Abstract
Background
We investigated the impact of weight change on mortality in a population-based cohort setting.
Methods
We conducted two weight measurements for 5436 participants aged ≥ 30 years with an approximate 3-year interval. Based on their weight change, we categorized participants to: > 5% weight loss, 3–5% weight loss, stable weight (± < 3%), 3–5% weight gain, > 5% weight gain. We followed participants for mortality annually up to March 20th 2018. We applied the multivariable Cox proportional hazard models to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) of weight change categories for all-cause, cardiovascular (CV), and cancer mortality, considering stable weight as reference. The Cox models was adjusted for age, sex, educational level, body mass index, smoking status, hypertension, hypercholesterolemia, diabetes, and cardiovascular disease (CVD) at baseline.
Results
During a median follow-up of 14.4 years, 629 deaths (247 CV and 126 cancer deaths) have occurred. Over 5% weight loss and gain were associated with increased risk of all-cause mortality in multivariable analysis with HRs of 1.47 [95% CI: 1.17–1.85] and 1.27 [1.02–1.57], respectively; however, a 3–5% loss or gain did not alter the risk of all-cause mortality significantly. These significant risks for wight change > 5% were not modified by the presence of diabetes, obesity, and smoking status; however, the unfavorable impact of weight change on mortality events was more prominent in those older than > 65 years (P-value for interaction: 0.042). After excluding those with history of CVD, diabetes, and cancer during the weight measurements period, these associations significantly attenuated (HR: 1.29 [0.89–1.87] for > 5% weight loss and 1.12 [0.84–1.50] for > 5% weight gain). Additionally, a > 5% weight loss was also associated with about 60% higher risk for CV mortality (HR: 1.62 [1.15–2.28]), and a 3–5% weight loss was associated with about 95% higher risk of cancer mortality (HR: 1.95 [1.13–3.38]).
Conclusions
Our findings showed a U-shaped association across weight change categories for all-cause mortality risk with over 5% weight gain and loss causing higher risk. Moreover, weight loss can have adverse impact on CV and cancer mortality events.
Similar content being viewed by others
Introduction
Obesity is a major public health concern. In 2016, the prevalence of obesity was more than 20% among men and more than 30% among women in most of the countries of the Middle East and North Africa (MENA) region; however, the worldwide prevalence of obesity was 11.6% for men and 15.7% for women [1]. Almost all countries of the MENA region are in nutritional transition from a traditional to a modern diet that is heavy in processed foods and fast. Therefore, their burden of disease has already shifted from communicable to non-communicable diseases (NCD). In 2013, the mean energy intake in most countries of MENA region was reported higher than the global average [1]. Moreover, a progressive increase of the fat contribution in the diet was found in most countries of this region [2]. Furthermore, air pollution is of crucial significance in the MENA, since it has some of the highest levels of ambient air pollution worldwide. A potential role of ambient air pollution in the development of obesity has also been previously proposed [3].
According to the data from the STEPwise approach to surveillance (STEPS) survey, the prevalence of overweight/obesity among Iranian adults aged 20–65 years increased from 57.8% in 2007 to 62.8% in 2016 [4]. Moreover, according to STEPs 2016, the prevalence of overweight/obesity among Iranian adults aged 65–69 years and ≥70 years were 69.7 and 55.5%, respectively [5].
As a major risk factor, high body mass index (BMI) attributed to 18.8% of deaths and 12.9% of disability-adjusted life years (DALYs) of NCDs in 2019 in Iran [6]. A J- or U-shaped relation between BMI and mortality was already established that both underweight and obesity categories were at higher mortality risk [7, 8]. Only a single measurement of BMI/weight was included in several previous cohort studies [7,8,9], which ignores the dynamic aspect of body weight over time. Therefore, the evaluation of long/short term consequences of weight change during certain life periods is also of high importance.
A meta-analysis of 25 cohort studies reported that among individuals aged 40–65 years, weight loss and weight gain were associated with almost 45% and 7% increased all-cause mortality risk, respectively; the corresponding values were 50% and 21% for cardiovascular (CV) mortality risk, respectively [10]. Similarly, a recent meta-analysis of 30 prospective studies reported that compared with stable weight, both weight loss and weight gain were associated with 59% and 10% increased risk of all-cause mortality, respectively, among older adults[11]. It should be noted that in both of these meta-analyses, significant heterogeneities were reported among included studies (I2 ranged from 41%-89%). Ethnic/Racial differences have also been evidenced in body composition [12], obesity status [13], as well as weight management behavior [14]. Consequently, the association between weight change and longevity could also vary across ethnic/racial groups [15]. To the best of our knowledge, no study has evaluated the impact of weight change on all-cause, CV, and cancer mortality risk in the MENA region. We aimed to investigate the impact of 3-year weight change on mortality rates using a large-scale, population-based cohort of Iranian adults with more than a decade of follow-up.
Materials and methods
Study design and study population
The Tehran Lipid and Glucose Study (TLGS) is a prospective cohort study conducted on a representative sample of residents of Tehran, the capital of the Islamic Republic of Iran.
The TLGS was designed to investigate the prevalence and incidence of NCDs and their risk factors among Iranian population [16]. Tehran was comprised of 20 urban districts at the start of the TLGS. The district no. 13 was chosen for sample selection. The rationales for selecting district 13 were: (1) high stability of the population residing in district 13 compared to other districts of Tehran, and (2) the age distribution of the population of district 13 was similar to the age distribution of the overall Tehran population [16]. Details, measurement methods, and enrollment strategy of the TLGS have been described elsewhere [17]. Briefly, in the first phase (1999–2002), 15,005 individuals aged ≥ 3 years were enrolled in the study using a multistage stratified cluster random sampling technique, and re-examinations were conducted at approximately 3-year intervals. Another 3550 individuals were added in the second phase (2002–2005) and were followed in a triennial manner.
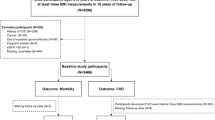
For this study, we selected 9558 participants aged ≥ 30 years from phase 1 and 2, as the baseline population, and identified their weight change in the next phase with an interval of about 3 years. For those individuals who were enrolled at phase 1, weight change was identified in phase 2, and for participants who were enrolled at phase 2, weight change was measured in phase 3 (2005–2008). From the 9558 eligible participants, 4084 participants were excluded due to missing data on weight measurement (at baseline or next follow-up visit) or covariates at baseline. Moreover, we excluded 38 participants with no follow-up data. Finally, 5436 participants remained, who were followed up for all-cause death. Participants were censored at the date of loss to follow-up or study end (20 march 2018) (Fig. 1).
We obtained written informed consent from all participants. This study was approved by the ethical committee of the Research Institute for Endocrine Sciences of Shahid Beheshti University of Medical sciences.
Clinical and laboratory measurements
At each visit, we used interviewer-administered questionnaires to obtain demographic information, medication usage, past medical history, educational level, and smoking habits. We measured weight by a digital scale to the nearest 100 g and height in a standing position while participants had light clothing and no shoes on. Furthermore, we calculated BMI as weight in kilograms divided by the square of height in meters. Subsequent to 15 min of rest, two physician-measured blood pressures were performed on the right arm using a standard sphygmomanometer. We assessed systolic blood pressure (SBP) and diastolic blood pressure (DBP) as the mean of these two blood pressure measurements. We took morning blood samples from all participants after at least 12 h of fasting. We also performed measurements of fasting plasma glucose (FPG) and total cholesterol (TC) by standard methods, as described in detail before [16].
Definition of terms
We defined diabetes mellitus as one of these criteria: a) FPG ≥ 7 mmol/L and b) taking any glucose-lowering drugs. Furthermore, we defined hypertension as these three criteria: SBP ≥ 140 mmHg, or DBP ≥ 90 mmHg, or using antihypertensive drugs as hypertension. Also, we defined having TC ≥ 5.18 mmol/L or using lipid-lowering drugs as hypercholesterolemia [17].
Based on smoking habits, we divided our participants into two groups: a) current smokers, b) past/never smokers. We categorized educational levels into 3 groups: 1) more than 12 years, 2) between 6–12 years, and 3) less than 6 years of academic education.
We calculated weight change as: \(\frac{\mathrm{Follow}-\mathrm{up}\;\mathrm{measurement}-\mathrm{Baseline}\;\mathrm{measurement}}{\mathrm{Baseline}\;\mathrm{measurement}}\times100\). Based on 3-year weight change percentage, as recommended by Stevens et al. [18], we categorized participants into five groups: a) more than 5% weight loss; b) 3% to 5% weight loss; c) less than 3% weight change [reference group]; d) 3% to 5% weight gain; e) more than 5% weight gain.
Outcome assessment
Details of the TLGS outcome collection have been explained previously [19]. To summarize, through an annual phone call, a trained nurse interviewed participants for any new medical events. In cases of mortality, a verbal autopsy was performed using a standard questionnaire. The questionnaire consists of time and location (in home or hospital) of death plus medical events or complications leading to death. We collected medical data for each deceased person by referring to medical record departments of service providers (outpatient or hospital). The collected data was assessed by a panel of specialists included an internist, a cardiologist, an endocrinologist, a pathologist, and an epidemiologist. The outcome committee adjudicated an underlying cause of death for each deceased participant.
Statistical analyses
Baseline characteristics of the respondents (study participants) and non-respondents (those with missing data of main exposure/covariates or those without follow-up data) were compared. The Student’s t-test and the Chi-square test for continuous and categorical variables were used, respectively. We also illustrated baseline characteristics across weight change categories as number (%) for categorical variables and mean ± standard deviation (SD) for continuous variables.
Based on literature review[10, 11, 20], confounding factors were selected. Then, to assess the relation of weight change categories with incident all-cause, CV, and cancer mortality, we applied the multivariable Cox proportional regression analysis, and the hazard ratios (HRs) with 95% confidence intervals (CIs) were reported in two models: Model 1: adjusted for age and sex; Model 2: Model 1 + further adjusted for educational level, BMI, smoking status, hypertension, hypercholesterolemia, diabetes, and cardiovascular disease (CVD) at baseline. Multicollinearity of independent variables was checked via the variance inflation factor (VIF) statistic; given the VIF of < 4, we did not find evidence of collinearity in the model.
As a sensitivity analysis, to eliminate the effects of unintentional weight loss, participants with CVD, diabetes, and cancer at baseline or first follow-up were excluded and the association of weight change categories with all-cause mortality was reassessed.
We also checked the interactions of weight change categories with age groups (≥ 65 years versus < 65 years), sex (men versus women), BMI groups (≥ 30 kg/m2 versus < 30 kg/m2), diabetes (yes versus no), and smoking status (past/never versus current) via the log–likelihood ratio test in the multivariable model, in separate models.
Time to event is described as the time of censoring or the death occurring, whichever came first. We censored individuals in the case of leaving the district, lost to follow-up, or being alive in the study until March 20th 2018.
To assess proportionality in the Cox models, we used the Schoenfeld residual test; our proportionality assumptions were all appropriate. We employed STATA version 14 (StataCorp LP, College Station, Texas) for statistical analyses. P-values of < 0.05 were considered statistically significant.
Results
Our study population consisted of 5436 participants (2395 men) with a mean age of 47.9 (SD: 12.1) years at baseline.
As shown in Additional file 1: Table S1, compared to non-respondents, respondents were older, less educated, had higher BMI and total cholesterol, but had lower prevalence of CVD and current smoking. Moreover, no difference was found for mortality events between groups.
Baseline and the first follow-up characteristics of the individuals across weight change categories are presented in Table 1. During the first three years of the follow-up, almost 42% of the subjects had a stable weight (-3% to + 3%). Furthermore, 27% and 9% of the participants had a weight gain or weight loss of more than 5%, respectively. Generally, in the total population, after 3 years of follow-up, BMI and FPG increased among continuous variables. Moreover, the prevalence of CVD and usage of glucose lowering, antihypertensive, and lipid-lowering drugs were increased; while SBP, DBP, total cholesterol, and current smoking were decreased.
During a median follow-up of 14.4 years of [interquartile range: 12.7–15.5], 629 deaths (373 among men) have been recorded. The distribution of different causes of death is shown in Fig. 2. Underlying causes of mortality in the total population were CV (n = 247), cancer (n = 126), infectious diseases (n = 96), accidents (n = 20), diabetes complications (n = 22), and others (n = 11). Moreover, 107 cases of death had not a classified cause.
The multivariable HRs and 95% CIs of the association between weight change categories and all-cause mortality risk are shown in Table 2. Compared to subjects with stable weight, those who lost and gained more than 5% of weight had age- and sex-adjusted HRs of 1.61 [95% CI: 1.29–2.02] and 1.22 [0.99–1.50; P-value: 0.066] for the risk of all-cause mortality, respectively; the corresponding risks in model 2 were 1.47 [1.17 -1.85] and 1.27 [1.02–1.57], respectively. Importantly, male sex, older age, having less than 6 years of education, current smoking, history of CVD, diabetes, and hypertension were significantly associated with increased risk of all-cause mortality in model 2 (data not shown). After exclusion of those with history of CVD, diabetes, and cancer at baseline or first follow-up, 4294 participants remained, with a total number of 321 cases of death during follow-up. Generally, no significant association was remained; however, a suggestive (but not significant) 30% higher risk was found for the weight loss of over 5%. (Additional file 2: Table S2).
Fig. 3 shows the associations of weight change categories with CV and cancer mortality events. As shown in Fig. 3-A, for CV mortality, a > 5% weight loss was significantly associated with increased risk (HR: 1.62 [1.15–2.28]). Moreover, after excluding those with prevalent CVD at baseline (313 participants), the results did not change (Additional file 3: Fig. S1). In our data analysis, a 3–5% weight loss was also associated with an increased risk for cancer mortality events by a HR of 1.95 [1.13–3.38] (Fig. 3-B).
Multivariable hazard ratios (HR) and 95% confidence intervals (CI) for the association of weight change categories with cardiovascular mortality (A) and cancer mortality (B). Model 1: adjusted for age and sex; Model 2: further adjusted for body mass index, educational level, Smoking status, hypertension, hypercholesterolemia, diabetes mellitus, and history of CVD at baseline
Multivariable HRs and 95% CIs of the subgroup analysis are presented in Fig. 4. Considering age stratification, the interaction between age groups (≤ 65 years versus > 65 years) and weight change categories was significant with a P-value of 0.042. Weight loss of > 5% increased the risk of all-cause mortality in both age groups with a greater effect size for those aged > 65 years (HR: 2.01 versus 1.38); however, weight gain had a significant impact only among the older population (HR: 1.44 [1.03–2.00]). The interaction of weight change categories with sex had also a P-value of 0.088; weight gain caused more prominent adverse effects among men; however, weight loss of over 5% increased the risk of mortality in both sexes. Moreover, although the interactions of weight change categories with BMI categories, diabetes, and smoking status were not significant, in line with the total population, generally, gaining and losing weight of more than 5% was found to be significantly associated with higher risk of all-cause mortality among non-obese (BMI < 30 kg/m2), non-diabetes participants, as well as never/past smokers.
Multivariable hazard ratios and 95% confidence intervals, stratified by age (A), sex (B), BMI (C), Diabetes (D), and smoking status (E). E/N: Number of event/ Number of participants; BMI: body mass index; Multivariable hazard ratios were adjusted for age, sex, BMI, educational level, SMK, hypertension, hypercholesterolemia, and history of CVD at baseline; considering that age in A, sex in B, BMI in C, Diabetes in D, and smoking status in E were excluded from the models
Discussion
In this study, with more than a decade of follow-up, after adjustment for a large set of covariates, compared to the stable weight, participants with a > 5% weight loss or weight gain had significantly higher risk of all-cause mortality. These significant risks were not modified by the presence of diabetes, obesity, and smoking status; however, the unfavorable impact of weight change on mortality events was more prominent in the older population. Moreover, compared to women, men were more sensitive to the impact of weight gain on mortality events. Additionally, a >5% weight loss was also associated with about 60% higher risk for CV mortality, and a 3-5% weight loss was associated with about 95% higher risk of cancer mortality.
Comparing the findings of this study with other studies is not simple due to the differences in the mean age and other baseline characteristics of the participants, the sample size, considerable variations in the definitions of weight change categories, and level of adjustments for confounders. In the current study, we found a U-shaped association between weight change and all-cause mortality events. A large-scale Korean cohort reported a reverse J-shaped association between 4-year weight change and all-cause mortality risk, regardless of BMI categories [21]. A similar association was also recently reported in a multi-ethnic cohort in the United States among native Hawaiians, Japanese Americans, African Americans, whites, and Latinos [22]. A large population-based cohort study on middle-aged and elderly Chinese demonstrated a U-shaped association between weight change and all-cause/CV mortality risk, with both moderate-to-large weight gain and loss conferring excess risk compared to the nadir risk for stable weight [23]. Among the UK population in the European Prospective Investigation into Cancer in Norfolk cohort, it was shown that compared to the stable weight, weight loss was associated with higher mortality; however, findings for weight gain were inconclusive [24].
The significantly higher risk of weight loss for all-cause mortality was also addressed in two important meta-analyses. Firstly, in a meta-analysis of 25 prospective studies, it is reported that weight loss was related to 45% increased risk of all-cause mortality in middle and older age [10]. Another one showed that weight loss increased all-cause mortality risk by 59% in older adults ≥ 65 years [11]. Likely, in our data analysis, the impact of > 5% weight loss was more pronounced among older participants than the younger age group (100% versus 38% increased risk for mortality, respectively). Weight loss can be related to loss in fat and also muscle or lean body mass, particularly relevant among an aging population (sarcopenia). Since the recovery of muscle mass loss is difficult, weight loss in older adults is regarded problematic [25,26,27]. While on the contrary, individuals who maintain body weight in later life could be more likely to maintain muscle and bone mass compared to those losing weight [28, 29]. Undiagnosed pre-existing diseases could also be a plausible explanation for the observed increase in mortality risk among those who lost weight, especially for unintentional weight loss; however, in the current study, only 46 (7.3% of total mortality) deaths have occurred during the first two years of follow up; hence, this issue might not play a significant role in our population.
Additionally, in our study, individuals with a weight gain of > 5% were also at higher risk of mortality; the association was more prominent in older adults. This is in line with findings from the two previous meta-analyses conducted among adults aged 40–65 years [10] and specifically among older adults aged 65 years or above [11]. Since excess adiposity is proved to increase the mortality risk [7, 30], weight gain is assumed to heighten mortality risk. Weight gain is also known to increase the risk of CVD, which may also heighten mortality risk [31]. Importantly, we found that gaining weight was associated with more unfavorable impact among men, and its association was demonstrated even as little as more than 3% weight gain. It was suggested that weight gain was more attributable to the accumulation of visceral adipose tissue among men that significantly associated with poor outcomes [32].
Regarding cause specific mortality, in this study, a weight loss of > 5% showed a significant increased risk of CV mortality in the multivariable model; however, such association was not observed for weight gain. The meta-analysis of 25 studies [10], as well as two recent Chinese studies [33, 34], reported an association of both weight loss and weight gain with increased risk of CV mortality. Additionally, a 3 to 5% weight loss was associated with an increased risk of cancer mortality. This can be described by the fact that cancer-associated weight loss is associated with poor prognosis in advanced malignancy [35]. The study by Li et al. did not report significant risk of cancer related mortality among BMI change groups in overall population; however, a 5% decrease in BMI was associated with 14% increase in the risk of cancer-related mortality among men [36]. Another study from UK also reported that both weight gain and loss could increase the risk of cancer-related mortality by 17 and 14%, respectively [37].
This study has important strengths, including its prospective nature with long comprehensive follow-up. Furthermore, to the best of our knowledge, this study is the first to examine weight change and the risk of all-cause mortality in the MENA region. Finally, some previous studies related to the effect of weight change were based on self-reported questionnaires, which may have a recall information bias; however, based on the physical examination, our study used actual measurements of anthropometric indices and confounding factors.
We also acknowledge several limitations. First, due to the lack of available data, it was unknown whether weight change was unintentional or intentional. Intentional weight loss for health improvement is proved to be associated with lower mortality [38], particularly for obese individuals; therefore the exclusion of those intentionally losing weight could affect the findings of this study. Importantly, when we excluded those with prevalent comorbidity at the baseline, which potentially might have unintentional weight loss, those with weight loss more than 5% still had about 30% higher risk of mortality events that did not reach to the significant level. Second, data on some potential residual confounders, including silent comorbidities, previous weight fluctuations, socioeconomic status (excluding educational level), diet, and daily energy intake were not available; the issue might affect our results. Moreover, due to using different tools for physical activity level assessment in phases I (Lipid Research Clinic questionnaire) and II (Modifiable Activity Questionnaire), physical activity and its change were not considered as covariates; however, in national studies, it was shown that more than 21% of Iran population were physically inactive in 2011 [39]. Third, certain subgroup analyses could still be underpowered due to the small number of participants in certain strata, which may have led to insignificant associations in some categories. Therefore, the subgroup analyses findings should be extrapolated with caution. Forth, about 40% of eligible population at the baseline did not enter the data analysis; however, the mortality rate did not differ between respondents versus not respondents. This issue might indicate that the impact of older age, lower education, higher BMI and total cholesterol among respondents for mortality events was attenuated by the lower prevalence of CVD and current smoking. So, the selection bias might not apply to our data analysis. Fifth, we could not investigate the weight change in different age stages or through a longer period due to the limited sample size. Finally, the present study only included Tehranian participants and is not a national representative; hence, results cannot be generalized to the other ethnicities or rural populations.
Conclusion
In this large-scale population-based cohort study of Iranian adults, during more than 14 years of follow-up, 3-year weight change demonstrated a U-shaped association with all-cause mortality risk; both weight gain and weight loss of > 5% were associated with increased all-cause mortality risk. It was also found that weight loss of over 5% and 3–5% was significantly associated with CV and cancer mortality events, respectively.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- BMI:
-
Body mass index
- DALYs:
-
Disability-adjusted life years
- NCDs:
-
Non-communicable diseases
- CVD:
-
Cardiovascular disease
- MENA:
-
Middle east and north Africa
- TLGS:
-
Tehran lipid and glucose study
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- FPG:
-
Fasting plasma glucose
- TC:
-
Total cholesterol
- SD:
-
Standard deviation
- HR:
-
Hazard ratios
- CIs:
-
Confidence intervals
- CVD:
-
Cardiovascular disease
- VIF:
-
Variance inflation factor
References
Azizi F, Hadaegh F, Hosseinpanah F, Mirmiran P, Amouzegar A, Abdi H, Asghari G, Parizadeh D, Montazeri SA, Lotfaliany M. Metabolic health in the Middle East and north Africa. Lancet Diabetes Endocrinol. 2019;7(11):866–79.
Nasreddine L, Ayoub JJ, Al Jawaldeh A. Review of the nutrition situation in the Eastern Mediterranean Region. East Mediterr Health J. 2018;24(1):77–91.
Addressing the impact of air pollution on health in the eastern Mediterranean region. 2014. http://www.emro.who.int/ about-who/rc61/impact-air-pollution.html (accessed March 20, 2019).
Rahmani F, Asgari S, Khalili D, Moeini ASH, Tohidi M, Azizi F, Hadaegh F. National trends in cardiovascular health metrics among Iranian adults using results of three cross-sectional STEPwise approaches to surveillance surveys. Sci Rep. 2021;11(1):1–11.
Djalalinia S, Saeedi Moghaddam S, Sheidaei A, Rezaei N, Naghibi Iravani SS, Modirian M, Zokaei H, Yoosefi M, Gohari K, Kousha A, Abdi Z, Naderimagham S, Soroush AR, Larijani B, Farzadfar F. Patterns of Obesity and Overweight in the Iranian Population: Findings of STEPs 2016. Front Endocrinol (Lausanne). 2020;11:42. https://doi.org/10.3389/fendo.2020.00042.
Global Burden of Disease Study 2019. (GBD 2019) Results [database on the Internet]. Institute for Health Metrics and Evaluation (IHME). 2020. Available from: http://ghdx.healthdata.org/gbd-results-tool. Accessed 21 Nov 2020.
Di Angelantonio E, Bhupathiraju SN, Wormser D, Gao P, Kaptoge S, De Gonzalez AB, Cairns BJ, Huxley R, Jackson CL, Joshy G. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. The Lancet. 2016;388(10046):776–86.
Bhaskaran K, dos-Santos-Silva I, Leon DA, Douglas IJ, Smeeth L. Association of BMI with overall and cause-specific mortality: a population-based cohort study of 3· 6 million adults in the UK. Lancet Diabetes Endocrinol. 2018;6(12):944–53.
Troiano RP, Frongillo EA Jr, Sobal J, Levitsky DA. The relationship between body weight and mortality: a quantitative analysis of combined information from existing studies. Int J Obes Relat Metab Disord. 1996;20(1):63–75.
Karahalios A, English DR, Simpson JA. Change in body size and mortality: a systematic review and meta-analysis. Int J Epidemiol. 2017;46(2):526–46.
Alharbi TA, Paudel S, Gasevic D, Ryan J, Freak-Poli R, Owen AJ. The association of weight change and all-cause mortality in older adults: a systematic review and meta-analysis. Age Ageing. 2021;50(3):697–704.
Heymsfield SB, Peterson CM, Thomas DM, Heo M, Schuna J Jr. Why are there race/ethnic differences in adult body mass index–adiposity relationships? A quantitative critical review Obesity reviews. 2016;17(3):262–75.
Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–14.
Dorsey RR, Eberhardt MS, Ogden CL. Racial and ethnic differences in weight management behavior by weight perception status. Ethn Dis. 2010;20(3):244.
Park SY, Wilkens LR, Maskarinec G, Haiman CA, Kolonel LN, Marchand LL. Weight change in older adults and mortality: the Multiethnic Cohort Study. Int J Obes (Lond). 2018;42(2):205–12.
Azizi F, Ghanbarian A, Momenan AA, Hadaegh F, Mirmiran P, Hedayati M, Mehrabi Y, Zahedi-Asl S. Prevention of non-communicable disease in a population in nutrition transition: Tehran Lipid and Glucose Study phase II. Trials. 2009;10(1):5.
National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–421.
Stevens J, Truesdale KP, McClain JE, Cai J. The definition of weight maintenance. Int J Obes. 2006;30(3):391–9.
Khalili D, Mosavi-Jarrahi A, Eskandari F, Mousavi-Jarrahi Y, Hadaegh F, Mohagheghi M, Azizi F. Evaluation of cause of deaths’ validity using outcome measures from a prospective, population based cohort study in Tehran. Iran PloS one. 2012;7(2):e31427–e31427.
Moazzeni SS, Hizomi Arani R, Deravi N, Hasheminia M, Khalili D, Azizi F, Hadaegh F. Weight change and risk of cardiovascular disease among adults with type 2 diabetes: more than 14 years of follow-up in the Tehran Lipid and Glucose Study. Cardiovasc Diabetol. 2021;20(1):1–13.
Kim Y-H, Kim SM. Han K-d, Son J-W, Lee S-S, Oh SW, Lee W-Y, Yoo SJ, Obesity TTotOFSotKSftSo: Change in weight and body mass index associated with all-cause mortality in Korea: a nationwide longitudinal study. J Clin Endocrinol Metab. 2017;102(11):4041–50.
Park S-Y, Wilkens LR, Maskarinec G, Haiman CA, Kolonel LN, Marchand L. Weight change in older adults and mortality: the Multiethnic Cohort Study. Int J Obes. 2018;42(2):205–12.
Pan X-F, Yuan J-M, Koh W-P, Pan A. Weight change in relation to mortality in middle-aged and elderly Chinese: the Singapore Chinese Health Study. Int J Obes. 2019;43(8):1590–600.
Mulligan AA, Lentjes MA, Luben RN, Wareham NJ, Khaw K-T. Weight change and 15 year mortality: results from the European Prospective Investigation into Cancer in Norfolk (EPIC-Norfolk) cohort study. Eur J Epidemiol. 2018;33(1):37–53.
Beavers KM, Lyles MF, Davis CC, Wang X, Beavers DP, Nicklas BJ. Is lost lean mass from intentional weight loss recovered during weight regain in postmenopausal women?–. Am J Clin Nutr. 2011;94(3):767–74.
Lee JS, Visser M, Tylavsky FA, Kritchevsky SB, Schwartz AV, Sahyoun N, Harris TB, Newman AB. Study HA Weight loss and regain and effects on body composition: the Health, Aging, and Body Composition Study. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences. 2010;65(1):78–83.
Suetta C, Hvid LG, Justesen L, Christensen U, Neergaard K, Simonsen L, Ortenblad N, Magnusson SP, Kjaer M, Aagaard P. Effects of aging on human skeletal muscle after immobilization and retraining. J Appl Physiol. 2009;107(4):1172–80.
Ashwell M, Gibson S. Waist to height ratio is a simple and effective obesity screening tool for cardiovascular risk factors: analysis of data from the British National Diet and Nutrition Survey of adults aged 19–64 years. Obes Facts. 2009;2(2):97–103.
Villareal DT, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento-Villareal R, Napoli N, Qualls C, Shah K. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364(13):1218–29.
Aune D, Sen A, Prasad M, Norat T, Janszky I, Tonstad S, Romundstad P, Vatten LJ. BMI and all cause mortality: systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ. 2016;353:i2156. https://doi.org/10.1136/bmj.i2156.
Jayedi A, Rashidy-Pour A, Soltani S, Zargar MS, Emadi A, Shab-Bidar S. Adult weight gain and the risk of cardiovascular disease: a systematic review and dose-response meta-analysis of prospective cohort studies. Eur J Clin Nutr. 2020;74(9):1263–75.
Nauli AM, Matin S. Why do men accumulate abdominal visceral fat? Front Physiol. 2019;10:1486.
Chen C, Ye Y, Zhang Y, Pan X-F, Pan A. Weight change across adulthood in relation to all cause and cause specific mortality: prospective cohort study. BMJ. 2019;367: l5584.
Pan XF, Yuan JM, Koh WP, Pan A. Weight change in relation to mortality in middle-aged and elderly Chinese: the Singapore Chinese Health Study. Int J Obes (Lond). 2019;43(8):1590–600.
Gannavarapu BS, Lau SKM, Carter K, Cannon NA, Gao A, Ahn C, Meyer JJ, Sher DJ, Jatoi A, Infante R, et al. Prevalence and survival impact of pretreatment cancer-associated weight loss: a tool for guiding early palliative care. Journal of Oncology Practice. 2018;14(4):e238–50.
Li J-B, Luo S, Wong MCS, Li C, Feng L-F, Peng J-H, Li J-H, Zhang X. Longitudinal associations between BMI change and the risks of colorectal cancer incidence, cancer-relate and all-cause mortality among 81,388 older adults : BMI change and the risks of colorectal cancer incidence and mortality. BMC Cancer. 2019;19(1):1082–1082.
Zhang J, Hayden K, Jackson R, Schutte R. Associations of weight changes with all-cause, cancer and cardiovascular mortality: A prospective cohort study. Public Health in Practice. 2021;2: 100065.
Gregg EW, Gerzoff RB, Thompson TJ, Williamson DF. Intentional weight loss and death in overweight and obese U.S. adults 35 years of age and older. Ann Intern Med. 2003;138(5):383–9.
Koohpayehzadeh J, Etemad K, Abbasi M, Meysamie A, Sheikhbahaei S, Asgari F, Noshad S, Hafezi-Nejad N, Rafei A, Mousavizadeh M. Gender-specific changes in physical activity pattern in Iran: national surveillance of risk factors of non-communicable diseases (2007–2011). Int J Public Health. 2014;59(2):231–41.
Acknowledgements
The authors would like to express their appreciation to the TLGS participants and staff for their kind cooperation.
Funding
No funding from any source was obtained for this study.
Author information
Authors and Affiliations
Contributions
Study conception and design: S.S.M and F.H; analysis and interpretation of data: M.H, S.S.M, and F.H; drafting of the manuscript: N.D, S.S.M, and F.H; critical revision: S.S.M, R.H.A, F.A, and F.H. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board (IRB) of the Research Institute for Endocrine Sciences (RIES), Shahid Beheshti University of Medical Sciences, and all participants provided written informed consent. All methods were done in accordance with the relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Table S1. Baseline characteristics of the respondents and non-respondents: the Tehran Lipid and Glucose Study, Iran, 1999-2018.
Additional file 2:
Table S2. Multivariable hazard ratios (HR) and 95% confidence intervals (CI) of association between weight change categories and all-cause mortality among those without cardiovascular disease, diabetes, and cancer at baseline or first follow-up: the Tehran Lipid and Glucose Study, Iran, 1999-2018.
Additional file 3:
Figure S1. Multivariable hazard ratios (HR) and 95% confidence intervals (CI) of association between weight change categories and cardiovascular (CV) mortality among those without history of CVD at baseline. Model 1: adjusted for age and sex; Model 2: further adjusted for Body mass index, educational level, Smoking status, hypertension, hypercholesterolemia, diabetes mellitus, and history of CVD at baseline.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Deravi, N., Moazzeni, S.S., Hasheminia, M. et al. Three-year weight change and risk of all-cause, cardiovascular, and cancer mortality among Iranian adults: over a decade of follow-up in the Tehran Lipid and Glucose Study. BMC Public Health 22, 1762 (2022). https://doi.org/10.1186/s12889-022-14126-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-022-14126-4