg/m2), normal weight (18.5 to < 25 kg/m2), overweight (25.0 to < 30) kg/m2, and obesity (≥ 30.0 kg/m2); and 16–18 years old, underweight (below the 5th percentile for the same age and sex) and normal weight (between the 5th and 85th percentiles for the same age and sex), overweight (between the 85th and 95th percentiles for the same age and sex), and obesity (above or equal to the 95th percentile for the same age and sex). Subsequently, the study participants were classified into five different body shape patterns based on WC: underweight, normal weight, overweight/general obesity (OGO) (male WC < 102 cm, female WC < 88 cm, with an elevated BMI), abdominal obesity (male WC ≥ 102 cm, female WC ≥ 88 cm, with normal BMI), and compound obesity (presence of both general obesity and abdominal obesity) [33].
CVH scores
The CVH scores include health behaviors such as diet, physical activity, nicotine exposure, and sleep health as well as health factors such as BMI, blood lipids, blood glucose, and blood pressure. In the NHANES, trained healthcare technicians recorded participants’ anthropometric data at mobile examination centers. The data for all the variables were collected across multiple NHANES survey cycles. Dietary scores are determined based on dietary intake data, which are assessed through two 24-h dietary recalls and dietary studies utilizing nutrition databases. Physical activity scores are determined based on self-reported physical activity levels from the Global Physical Activity Questionnaire (GPAQ), while sleep scores are determined based on self-reported average sleep duration during weekdays and nonworking days. Blood lipid scores are determined based on non-HDL levels, which are derived from the difference between total cholesterol levels measured using enzymatic methods and high-density lipoprotein (HDL) cholesterol levels directly measured using immunoturbidimetric assay. Blood glucose scores are determined based on blood glucose or glycated hemoglobin levels, as well as self-reported history of diabetes and oral antidiabetic medication usage. Blood pressure scores are determined based on the level of blood pressure, which is measured multiple times by trained professionals using a mercury sphygmomanometer. Systolic and diastolic pressures are calculated based on up to four readings. If multiple measurements were available, the average of the remaining measurements was used; otherwise, the first measurement was used. The scoring algorithm for each statistical data point is provided in Supplementary Table S1 and presidential advisory [26]. In the preliminary analysis of the present study, records of nicotine exposure related to nicotine delivery systems were excluded from the total score for accuracy because these records were collected only after 2013. The scores of each of the 7 CVH indicators were averaged (unweighted), excluding the nicotine exposure score, to calculate the total score, which ranged from 0 to 100. According to the recommendations of the AHA, the classification of CVH status was as follows: scores between 0 and 49 were classified as low CVH, scores between 50 and 79 were classified as moderate CVH, and scores between 80 and 100 were classified as high CVH. The main outcome variable of this study was the summary of the scores of the 7 CVH indicators, either as a continuous variable or a categorical variable (high, moderate, or low).
Statistical analyses
Baseline features are presented as the mean and standard deviation or median (interquartile range [IQR]) of continuous variables and as the proportion of categorical variables, with CVH based on overall CVH scores (high, moderate, or low CVH). Differences between features were compared using analyses of variance (ANOVAs) or Chi-square tests, as appropriate. Univariate and multivariate linear regression analyses were employed to assess the correlation between the weight loss percentage and the CVH scores. Covariates were selected based on medical knowledge and impact on the main effect estimate (≥ 10%). The multivariable models were adjusted for sex, age, race, family PIR, educational attainment of the head of household, attempted weight loss in the past year, ALT levels, AST levels, and uric acid levels. Logistic regression models were used to calculate the odds ratios (ORs) and 95% confidence intervals (CIs) for the correlation between the weight loss percentage and CVH category. Restricted cubic splines (RCSs) were successively used to evaluate the nonlinear relationship between the weight loss percentage and the CVH scores as well as to simultaneously assess the nonlinear dose‒response relationship between WC and poor CVH risk. The discriminatory ability of WC in identifying poor CVH risk was evaluated using receiver operating characteristic (ROC) curves. Subgroup analyses were conducted using linear regression models and are presented using forest plots. In the case of categorical variables, the missing values were imputed as the most frequent value among those with data. Missing values were imputed as “college or above” for the categorical variable educational attainment of the household head, for example. The missing values for continuous variables were imputed as the median.
To examine the robustness of the associations, we conducted several sensitivity analyses. We performed analyses with a subset of participants aged ≥ 20 years (n = 11,141) after excluding pregnant individuals (n = 90) and those with a history of CVD (congestive heart failure, coronary heart disease, angina pectoris, myocardial infarction, or stroke; n = 783) (Supplementary Tables S5, S6). Additionally, we conducted a reevaluation of the participants (n = 6259) from the 2013–2018 NHANES cycles. This reevaluation involved recalculating the cardiovascular health (CVH) score by incorporating the factor of nicotine exposure and excluding missing data (n = 1818) associated with nicotine exposure (see Additional file 7, Supplementary Tables S7, S8).
The statistical software package R 3.3.2 (http://www.R-project.org, The R Foundation) was used for all analyses. A two-tailed test was used, and a result was considered statistically significant when p < 0.05.
Results
Demographic characteristics of participants
Regarding the baseline characteristics of the research population, divided into groups according to CVH (as shown in Table 1), among a total of 12,835 participants (mean age: 42.7 ± 19.0 years, 53.1% male), 5.5%, 61.7%, and 32.8% had low, moderate, and high CVH, respectively. Compared to participants with lower CVH scores, individuals with higher CVH scores were more likely to be younger, female, have higher incomes, have higher educational attainment of the head of household, be non-Hispanic White in ethnicity, have a normal body weight, have a history of attempting weight loss, have lower liver function indicators, have lower uric acid levels, have a smaller WC and have a smaller weight loss percentage. Notably, 42.3% of the participants attempted weight loss, among whom 37.2% achieved weight reduction, but only 11.6% experienced weight loss greater than 5%. Within the group of participants who had lost weight, those with a weight loss percentage of 0–5% more often exhibited healthier eating habits and engaged in more physical activity (Supplementary Table S3).
Association between weight loss percentage and CVH scores across individuals with various obesity patterns
Table 2 presents the relationship between the weight loss percentage and the CVH scores across individuals with various obesity patterns. Among all participants, 4564 were normal weight (35.6%), 226 were underweight (1.8%), 4359 had OGO (34.0%), 313 had abdominal obesity (2.4%), and 3373 had compound obesity (26.3%). After adjusting for potential confounding factors, a 1% decrease in body weight was associated with a CVH score increase of 0.18 points. Additionally, within the OGO group, there was an increase of 0.09 points in the CVH score with a 1% decrease in body weight. Compared to those with a weight loss percentage < 0%, participants with weight loss percentages of 0–5% and 5.1–10% demonstrated an improved CVH score, with β values of 2.85 (95% CI 2.32–3.38) and 2.55 (95% CI 1.69–3.4), respectively. Furthermore, within different obesity pattern groups, participants with a weight loss percentage of 0–5% exhibited an increased CVH score in the normal weight and OGO groups compared to those with a weight loss percentage < 0%, with β values of 1.45 (95% CI 0.7–2.19) and 1.22 (95% CI 0.46–1.97), respectively. However, participants with a weight loss percentage of 15.1–20% exhibited decreased CVH scores in the normal weight, abdominal obesity, and compound obesity groups, with β values of − 3.21 (95% CI − 6.44 to 0.03), − 14.15 (95% CI: − 23.72 to − 4.59), and − 6.38 (95% CI − 11.79 to − 0.97), respectively.
Multivariable adjusted RCS analysis revealed a significant inverted U-shaped association between the weight loss percentage and the CVH scores, as depicted in Fig. 2. Specifically, the regression coefficient related to the CVH scores was highest when the weight loss percentage was 3% (Table 3). Furthermore, within different obesity pattern groups, we observed similar relationships between the normal weight and OGO groups. The relationship between the weight loss percentage and CVH category is displayed in Supplementary Table S2. For every 1% decrease in weight loss percentage, the odds of moderate CVH increased by 3%, and the risk of poor CVH increased by 4%. Compared to individuals with a weight loss percentage < 0%, individuals with weight loss percentages of 0–5% and 5.1–10% had adjusted ORs for high CVH of 1.87 (95% CI 1.5–2.32) and 1.83 (95% CI 1.28–2.61), respectively.
Association between weight loss percentage and CVH scores across individuals with various obesity patterns beta-coefficients. A All participants, B normal weight, C underweight, D overweight and general obesity, E abdominal obesity, F compound obesity. Solid and dashed lines represent the predicted value and 95% confidence intervals. The models were adjusted for sex, age, race, family poverty–income ratio, educational attainment of household head, attempts to lose weight in past year, ALT, AST, and uric acid
Association between the weight loss percentage and aspects of CVH
After establishing the relationship between the weight loss percentage and overall CVH scores, we next examined specific aspects of CVH. According to our findings (Supplementary Table S4 and Fig. 3), there was a positive correlation between weight loss percentage and the scores for sleep, BMI, blood lipid levels, and blood pressure. Specifically, for every 1% decrease in weight, the corresponding scores increased by 0.09, 0.66, 0.18, and 0.08 points, respectively. Furthermore, the results indicated that participants who achieved a weight loss percentage of 0–5% demonstrated improvements in various cardiovascular scores (except for blood glucose) compared to those with a weight loss percentage < 0%. At a weight loss percentage of 5.1–10%, the scores for blood lipid levels and blood pressure showed further increases. However, when the weight loss percentage reached 10–15%, there was a decrease in scores, including those for diet, sleep health, and blood glucose. Overall, moderate weight loss appeared to lead to better CVH outcomes than severe weight loss.
Association between the weight loss percentage and aspects of CVH beta-coefficients. A Diet, B physical activity, C sleep health, D body mass index, E blood lipids, F blood glucose, G blood pressure. Solid and dashed lines represent the predicted value and 95% confidence intervals. The models were adjusted for sex, age, race, family poverty–income ratio, educational attainment of household head, attempts to lose weight in past year, ALT, AST, and uric acid
Stratified analyses by potential effect modifiers
Figure 4 presents the results of subgroup analyses, revealing a consistent correlation between the weight loss percentage and the CVH scores across various subgroups, including age, sex, race, and attempts to lose weight in the past year. Notably, this correlation was found to be more pronounced within subgroups defined by economic income, educational level of the head of household, and OGO.
Subgroup analysis of the association between weight loss percentage and CVH scores. Each stratification was adjusted for sex, age, race, family poverty–income ratio, educational attainment of household head, tried to lose weight in past year, ALT, AST, and Uric acid except the stratification factor itself. Circles indicate β, with horizontal lines indicating 95% CIs
Association between WC and the risk of poor CVH
After full adjustment for variables such as age, race, family PIR, educational attainment of the head of household, history of weight loss attempts, ALT levels, AST levels, and uric acid levels, RCS analysis revealed a nonlinear relationship between WC and the risk of poor CVH. Beyond specific thresholds (94.9 cm for males and 90.2 cm for females), the risk of poor CVH increased rapidly. ROC analysis indicated that WC exhibited good discriminatory ability regarding the risk of poor CVH, with area under the curve (AUC) values of 0.743 (95% CI 0.731–0.755) for males and 0.773 (95% CI 0.761–0.785) for females (Fig. 5).
RCS analysis between WC and the risk of poor CVH and the ROC curve. A RCS analysis for the association between male WC and the risk of poor CVH. B RCS analysis for the association between female WC and the risk of poor CVH. C ROC curve of mela WC for poor CVH risk. D ROC curve of female WC for poor CVH risk
Sensitivity analyses
To examine the robustness of the association, we conducted several sensitivity analyses. First, we focused exclusively on analyzing the CVH scores of participants aged ≥ 20 years while excluding individuals with a history of baseline cardiac disease or pregnancy. Notably, we observed a persistently significant correlation between the weight loss percentage and the CVH scores (Supplementary Tables S5, S6). Finally, we conducted a reevaluation of the Cardiovascular Health (CVH) scores for the 2013–2018 NHANES cycles. In this analysis, we incorporated nicotine exposure as an additional scoring factor and performed a subsequent analysis in conjunction with the weight loss percentage. The obtained results reaffirmed our previous conclusions, demonstrating consistency (Supplementary Tables S5, S8).
Discussion
In this cross-sectional study, we conducted a preliminary investigation into the relationship between the percentage of weight reduction and CVH scores using NHANES data. The results revealed an inverted U-shaped relationship between the weight loss percentage and the CVH scores, with moderate weight reduction (0–10%, optimal value at 3%) potentially improving CVH scores, particularly in OGO patients. However, excessive weight loss (> 15–20%) did not confer any benefits. This association was robustly supported in the sensitivity analysis. Subgroup analysis further revealed a notably significant correlation between the weight loss percentage and the CVH scores among individuals with OGO. Additionally, high WC tended to be associated with low CVH in individuals with abdominal obesity or compound obesity.
Our findings suggest that there is a positive correlation between the weight loss percentage and the CVH scores, which is consistent with previous studies [34]. Additionally, we discovered a complex relationship between the weight loss percentage and various CVH indicators. First, compared to participants with a weight loss percentage < 0%, those with a weight loss percentage > 0% demonstrated better performance in terms of blood pressure, blood lipid levels, sleep, physical activity, and diet. Multiple studies have indicated that a balanced diet and regular exercise are closely related to weight loss [35,36,37,38,39,40], with exercise reducing blood pressure and blood lipid levels as well as improving sleep duration and quality [30, 41,42,43,44,45,46,47]. Moreover, previous research has shown that weight loss can alleviate insulin resistance and aid in blood sugar control [48,49,50]. The results from a multicenter trial on diabetes prevention programs revealed that weight loss > 5% can reduce risk factors for CVD, such as abnormal blood lipid levels, hypertension, and diabetes[30]. Furthermore, the likelihood of progressing from impaired glucose tolerance to diabetes was reduced by 58% among patients who achieved a weight loss of 7% [48]. We believe that the discrepancy in research results is due to the widespread use of hypoglycemic medications among diabetic participants in our study, which reduced the impact of weight loss on blood sugar levels. Additionally, we found this positive correlation only within individuals with a weight loss percentage of 0–10%. Once the weight loss percentage exceeded 10–15%, indicators such as exercise, blood pressure, and overall CVH scores became independent of the weight loss percentage, suggesting that individuals had attained a threshold for improvement in blood pressure and CVH. On the other hand, indicators such as diet, sleep, and blood sugar scores exhibit a negative correlation with the weight loss percentage, indicating that substantial weight loss may affect diet and sleep patterns. The results from Supplementary Table S9 can explain this phenomenon, as participants with a weight loss percentage of 0–5% demonstrated better adherence to a healthy diet, exercise, and sleep, with longer sleep durations and better average systolic blood pressure levels. However, participants with a weight loss percentage of 10.1–20% seemed to adopt some less recommended weight loss strategies, such as skipping meals and using weight loss medications, while also exhibiting shorter sleep durations. Prolonged energy restriction and excessive dieting can lead to a decrease in the metabolic rate and loss of muscle mass, which can adversely affect CVH. Excessive weight loss may also result in arrhythmias, hypotension, and electrolyte imbalances, further increasing the risk of CVD. Similar results were observed among different obesity pattern groups, with participants who lost 15.1–20% of their body weight exhibiting a decline in CVH scores in the normal weight, abdominal obesity, and compound obesity groups. The results of a randomized controlled trial showed that excessive weight fluctuations are detrimental to CVH [51]. However, the results of a multicenter trial conducted among patients with type 2 diabetes and BMI > 25 kg/m2 showed that weight loss ≥ 10% within the first year was associated with a reduced risk of fatal and nonfatal cardiovascular events after 10 years (HR: 0.79, 95% CI 0.64–0.98) in all participants [52]. Therefore, further research is required to elucidate the potential benefits and drawbacks of substantial weight loss on CVH. Overall, there appeared to be an inverted U-shaped association between the weight loss percentage and the CVH scores (Fig. 2), with the strongest positive effects on CVH observed at a weight loss percentage of 3%. Therefore, moderate weight loss (0–10%, optimal value of 3%) may potentially improve cardiovascular health (CVH) scores, while excessive weight loss (> 15–20%) may not provide any benefits.
Another interesting finding was that among different obesity pattern groups, only participants with normal weight or OGO demonstrated improvements in the CVH scores with a weight loss percentage of 0–5% compared to those who did not exhibit weight loss. In contrast, individuals with other obesity patterns, such as underweight, abdominal obesity, and compound obesity, did not exhibit similar improvements. Furthermore, stratified analysis highlighted a significant association primarily in the OGO group, which may be attributed to population heterogeneity. Specifically, individuals with OGO generally have a higher BMI, and weight loss significantly improves CVH in this population. Similar benefits were observed in individuals with normal weight, such as those with metabolically obese normal weight [53]. Underweight may signal debility, including malnutrition and comorbidities other than cardiovascular complications. However, debility itself increases the risk of CVD and death [54, 55]. Studies have shown an association between underweight and an increased risk of cardiovascular events and death [56,57,58,59], so further weight loss does not provide CVH benefits in this group. Additionally, WC is one of the most commonly used anthropometric measures for diagnosing abdominal obesity. Recent research has demonstrated an association between increased WC and increased risk of coronary heart disease, independent of other potential risk factors such as BMI [60]. A large WC indicates an increased risk of cardiac metabolism and is a risk factor for myocardial infarction [44, 61]. Similar manifestations can be observed even in individuals who are not overweight overall (i.e., abdominal obesity without general overweight) [62]. Patients with abdominal obesity have increased risks of CVD, diabetes, hypertension, dyslipidemia, and nonalcoholic fatty liver disease, as well as higher overall mortality rates [63,64,65]. Furthermore, we investigated the dose‒response relationship between WC and the risk of poor CVH, and the results showed a significant increase in the risk of poor CVH with a larger WC. Thus, for individuals with abdominal obesity, controlling WC is equally important as reducing BMI, and it may even be more beneficial in terms of improving CVH. Additionally, stratified analysis revealed differences in the impact of weight loss on CVH according to income and education level, which may be attributed to the fact that individuals with higher income levels and education levels tend to adopt healthier and more reasonable weight loss strategies [66,67,68], such as consulting health experts or physicians and choosing healthy and nutritious food options.
Strength and limits
We utilized data from the NHANES in our study, as the NHANES recruited a representative sample according to age, sex, and race in the United States, thereby enhancing the reliability and generalizability of our findings. However, our study also has certain limitations. First, due to the cross-sectional design, we could not establish causal relationships; thus, further prospective studies are needed to confirm our results. Second, despite considering numerous covariates in our regression models, residual confounding effects from unmeasured or unknown factors could not be completely eliminated. Furthermore, utilizing self-reported weight data from the previous year to ascertain weight loss may introduce the possibility of misreporting. However, previous research reports have shown that weight history recall is relatively stable and exhibits minimal deviation [69], thereby contributing to a certain degree of reliability in our research findings. Last, we solely relied on a nationally representative sample from the United States, but there are significant differences among racial groups regarding dietary habits, activity levels, sleep patterns, genetic variations, etc., which limit the generalizability of our findings to populations in other countries. In conclusion, given these limitations, it is imperative to validate our findings in subsequent studies.
Conclusions
Based on our research findings, it is evident that moderate weight loss may have a greater impact on improving CVH than excessive weight loss. These results emphasize the importance of targeted weight loss interventions for optimal health outcomes. Furthermore, our study indicates that in individuals with abdominal obesity or compound obesity, focusing on reducing WC may be more meaningful than solely reducing BMI. These novel insights not only contribute to the existing body of knowledge, but also underscore the necessity of tailored strategies to address specific obesity phenotypes. Further investigation is warranted to explore the broader applicability of our findings and delve into unanswered questions, ultimately deepening our understanding of the complex relationship between weight loss, cardiovascular health, and different obesity patterns.
What is already known on this subject?
Regarding the relationship between weight loss and cardiovascular health, the existing body of research has provided limited evidence, with the majority of studies focusing on populations with preexisting conditions such as hypertension and diabetes. However, there remains a paucity of exploration specifically targeting individuals with distinctive body types.
What this study adds?
Moderate weight loss may have a more significant impact on improving cardiovascular health compared to excessive weight loss. Additionally, in individuals with abdominal obesity or compound obesity, reducing waist circumference carries greater importance in enhancing CVH. This study has expanded our understanding of the relationship between different obesity patterns and cardiovascular health while emphasizing the necessity of developing personalized strategies for specific obesity phenotypes. These findings provide a feasible basis for future intervention measures.
Data availability
The datasets generated during and analyzed during the current study are available from the corresponding author on reasonable request.
References
World Health Organization (2000) Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 894(i-xii):1–253
Laslett LJ, Alagona P Jr, Clark BA 3rd, Drozda JP Jr, Saldivar F, Wilson SR, Poe C, Hart M (2012) The worldwide environment of cardiovascular disease: prevalence, diagnosis, therapy, and policy issues: a report from the American College of Cardiology. J Am Coll Cardiol 60(25 Suppl):S1-49. https://doi.org/10.1016/j.jacc.2012.11.002
GBD 2013 Mortality and Causes of Death Collaborators (2015) Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 385(9963):117–171. https://doi.org/10.1016/s0140-6736(14)61682-2
Roth GA, Huffman MD, Moran AE, Feigin V, Mensah GA, Naghavi M, Murray CJ (2015) Global and regional patterns in cardiovascular mortality from 1990 to 2013. Circulation 132(17):1667–1678. https://doi.org/10.1161/circulationaha.114.008720
NCD Risk Factor Collaboration (NCD-RisC) (2016) Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 387(10026):1377–1396. https://doi.org/10.1016/s0140-6736(16)30054-x
Skinner AC, Ravanbakht SN, Skelton JA, Perrin EM, Armstrong SC (2018) Prevalence of obesity and severe obesity in US children, 1999–2016. Pediatrics. https://doi.org/10.1542/peds.2017-3459
Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, Marczak L, Mokdad AH, Moradi-Lakeh M, Naghavi M, Salama JS, Vos T, Abate KH, Abbafati C, Ahmed MB, Al-Aly Z, Alkerwi A, Al-Raddadi R, Amare AT, Amberbir A, Amegah AK, Amini E, Amrock SM, Anjana RM, Ärnlöv J, Asayesh H, Banerjee A, Barac A, Baye E, Bennett DA, Beyene AS, Biadgilign S, Biryukov S, Bjertness E, Boneya DJ, Campos-Nonato I, Carrero JJ, Cecilio P, Cercy K, Ciobanu LG, Cornaby L, Damtew SA, Dandona L, Dandona R, Dharmaratne SD, Duncan BB, Eshrati B, Esteghamati A, Feigin VL, Fernandes JC, Fürst T, Gebrehiwot TT, Gold A, Gona PN, Goto A, Habtewold TD, Hadush KT, Hafezi-Nejad N, Hay SI, Horino M, Islami F, Kamal R, Kasaeian A, Katikireddi SV, Kengne AP, Kesavachandran CN, Khader YS, Khang YH, Khubchandani J, Kim D, Kim YJ, Kinfu Y, Kosen S, Ku T, Defo BK, Kumar GA, Larson HJ, Leinsalu M, Liang X, Lim SS, Liu P, Lopez AD, Lozano R, Majeed A, Malekzadeh R, Malta DC, Mazidi M, McAlinden C, McGarvey ST, Mengistu DT, Mensah GA, Mensink GBM, Mezgebe HB, Mirrakhimov EM, Mueller UO, Noubiap JJ, Obermeyer CM, Ogbo FA, Owolabi MO, Patton GC, Pourmalek F, Qorbani M, Rafay A, Rai RK, Ranabhat CL, Reinig N, Safiri S, Salomon JA, Sanabria JR, Santos IS, Sartorius B, Sawhney M, Schmidhuber J, Schutte AE, Schmidt MI, Sepanlou SG, Shamsizadeh M, Sheikhbahaei S, Shin MJ, Shiri R, Shiue I, Roba HS, Silva DAS, Silverberg JI, Singh JA, Stranges S, Swaminathan S, Tabarés-Seisdedos R, Tadese F, Tedla BA, Tegegne BS, Terkawi AS, Thakur JS, Tonelli M, Topor-Madry R, Tyrovolas S, Ukwaja KN, Uthman OA, Vaezghasemi M, Vasankari T, Vlassov VV, Vollset SE, Weiderpass E, Werdecker A, Wesana J, Westerman R, Yano Y, Yonemoto N, Yonga G, Zaidi Z, Zenebe ZM, Zipkin B, Murray CJL (2017) Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med 377(1):13–27. https://doi.org/10.1056/NEJMoa1614362
World Health Organization (2017) Cardiovascular Diseases. (Fact sheet)
Bastien M, Poirier P, Lemieux I, Després JP (2014) Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog Cardiovasc Dis 56(4):369–381. https://doi.org/10.1016/j.pcad.2013.10.016
Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, Qizilbash N, Collins R, Peto R (2009) Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 373(9669):1083–1096. https://doi.org/10.1016/s0140-6736(09)60318-4
Hennekens CH, Andreotti F (2013) Leading avoidable cause of premature deaths worldwide: case for obesity. Am J Med 126(2):97–98. https://doi.org/10.1016/j.amjmed.2012.06.018
Krauss RM, Winston M, Fletcher RN, Grundy SM (1998) Obesity: impact of cardiovascular disease. Circulation 98(14):1472–1476
Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS (2002) Obesity and the risk of heart failure. N Engl J Med 347(5):305–313. https://doi.org/10.1056/NEJMoa020245
Powell-Wiley TM, Poirier P, Burke LE, Després JP, Gordon-Larsen P, Lavie CJ, Lear SA, Ndumele CE, Neeland IJ, Sanders P, St-Onge MP (2021) Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation 143(21):e984–e1010. https://doi.org/10.1161/cir.0000000000000973
Klein S, Burke LE, Bray GA, Blair S, Allison DB, Pi-Sunyer X, Hong Y, Eckel RH (2004) Clinical implications of obesity with specific focus on cardiovascular disease: a statement for professionals from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation 110(18):2952–2967. https://doi.org/10.1161/01.Cir.0000145546.97738.1e
Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, Brenneman AT, Brown-Friday JO, Goldberg R, Venditti E, Nathan DM (2009) 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 374(9702):1677–1686. https://doi.org/10.1016/s0140-6736(09)61457-4
Clifton PM, Keogh JB (2018) Effects of different weight loss approaches on CVD risk. Curr Atheroscler Rep 20(6):27. https://doi.org/10.1007/s11883-018-0728-8
Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD (2010) Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation 121(4):586–613. https://doi.org/10.1161/circulationaha.109.192703
Yang Q, Cogswell ME, Flanders WD, Hong Y, Zhang Z, Loustalot F, Gillespie C, Merritt R, Hu FB (2012) Trends in cardiovascular health metrics and associations with all-cause and CVD mortality among US adults. JAMA 307(12):1273–1283. https://doi.org/10.1001/jama.2012.339
Ford ES, Greenlund KJ, Hong Y (2012) Ideal cardiovascular health and mortality from all causes and diseases of the circulatory system among adults in the United States. Circulation 125(8):987–995. https://doi.org/10.1161/circulationaha.111.049122
Hwang SJ, Onuma O, Massaro JM, Zhang X, Fu YP, Hoffmann U, Fox CS, O’Donnell CJ (2018) Maintenance of ideal cardiovascular health and coronary artery calcium progression in low-risk men and women in the Framingham Heart Study. Circ Cardiovasc Imaging 11(1):e006209. https://doi.org/10.1161/circimaging.117.006209
Dong C, Rundek T, Wright CB, Anwar Z, Elkind MS, Sacco RL (2012) Ideal cardiovascular health predicts lower risks of myocardial infarction, stroke, and vascular death across whites, blacks, and hispanics: the northern Manhattan study. Circulation 125(24):2975–2984. https://doi.org/10.1161/circulationaha.111.081083
Akesson A, Larsson SC, Discacciati A, Wolk A (2014) Low-risk diet and lifestyle habits in the primary prevention of myocardial infarction in men: a population-based prospective cohort study. J Am Coll Cardiol 64(13):1299–1306. https://doi.org/10.1016/j.jacc.2014.06.1190
Chomistek AK, Chiuve SE, Eliassen AH, Mukamal KJ, Willett WC, Rimm EB (2015) Healthy lifestyle in the primordial prevention of cardiovascular disease among young women. J Am Coll Cardiol 65(1):43–51. https://doi.org/10.1016/j.jacc.2014.10.024
Li Y, Pan A, Wang DD, Liu X, Dhana K, Franco OH, Kaptoge S, Di Angelantonio E, Stampfer M, Willett WC, Hu FB (2018) Impact of healthy lifestyle factors on life expectancies in the US population. Circulation 138(4):345–355. https://doi.org/10.1161/circulationaha.117.032047
Lloyd-Jones DM, Allen NB, Anderson CAM, Black T, Brewer LC, Foraker RE, Grandner MA, Lavretsky H, Perak AM, Sharma G, Rosamond W (2022) Life.s essential 8: updating and enhancing the American Heart Association’s Construct of Cardiovascular Health: a presidential advisory from the American Heart Association. Circulation 146(5):e18–e43. https://doi.org/10.1161/cir.0000000000001078
Zhang J, Chen G, Habudele Z, Wang X, Cai M, Li H, Gao Y, Lip GYH, Lin H (2023) Relation of life’s essential 8 to the genetic predisposition for cardiovascular outcomes and all-cause mortality: results from a national prospective cohort. Eur J Prev Cardiol. https://doi.org/10.1093/eurjpc/zwad179
Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF, Loria CM, Millen BE, Nonas CA, Pi-Sunyer FX, Stevens J, Stevens VJ, Wadden TA, Wolfe BM, Yanovski SZ (2014) 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol 63(25 Pt B):2985–3023. https://doi.org/10.1016/j.jacc.2013.11.004
Raynor HA, Champagne CM (2016) Position of the academy of nutrition and dietetics: interventions for the treatment of overweight and obesity in adults. J Acad Nutr Diet 116(1):129–147. https://doi.org/10.1016/j.jand.2015.10.031
Douketis JD, Macie C, Thabane L, Williamson DF (2005) Systematic review of long-term weight loss studies in obese adults: clinical significance and applicability to clinical practice. Int J Obes 29(10):1153–1167. https://doi.org/10.1038/sj.ijo.0802982
Expert panel on the identification, evaluation, and treatment of overweight in adults (1998) Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: executive summary. Am J Clin Nutr 68(4):899–917. https://doi.org/10.1093/ajcn/68.4.899
Hampl SE, Hassink SG, Skinner AC, Armstrong SC, Barlow SE, Bolling CF, Avila Edwards KC, Eneli I, Hamre R, Joseph MM, Lunsford D, Mendonca E, Michalsky MP, Mirza N, Ochoa ER, Sharifi M, Staiano AE, Weedn AE, Flinn SK, Lindros J, Okechukwu K (2023) Clinical practice guideline for the evaluation and treatment of children and adolescents with obesity. Pediatrics. https://doi.org/10.1542/peds.2022-060640
Nishida C, Ko GT, Kumanyika S (2010) Body fat distribution and noncommunicable diseases in populations: overview of the 2008 WHO Expert Consultation on Waist Circumference and Waist-Hip Ratio. Eur J Clin Nutr 64(1):2–5. https://doi.org/10.1038/ejcn.2009.139
Pack QR, Rodriguez-Escudero JP, Thomas RJ, Ades PA, West CP, Somers VK, Lopez-Jimenez F (2014) The prognostic importance of weight loss in coronary artery disease: a systematic review and meta-analysis. Mayo Clin Proc 89(10):1368–1377. https://doi.org/10.1016/j.mayocp.2014.04.033
Hankinson AL, Daviglus ML, Bouchard C, Carnethon M, Lewis CE, Schreiner PJ, Liu K, Sidney S (2010) Maintaining a high physical activity level over 20 years and weight gain. JAMA 304(23):2603–2610. https://doi.org/10.1001/jama.2010.1843
Lee IM, Djoussé L, Sesso HD, Wang L, Buring JE (2010) Physical activity and weight gain prevention. JAMA 303(12):1173–1179. https://doi.org/10.1001/jama.2010.312
Wing RR, Phelan S (2005) Long-term weight loss maintenance. Am J Clin Nutr 82(1 Suppl):222s–225s. https://doi.org/10.1093/ajcn/82.1.222S
Esposito K, Marfella R, Ciotola M, Di Palo C, Giugliano F, Giugliano G, D’Armiento M, D’Andrea F, Giugliano D (2004) Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA 292(12):1440–1446. https://doi.org/10.1001/jama.292.12.1440
Hooper L, Abdelhamid AS, Jimoh OF, Bunn D, Skeaff CM (2020) Effects of total fat intake on body fatness in adults. Cochrane Database Syst Rev 6(6):Cd013636. https://doi.org/10.1002/14651858.Cd013636
Hall KD, Guo J (2017) Obesity energetics: body weight regulation and the effects of diet composition. Gastroenterology 152(7):1718-1727.e1713. https://doi.org/10.1053/j.gastro.2017.01.052
Stevens VJ, Corrigan SA, Obarzanek E, Bernauer E, Cook NR, Hebert P, Mattfeldt-Beman M, Oberman A, Sugars C, Dalcin AT et al (1993) Weight loss intervention in phase 1 of the trials of hypertension prevention: the TOHP Collaborative Research Group. Arch Int Med 153(7):849–858
Horvath K, Jeitler K, Siering U, Stich AK, Skipka G, Gratzer TW, Siebenhofer A (2008) Long-term effects of weight-reducing interventions in hypertensive patients: systematic review and meta-analysis. Arch Intern Med 168(6):571–580. https://doi.org/10.1001/archinte.168.6.571
Rothberg AE, McEwen LN, Kraftson AT, Ajluni N, Fowler CE, Nay CK, Miller NM, Burant CF, Herman WH (2017) Impact of weight loss on waist circumference and the components of the metabolic syndrome. BMJ Open Diabetes Res Care 5(1):e000341. https://doi.org/10.1136/bmjdrc-2016-000341
Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF, Loria CM, Millen BE, Nonas CA, Pi-Sunyer FX, Stevens J, Stevens VJ, Wadden TA, Wolfe BM, Yanovski SZ, Jordan HS, Kendall KA, Lux LJ, Mentor-Marcel R, Morgan LC, Trisolini MG, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF (2014) 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation 129(25 Suppl 2):S102-138. https://doi.org/10.1161/01.cir.0000437739.71477.ee
Semlitsch T, Krenn C, Jeitler K, Berghold A, Horvath K, Siebenhofer A (2021) Long-term effects of weight-reducing diets in people with hypertension. Cochrane Database Syst Rev 2(2):Cd008274. https://doi.org/10.1002/14651858.CD008274.pub4
Stevens VJ, Obarzanek E, Cook NR, Lee IM, Appel LJ, Smith West D, Milas NC, Mattfeldt-Beman M, Belden L, Bragg C, Millstone M, Raczynski J, Brewer A, Singh B, Cohen J (2001) Long-term weight loss and changes in blood pressure: results of the trials of hypertension prevention, phase II. Ann Intern Med 134(1):1–11. https://doi.org/10.7326/0003-4819-134-1-200101020-00007
Janus C, Jensen S, Lundgren JR, Juhl CR, Olsen LM, Stallknecht B, Holst JJ, Madsbad S, Torekov SS (1974) Diet-induced weight loss improves sleep quality and these improvements are sustained with one year of weight maintenance with exercise: the S-LITE randomized trial. Diabetes. https://doi.org/10.2337/db20-1974-p
Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM (2002) Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346(6):393–403. https://doi.org/10.1056/NEJMoa012512
Hamman RF, Wing RR, Edelstein SL, Lachin JM, Bray GA, Delahanty L, Hoskin M, Kriska AM, Mayer-Davis EJ, Pi-Sunyer X, Regensteiner J, Venditti B, Wylie-Rosett J (2006) Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care 29(9):2102–2107. https://doi.org/10.2337/dc06-0560
Pi-Sunyer X, Blackburn G, Brancati FL, Bray GA, Bright R, Clark JM, Curtis JM, Espeland MA, Foreyt JP, Graves K, Haffner SM, Harrison B, Hill JO, Horton ES, Jakicic J, Jeffery RW, Johnson KC, Kahn S, Kelley DE, Kitabchi AE, Knowler WC, Lewis CE, Maschak-Carey BJ, Montgomery B, Nathan DM, Patricio J, Peters A, Redmon JB, Reeves RS, Ryan DH, Safford M, Van Dorsten B, Wadden TA, Wagenknecht L, Wesche-Thobaben J, Wing RR, Yanovski SZ (2007) Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care 30(6):1374–1383. https://doi.org/10.2337/dc07-0048
Bangalore S, Fayyad R, Laskey R, DeMicco DA, Messerli FH, Waters DD (2017) Body-weight fluctuations and outcomes in coronary disease. N Engl J Med 376(14):1332–1340. https://doi.org/10.1056/NEJMoa1606148
Gregg EW, Jakicic JM, Blackburn G, Bloomquist P, Bray GA, Clark JM, Coday M, Curtis JM, Egan C, Evans M, Foreyt J, Foster G, Hazuda HP, Hill JO, Horton ES, Hubbard VS, Jeffery RW, Johnson KC, Kitabchi AE, Knowler WC, Kriska A, Lang W, Lewis CE, Montez MG, Nathan DM, Neiberg RH, Patricio J, Peters A, Pi-Sunyer X, Pownall H, Redmon B, Regensteiner J, Rejeski J, Ribisl PM, Safford M, Stewart K, Trence D, Wadden TA, Wing RR, Yanovski SZ (2016) Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: a post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol 4(11):913–921. https://doi.org/10.1016/s2213-8587(16)30162-0
Ruderman NB, Schneider SH, Berchtold P (1981) The “metabolically-obese,” normal-weight individual. Am J Clin Nutr 34(8):1617–1621. https://doi.org/10.1093/ajcn/34.8.1617
Adabag S, Vo TN, Langsetmo L, Schousboe JT, Cawthon PM, Stone KL, Shikany JM, Taylor BC, Ensrud KE (2018) Frailty as a risk factor for cardiovascular versus noncardiovascular mortality in older men: results from the MrOS sleep (outcomes of sleep disorders in older men) study. J Am Heart Assoc. https://doi.org/10.1161/jaha.118.008974
Veronese N, Sigeirsdottir K, Eiriksdottir G, Marques EA, Chalhoub D, Phillips CL, Launer LJ, Maggi S, Gudnason V, Harris TB (2017) Frailty and risk of cardiovascular diseases in older persons: the age gene/environment susceptibility-Reykjavik study. Rejuvenation Res 20(6):517–524. https://doi.org/10.1089/rej.2016.1905
Flegal KM, Graubard BI, Williamson DF, Gail MH (2005) Excess deaths associated with underweight, overweight, and obesity. JAMA 293(15):1861–1867. https://doi.org/10.1001/jama.293.15.1861
Global BMIMC, Di Angelantonio E, Bhupathiraju ShN, Wormser D, Gao P, Kaptoge S, Berrington de Gonzalez A, Cairns BJ, Huxley R, Jackson ChL, Joshy G, Lewington S, Manson JE, Murphy N, Patel AV, Samet JM, Woodward M, Zheng W, Zhou M, Bansal N, Barricarte A, Carter B, Cerhan JR, Smith GD, Fang X, Franco OH, Green J, Halsey J, Hildebrand JS, Jung KJ, Korda RJ, McLerran DF, Moore SC, O’Keeffe LM, Paige E, Ramond A, Reeves GK, Rolland B, Sacerdote C, Sattar N, Sofianopoulou E, Stevens J, Thun M, Ueshima H, Yang L, Yun YD, Willeit P, Banks E, Beral V, Chen Z, Gapstur SM, Gunter MJ, Hartge P, Jee SH, Lam TH, Peto R, Potter JD, Willett WC, Thompson SG, Danesh J, Hu FB (2016) Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 388(10046):776–786. https://doi.org/10.1016/s0140-6736(16)30175-1
Zheng W, McLerran DF, Rolland B, Zhang X, Inoue M, Matsuo K, He J, Gupta PC, Ramadas K, Tsugane S, Irie F, Tamakoshi A, Gao YT, Wang R, Shu XO, Tsuji I, Kuriyama S, Tanaka H, Satoh H, Chen CJ, Yuan JM, Yoo KY, Ahsan H, Pan WH, Gu D, Pednekar MS, Sauvaget C, Sasazuki S, Sairenchi T, Yang G, Xiang YB, Nagai M, Suzuki T, Nishino Y, You SL, Koh WP, Park SK, Chen Y, Shen CY, Thornquist M, Feng Z, Kang D, Boffetta P, Potter JD (2011) Association between body-mass index and risk of death in more than 1 million Asians. N Engl J Med 364(8):719–729. https://doi.org/10.1056/NEJMoa1010679
Kwon H, Yun JM, Park JH, Cho BL, Han K, Joh HK, Son KY, Cho SH (2021) Incidence of cardiovascular disease and mortality in underweight individuals. J Cachexia Sarcopenia Muscle 12(2):331–338. https://doi.org/10.1002/jcsm.12682
Gaglioti AH, Rivers D, Ringel JB, Judd S, Safford MM (2022) Individual and neighborhood influences on the relationship between waist circumference and coronary heart disease in the REasons for geographic and racial differences in stroke study. Prev Chronic Dis 19:E20. https://doi.org/10.5888/pcd19.210195
Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L (2004) Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 364(9438):937–952. https://doi.org/10.1016/s0140-6736(04)17018-9
St-Onge MP, Janssen I, Heymsfield SB (2004) Metabolic syndrome in normal-weight Americans: new definition of the metabolically obese, normal-weight individual. Diabetes Care 27(9):2222–2228. https://doi.org/10.2337/diacare.27.9.2222
Janssen I, Katzmarzyk PT, Ross R (2004) Waist circumference and not body mass index explains obesity-related health risk. Am J Clin Nutr 79(3):379–384. https://doi.org/10.1093/ajcn/79.3.379
Ala M (2023) Eftekhar SP Weight loss breaks the bond between nonalcoholic fatty liver disease and cardiovascular diseases: a clinical and epidemiological perspective. Obes Rev. https://doi.org/10.1111/obr.13563
Gnatiuc L, Alegre-Díaz J, Wade R, Ramirez-Reyes R, Tapia-Conyer R, Garcilazo-Ávila A, Chiquete E, Gonzáles-Carballo C, Solano-Sanchez M, Clarke R, Collins R, Herrington WG, Hill M, Lewington S, Peto R, Emberson JR, Kuri-Morales P (2019) General and abdominal adiposity and mortality in Mexico City: a prospective study of 150 000 adults. Ann Intern Med 171(6):397–405. https://doi.org/10.7326/m18-3502
Lorts C, Ohri-Vachaspati P (2016) Disparities in who receives weight-loss advice from a health care provider: does income make a difference? Prev Chronic Dis 13:E142. https://doi.org/10.5888/pcd13.160183
Kakinami L, Gauvin L, Barnett TA, Paradis G (2014) Trying to lose weight: the association of income and age to weight-loss strategies in the U.S. Am J Prev Med 46(6):585–592. https://doi.org/10.1016/j.amepre.2014.01.022
Kant AK, Graubard BI (2013) Family income and education were related with 30-year time trends in dietary and meal behaviors of American children and adolescents. J Nutr 143(5):690–700. https://doi.org/10.3945/jn.112.165258
Chang VW, Christakis NA (2003) Self-perception of weight appropriateness in the United States. Am J Prev Med 24(4):332–339. https://doi.org/10.1016/s0749-3797(03)00020-5
Acknowledgements
Grateful acknowledgment is given to the anonymous reviewers for their indispensable assistance.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
FC designed the study; FC wrote the manuscript. FC and YZ collected, analyzed and interpreted the data. FC, SC and YZ critically reviewed, edited and approved the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The National Center for Health Statistics (NCHS) Ethics Review Board and the Centers for Disease Control and Prevention (CDC) both gave their approval to the survey protocol (Protocol #2005-06, Continuation of Protocol #2005-06, Protocol #2011-17, Continuation of Protocol #2011-17), and this study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All participants gave written informed consent.
Consent for publication
Informed consent for publication was obtained from all individual participants included in the study.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, F., Zhang, Y. & Chen, S. The inverted U-shaped relationship between weight loss percentage and cardiovascular health scores. Eat Weight Disord 28, 87 (2023). https://doi.org/10.1007/s40519-023-01619-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40519-023-01619-3