Abstract
Obesity is a public health crisis, presenting a huge burden on health care and the economic system in both developed and developing countries. According to the WHO’s latest report on obesity, 39% of adults of age 18 and above are obese, with an increase of 18% compared to the last few decades. Metabolic energy imbalance due to contemporary lifestyle, changes in gut microbiota, hormonal imbalance, inherent genetics, and epigenetics is a major contributory factor to this crisis. Multiple studies have shown that probiotics and their metabolites (postbiotics) supplementation have an effect on obesity-related effects in vitro, in vivo, and in human clinical investigations. Postbiotics such as the SCFAs suppress obesity by regulating metabolic hormones such as GLP-1, and PPY thus reducing feed intake and suppressing appetite. Furthermore, muramyl di-peptides, bacteriocins, and LPS have been tested against obesity and yielded promising results in both human and mice studies. These insights provide an overview of targetable pharmacological sites and explore new opportunities for the safer use of postbiotics against obesity in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the decades, researchers have faced a global challenge in understanding preventing and treating obesity and its accompanying metabolic consequences. Obesity prevalence and its related metabolic conditions have skyrocketed worldwide, particularly in developed countries [1, 2]. Given the link between obesity and both short- and long-term poor somatic, psychological, and socioeconomic circumstances, various studies support the WHO's assessment that obesity is one of the most serious threats to global public health today [3,4,5]. The development of obesity is linked to several variables. Along with the genetics, hormonal, and environmental factors, the utilization of high-calorie junk foods, a high consumption rate, less physically demanding occupations, a lack of physical activities, insufficient sleep, and repeated use of some medications contribute significantly to the onset of obesity [6]. Obesity is a complex and heritable illness caused by the interaction of genetic predisposition, epigenetics, metagenomics, and the environment. Numerous genes related to syndromic monogenic, non-syndromic monogenic, oligogenic, and polygenic obesity have been found in attempts to understand the genetic basis of obesity [7]. The genetics of leanness are also regarded as important since they reflect some of the etiologies of obesity. Various studies have witnessed different genes linked to monogenic obesity in humans. The mutations in leptin (an adipocyte-specific secreted protein associated with energy expenditure and appetite), leptin receptor, melanocortin 4 receptor (a G-protein-coupled receptor involved in energy homeostasis), and prohormone convertase 1 (involved in prohormone management), defects in pro-opiomelanocortin precursor (precursor of adrenocorticotrophin, melanocyte-stimulating hormone) [8]. The variations in these genes were also associated with severe consequences including a defective immune system, cardiovascular diseases, insulin resistance, metabolic dysfunctions, type-2 diabetes, ageing, and cancer [9]. The hormones secreted from the endocrine tissue, adipose tissue, and neuroendocrine cells mediate appetite, body composition, and glucose homeostasis [10, 11].
Improper nutrition not only affects the composition and function of the gut microbiota, but it also has a direct impact on energy intake and can contribute to the development of obesity [12]. The neural system regulates energy expenditure through the stimulants from the gastrointestinal tract in the form of neurotransmitters and other neuropeptides generated by gut microbiota [7, 13]. The regulatory chemicals generated by the microbiota have an impact on brain areas that are in charge of cognitive processes, emotions, and food consumption [14]. In obesity, the negative energy balance (due to increased physical activity or decreased food consumption, or both) is important concerning energy expenditure, physical and metabolic activities, and orexigenic signals [15].
The fact that probiotics and their metabolites play an important role in maintaining health and help in treating and mitigating various gastrointestinal (GIT) diseases/conditions via maintaining intestinal homeostasis cannot be ignored [16, 17]. “Postbiotics” is a term used to describe biological components produced by probiotics that have beneficial effects on the host. These biological components such as short-chain fatty acids (SCFAs), bacteriocins, lipoteichoic acids, surface layer protein, and secreted protein were named postbiotics in recent scientific discoveries. [18,19,20,21]. It is worth noticing that the host microbiota varies among individuals and populations and so as well as its metabolites, which are linked to the difference in functional phenotype as well as the metabolic status of the host [22]. The use of SCFAs and other microbial compounds produced by the host’s gut microbiota may also explain the intricacy of the pathogenic pathways linked to obesity [23, 24]. Therefore, this review aims to describe the physiology and molecular mechanism that directly and indirectly lead to obesity, furthermore, highlighting the nutritional strategy of using postbiotics and its action mechanism in encountering obesity and weight gain.
Current global situation of obesity
The body mass index (BMI) scale is the most widely used to assess obesity [25]. According to the World Health Organization, BMI is “a basic indicator of weight-for-height that is routinely used to classify adults as underweight, overweight, or obese”. The most recent report published by WHO (2022) obesity is an emerging epidemic in developed and developing countries worldwide. The WHO fact sheet (https://worldpopulationreview.com/country-rankings/obesity-rates-by-country) about obesity updated in 2022 reported about 39% of the adult population aged 18 and above as obese, while those lower than 18 has a rise of 18% in 2016 compared to the 4% in 1975.
Obesity and its consequences are important factors contributing to morbidity, mortality, and compromise living standards, its complications can have a major effect on the financial and social life of an individual and population [26]. As it is strongly linked with mortality due to high-risk diseases such as cardiovascular, liver diseases, and certain types of cancer [26,27,28]. A recent report by “Statista, 2022” (https://lb-aps-frontend.statista.com/statistics/1287734/rate-of-deaths-attributable-to-obesity-leading-countries-worldwide/) showed that the mortality credited for obesity is 62.6 per 100,000 population.
Obesity and energy metabolism linkage/consequences
In the recent era of industrialization, easy transportation, urbanization, and developments, a significant decline in physical activities leading to an imbalance of energy homeostasis cannot be ignored [29, 30]. These factors hugely favoured the condition of obesity and increased body weight by an easily and increased food access. Energy homeostasis refers to the intake of energy compared to its expenditure within the frontiers of thermodynamics law [31, 32]. A persistent positive energy results in obesity, it just takes a 1% increase in daily energy consumption for the average person to accumulate a 10-kg gain in fat mass over a decade [32, 33]. The energy intake and energy expenditure balance maintain the whole body’s energy homeostasis, when this balance is disturbed due to the contemporary lifestyle tied to the energy rich diet, the surplus energy is stored in the form of adipose tissue leading to obesity [34]. Obesity causes increased circulation of free fatty acids (FFA), which in turn induces oxidative stress by stimulating the reactive oxygen species (ROS) [35]. The elevation in ROS is the factual cause of insulin resistance. The decrease in liver antioxidant enzymes glutathione (GSH) is strongly linked with a high-fat diet (HFD), whereas the NADPH oxidase, which is involved in (ROS) production, is increased [36, 37]. Markers of oxidative stress increase in skeletal muscle because of HFD, which induces peripheral insulin resistance and ectopic fat storage [38, 39]. Over time, the pancreas gets exhausted and blood glucose levels begin to rise because there is not enough production of insulin to overcome the resistance. Once hyperglycemia occurs, the toxic effect on islet cells (glucotoxicity) intensifies the problem, thus lipotoxicity takes place as a result of increased FFAs levels [26, 40]. Insulin resistance in the liver, muscles, and adipose tissue escalates proinflammatory cytokines and de-escalates anti-inflammatory cytokines, which results in chronic inflammation [26]. No wonder how risky is obesity, and its consequences, as it is significantly associated with life-threatening diseases such as cardiovascular diseases, type two diabetes, cancer, osteoarthritis, and liver diseases [26, 27]. These risks arise from the enlarged number of adipocytes formation and their metabolism. Considering these consequences, obesity increases overall mortality, which needs serious attention.
Endocrinal regulation of obesity
The hormonal imbalance and its resulting abnormalities are significantly associated with obesity [41]. The lean body maintains the normal regulation of the endocrinal system, an increase in weight causes the disproportion of several hormones and affects normal physiology [14, 41]. The hormones secreted from the endocrine tissue, adipose tissue, and neuroendocrine cells mediate appetite, body composition, and glucose homeostasis [10, 11]. These hormonal signals are strictly controlled in order to keep body weight/adiposity within a restricted, individually determined range, which can be influenced by factors such as calorie intake, meal composition, and lifestyle [10, 42]. In response to changed energy balance, the hypothalamus analyses and integrates a variety of neuronal and humoral cues to coordinate eating and energy expenditure. Long-term signals from the hypothalamus provide information about the body’s energy resources, an endocrine condition, and overall body condition [43]. Meal initiation and termination are supervised by short-term cues such as gut hormones and neurological impulses from the brain centre and gut. Both these short-term and long-term cues significantly influence energy expenditure by affecting sympathetic nerve outflow to brown adipose tissue and pituitary hormone release [44, 45]. Parallel to the neurological system’s control of appetite, the gut–brain axis communicates continuously from the stomach to the brain in both health and sickness. Not only does the gut microbiota connect with adjacent cells, but also produces and releases chemicals that can communicate with distant cells [46, 47]. In this regard, any changes to it may have a significant effect on appetite control. Gut microbiota and their metabolites (postbiotics) target the central nervous system (CNS) directly through vagal stimulation or indirectly through immune–neuroendocrine processes. Indeed, fat tissues are metabolic/endocrine organ that secretes adipokines, chemokines, and proinflammatory cytokines such as tumour necrosis factor-alpha (TNF-α), and interleukin-6 (IL-6), and others, thus play an important role related to obesity, and inflammation [48]. The adipose tissue releases three major hormones leptin, adiponectin, and visfatin [10]. Leptin is released by the white adipose tissue according to the body fat mass which induces an anorexigenic reaction and increases the expenditure of energy [49, 50]. The administration of leptin both peripheral and central significantly reduced the feed intake and feeding behaviour in mice [51]. Six alternative splice isoforms have been identified yet, and Ob-Rb among them is found high in the hypothalamus and other cells and acts as a primary signal transducer in the JAK–STAT signalling pathway [52]. Overall leptin acts as a mediator for energy homeostasis, through blood glucose regulation, feed intake, and eating behaviour in humans and mice [53, 54].
Adiponectin regulates insulin and acts as an anti-inflammatory agent, which is reduced in obese conditions [28]. The synthesis of adiponectin is triggered by glucocorticoids, prolactin, growth hormone, and catecholamine, while inhibited by androgens and the paracrine actions of TNFα [55, 56]. Adiponectin stimulates glucose metabolism and fatty acid oxidation in muscle tissue [57], while in the liver it increases insulin sensitivity, limits non-esterified fatty acids inflow stimulates fatty acids oxidation, and minimizes glucose synthesis and release [58]. A study performed in adiponectin knockout mice showed reduced hepatic insulin sensitivity and glucose intolerance [59].
Visfatin is an insulin-like peptide hormone generated by adipocytes that stimulates glucose absorption in muscles and skin while blocking its release from the liver [60]. A study in mice revealed visfatin lower glucose levels in an insulin-independent mode [60, 61]. Visfatin promotes the accumulation of triglycerides from pre-adipocytes, enhances glucose to lipid conversion, and upregulates the expression of various genes including PPAR gamma and adiponectin. However, visfatin did not alter the food intake or body weight in a knockout heterozygous mouse compared to the wild type [60,61,62]. Besides these, there are other hormones like insulin, ghrelin, obestatin, and so on (Table 1) which directly or indirectly affect body weight showing a deep connection between endocrinology and obesity.
Genetics of obesity
The genetic contribution to obesity cannot be underestimated due to the significant heritability of the BMI (20–40%) [82, 83]. Evidence showed that there has been a considerable link between genetics and obesity, with two studies claiming a heritability value of 0.77 at different ages in different regions [84, 85]. To date, there have been discoveries of some important genes strongly related to severe obesity, which give enough evidence of genetic and obesity linkage, as shown in Table 1. Recently, through GWAS combined with the in vivo study in C. elegans, scientists discovered 14 genes that promote obesity and 3 genes that prevent diet-induced obesity as shown in Table. 2 [86]. Referencing the studies performed previously several hundred genetic loci have been found by genome-wide association analysis (GWAS) studies, where sequence variants are statistically linked with BMI at the population level, however, these links show only a 3–5% contribution of variation to the BMI [82, 87, 88]. Furthermore, the majority of obesity-predisposing gene variations are not linked to weight loss or regain due to lifestyle interactions.
Genetics of obesity studies conducted in humans and mice model
In order to improve the prevention, treatment, and management of obesity it is important to undermine and understand its molecular causes. Consequently, this has encouraged identifying the genes responsible for obesity using rodent and human models [40, 89]. The mouse model is widely used in studying the genetics of obesity due to its low cost, maintenance, small size, easy breeding, and short gestation period [90,91,92]. The complete genome sequence, genetically distinct strains availability, and cutting-edge genetic manipulative tools make it possible to conduct advanced genetic analysis associated with obesity in rodents. Furthermore, the occurrence of obesity and metabolic phenotypes alteration in mice are similar to humans, moreover, the measurement of these phenotypes in mice is more convenient and safe compared to humans [93, 94]. However, there are certain limitations in the mouse model used for obesity phenotypes compared to the human model, such as the difference in obesity phenotypes, and physiology, which leads to further and safe investigation in the human model [95]. From the literature, we have identified several genes that are directly related to obesity and both verified in mice and human models as shown in Table 2.
Novel genes in human obesity using the C. elegans model
Previously genetic selection using the Caenorhabditis elegans model has led to the discovery of drug targets for various diseases including depression and metabolic related disorders [116, 117]. C. elegans is considered, evolutionarily distant from humans, as the many pathways related to lipid, glucose, and protein metabolism are the same in both species [118]. In both organisms, identical genes such as TOR kinase and AMPK, as well as transcription factors like sterol response element binding protein (SREBP), peroxisomes proliferator-activated receptor gamma (PPAR), and transcription factor EB (TFEB), govern metabolic genes and cellular responses. Studies have also shown that the loss of function of such regulators in both species causes metabolic dysfunction [119,120,121,122]. Furthermore, obesity-related genes identified in human GWAS whose orthologue has been shown to contribute to obesity in C. elegans are more likely to be a robust anti-obesity target across human populations. Recently scientists discovered 11 novel and overall 16 genes as shown in Table 2, which promote or prevent C. elegans obesity, as well as the early beginning of organismal degradation and mortality linked with obesity [86]. The findings of in vivo research in C. elegans combined with assessments of mouse and human GWAS datasets revealed that the sign of the connection between the mouse and human gene expression levels and their associated clinical characteristics matched. The behavioural consequences of knocking down these genes in C. elegans revealed that these obesity genes had conserved causation and therapeutic potential [86] (Table 3).
Epigenetics of obesity
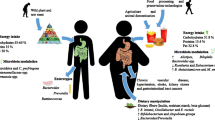
For the development of novel obesity causing DNA variations, the duration of the obesity as a pandemic is not long enough. In this case, the dynamic epigenetic regulations and environmental factors are leading contenders for explaining energy regulations [6, 7]. So far, DNA methylation has been the most thoroughly researched epigenetic mark for human disorders at the genome-wide or site-specific level, which takes place at the cytosine [83, 88, 123]. Various studies have identified the methylated loci through the epigenetic wide association studies linked with obesity. Furthermore, these studies uncovered that several genes had undergone methylation associated with obesity [124]. CpG promoter methylation of peroxisome proliferator-activated receptor gamma (PPARc) coactivator-1alpha (PGC-1a), a transcriptional coactivator for mitochondrial biogenesis, is elevated in obese women's subcutaneous adipose tissue (SAT) [88]. In obese people, adiponectin, an adipokine that controls systemic energy expenditure and insulin sensitivity, is diminished in adipose tissue [125]. In very obese patients, DNA methylation levels at the adiponectin gene locus in SAT were linked to BMI. The hypothalamus through regulation of the pro-opiomelanocortin (POMC) gene controls energy homeostasis, and methylation in the POMC gene was found to be significantly associated with obesity. Only a few particular genes and pathways have been consistently identified as being involved in the development of obesity [6]. Therefore, despite the genetic susceptibility to obesity, environmental and epigenetics seem to be important factors (Fig. 1).
Environment, genetics, and epigenetics contribute as the main factors causing obesity. Environmental factors such as a sedentary lifestyle, unhealthy food, stress, and abnormal sleep along with genetics and epigenetics are predisposing causes for obesity. Both these factors cause epigenetics alteration, which causes energy dysbiosis, tissue inflammation, decrease insulin resistance, and increase lipid accumulation. In turn, obesity is capable of causing severe health problems such as cancer, type-2 diabetes, ageing, and cardiovascular disease are the most common
Gut microbiota and obesity linkage
The gut microbiota plays an important role in the development of obesity due to its intimate nexus with energy metabolism. Any alteration in gut microbiota may lead to energy dysbiosis as well as energy homeostasis [14, 126]. The human gut hosts a diverse microbial population among which ~ 1000 bacteria are preponderant and belong to 40 different species [16, 127]. In order to disclose the predictive microbial markers of obesity, the Firmicutes phyla’s staphylococcus and lactobacillus, as well as Bifidobacterium from the Actinobacteria genus, were examined. Interestingly Bifidobacterium showed a higher number in normal-weight compared to the obese individuals, while staphylococcus were less [128, 129]. With the decrease of keystone microbial species in the guts, the symbiosis between the host and gut microbiota is disturbed, resulting in dysbiosis, which unsettles the host's metabolic health. On one side dysbiosis is considered to be a result of a decreased number of bacteria that are metabolically protective against obesity, and an increase in those that extract more energy from indigestible carbohydrates. Furthermore, various studies including clinical trials suggested that intervention of certain microbial species exerts a significant effect and mitigates obesity as shown in Table 4.
Various studies using the animal model have proposed that the gut microbiota energy homeostasis and adiposity through various mechanisms. The gut microbiota extracts energy from the diet while also modifying tissue fatty acid composition, secreting gut-derived peptides and hormones with CNS effects, and generating chronic low-grade inflammation via lipopolysaccharide release [48, 129]. One of the critical tasks of the gut microbiota is the enzymatic conversion of primary bile into secondary bile, which influences the absorption and emulsification of bile acids. Following this mechanism gut microbiota has an enormous impact on bile acid entero-hepatic distribution. The secondary form binds to G-protein and leads to glucagon-like-1 peptide stimulation, which lowers circulation and hepatic triglyceride levels [126, 139]. Both qualitative and quantitative variations in the gut microbiota can affect this pathway by encouraging fat formation in the body. For instance, gut microbiota on a high-fat diet may convert dietary choline into hepatic toxic methyl-amines, lowering choline availability, which is required for very low-density lipoprotein (VLDL) assembly and production, thus significantly enhancing hepatic steatosis and lipo-peroxidation. The intestinal microbial community plays an important role in the processing of dietary carbohydrates, and their fermentation into SCFAs. The acetic acid that is the most abundant in peripheral blood is vital for cholesterol synthesis, a stimulant for adipogenesis via the FFA2 receptor, and a suppressor of appetite via the hypothalamic mechanism. Propionic acid is the main precursor for protein synthesis, hepatic gluconeogenesis, and lipogenesis, as well as an inhibitor of fatty acid production, and an inflammation-reducing agent [16, 48, 126].
Host and gut microbial metabolites (postbiotics) interaction
The intestinal microbial community plays an important role in the processing of dietary carbohydrates, and their fermentation into microbial metabolites. Growing findings suggest that microbial metabolites (postbiotics) produced by microbial fermentation have an important role in regulating host metabolism, with implications for obesity [13, 135]. Clostridium and Eubacterium from the gut microbiota convert bile acid in the intestine to secondary forms such as deoxycholic acid and lithocholic acid, which bind to the TGR5 receptor (G-protein-coupled receptor) and stimulate the secretion of the incretin hormones GLP-1 and insulin, promoting energy expenditure [140]. Long chain fatty acids produced by gut microbiota, such as linoleic acid, modify the lipid profile, contributing to obesity. The acetic acid that is the most abundant in peripheral blood is vital for cholesterol synthesis, a stimulant for adipogenesis via the FFA2 receptor, and a suppressor of appetite via the hypothalamic mechanism. Propionic acid is the main precursor for protein synthesis, hepatic gluconeogenesis, and lipogenesis, as well as an inhibitor of fatty acid production, and an inflammation-reducing agent [16, 48, 126].
Short-chain fatty acids in control of energy regulation
Due to a lack of suitable enzymes, our gut bacteria ferment dietary components that are incompletely hydrolyzed, leading to the formation of SCFA such as acetate, butyrate, and propionate [141]. These SCFAs have important roles in the pathophysiology of obesity and related illnesses by regulating energy intake, energy harvesting, and host energy and substrate metabolism, all of which affect body weight [142,143,144,145]. Several pathways have also been hypothesized to link SCFA to insulin sensitivity and the progression of T2DM, including interorgan effects on adipose tissue function and lipid storage capacity, metabolism, and inflammatory activities [146,147,148]. SCFAs are monocarboxylic acids including acetate, lactate, propionate, and butyrate as the most abundant and common metabolites secreted by the gut microbiota [127]. These SCFAs are the main constituent of fibre fermentation because of gut microbiota and exert significant effects on host physiology, gut health, mucous production, promoting gut integrity, and protection of the gut epithelial [23, 149].
Evidence suggests that glucose is not the only source of energy utilized by the body. In addition, the body uses SCFAs and amino acids to carry out various physiological activities [141]. A study reported the involvement of butyrate and propionate in stimulating different gut hormones and reducing feed intake [150]. Propionate blocks lipogenesis by downregulating fatty acid synthase in the liver, while acetate is a lipogenic substrate, thus, the acetate/propionate ratio is thought to be critical for de novo lipogenesis. In addition, propionate and butyrate induce intestinal lipogenesis by upregulating the lipogenesis-related genes, thus mitigating obesity.
Acetate and obesity nexus
Acetate has been attributed to health benefits, whether derived from food or microbial fermentation in the gut. These health benefits include energy homeostasis, improved heart function, blood generation, and memory formation [151, 152]. The question of how acetate contributes to so many diverse biological functions is an area of intense research nowadays. Acetate is believed to be responsible for appetite regulation [153]. Supplementation of acetate can stimulate biochemical and physiological responses resulting in control of insulin regulation, weight loss, cardiac system safety, and anti-inflammatory responses [16]. Its function related to obesity is however still conflicting. On the one hand, acetate has been demonstrated to increase the expression of anorectic hormones in the hypothalamus, such as GLP-1 and peptide tyrosine-tyrosine (PYY), so decreasing food intake (Fig. 2) [14, 154] (Fig. 2). A study performed in mice showed that acetate generated in the intestine increases anorectic signalling in the arcuate nucleus ARC via the glutamate–glutamine transcellular cycle [154]. However, this statement was contradicted by another study that showed the increased level of acetate was involved in increasing insulin and ghrelin leading to obesity [155]. Therefore, further research is needed to confirm whether the acetate has a stimulating or suppressing effect on the appetite.
Propionate and obesity nexus
Propionate another SCFA has been reported to mitigate obesity and reduce feed intake through gut hormone modulation [153, 156]. Propionate suppresses the appetite by regulating free fatty acids receptor FFAR2/3 in the intestinal cells, which induces the glucagon-like peptide (GLP-1) and PPY peptides [157]. The presence of propionate in the hindgut activates the PPY and GLP-1 involved in reducing both feed intake and weight gain in obese individuals (Fig. 2) [153]. Furthermore, propionate has been reported in the suppression of the genes responsible for lipid synthesis. Various studies performed using the mouse model explained the mechanism of propionic acid in preventing obesity by inhibiting food intake, increasing insulin sensitivity, and energy expenditure [145, 153, 158].
Butyrate and obesity nexus
Among others, butyrate is one of the most used SCFAs used by the intestinal mucosa, as a primary source of energy [14, 142]. Dietary butyrate has been reported in insulin resistance and prevents diet-induced obesity in mice [142]. Furthermore, butyrate has also been involved in controlling weight by boosting energy expenditure through direct contact with skeletal muscle and inducing lipolysis in adipose tissue. Butyrate supplementation in the diet showed a significant reduction in diet-induced obesity and insulin resistance in obese mice models [143, 159]. SCFAs are the byproducts of bioconversion in the colon and play a major role in appetite regulation by boosting the release of anorectic gut hormones such as PYY and GLP-1 [153]. As a result, raising SCFA levels represents a viable target that could lower adiposity and weight in obese persons. A study in mice revealed that oral administration of sodium butyrate induces fat oxidation and energy expenditure leading to weight loss [23, 142]. Moreover, the microbiota transplant from human to mouse resulted in increased adiposity, decreased faecal SCFAs, and increased monosaccharide and disaccharide concentration after feeding a plant carbohydrate-rich diet compared to the one received microbiota from the lean individual [131]. These studies suggest that the microbes from the obese individual have lower capabilities to properly ferment and digest the polysaccharides compared to the microbiota from the lean individual. However, the molecular effect of butyrate needs further investigation due to its controversial position as it also acts as a substrate for energy in the host system. To explore the real scenario behind this controversy, the actual role of butyrate in the energy cycle shall be tested in different animals, while using equicaloric food in both control and test groups.
Peptidoglycans as postbiotics linkage with obesity
Adipose tissue inflammation and insulin resistance are some of the main consequences of obesity, however, certain microbial components can significantly protect against these damages [144]. For example, the postbiotics from the proximal gut microbiota showed a significant role in preventing insulin resistance because of a high-fat diet [160, 161]. Peptides are the important component of the bacterial cell wall present in the form of peptidoglycan. A recent study using a mouse model showed that peptide-based postbiotics (muramyl-dipeptide) reduced insulin resistance and adipose tissue inflammation in obese conditions through nucleotide oligomerization 2 protein receptors [20]. NOD2 acts as a bacterial peptidoglycan sensor and its activation stimulates metabolic, inflammatory, and antimicrobial activities. Furthermore, NOD2 keeps the gut microbiota healthy [162]. A study in mice reported that NOD2 knockout mice developed obesity due to a high-fat diet and caused metabolic disturbances including hyperglycemia, hyperlipidemia, and glucose intolerance. These repercussions consequentially lead to the accumulation of adipocytes and lipid droplet formation in the liver [163]. A single dose of MDP-based postbiotics reduced glucose intolerance via interaction with NOD2 receptors without damaging the gut microbiome [20]. Further insight into the NOD protein and postbiotics interaction related pathways will explore the molecular mechanism of action of postbiotics and recognition of specific pharmacological sites for treating obesity.
Bacteriocins’ role in obesity
Bacteriocins are ribosomal-synthesized heat-stable antimicrobial peptides produced by the gut microbiota, which show distinct characteristics related to their size, structure, and mechanism of action [22, 150]. It is well known that bacteriocins show broad spectrum and narrow spectrum antimicrobial activities, however, certain studies also showed that some of these species that produce bacteriocins play an important role in obesity and related metabolic activities [19, 150]. Recent studies have underlined the population of various microbiota that may be involved in obesity. A study claimed that the gut microbiota of genetically obese mice have a higher population of phyla Firmicutes and lower phyla Bacteroidetes [17, 24]. Other studies have established the role of a particular species or strain in obesity and T2D [17]. In germ-free mice, it was revealed that Enterobacter cloacae B29 produces endotoxins that cause obesity and insulin resistance [164]. Furthermore, Clostridium ramosum, previously shown to be enriched in patients with T2D, induced obesity in mice consuming a high-fat diet [165]. Gut bacteria that produce antibiotics with specific activity against some of these organisms may be beneficial for balancing metabolic health.
Lipopolysaccharides and obesity
Lipopolysaccharides (LPS), a component of Gram-negative bacteria’s cell membrane, the function of which has been ambiguous, act as a triggering factor, causing low-grade chronic inflammation and the development of insulin resistance (IR) [19]. LPS produced in the gastrointestinal tract enters the blood by direct diffusion via increasing intestinal permeability or absorption and chylomicron inclusion. High fat consumption reduces the expression of the tight junction proteins zonulin and occludin, increasing the intestinal permeability of LPS, the causative cause of endotoxemia [19, 69]. LPS interacts with toll-like receptor 4 (TLR-4) in immune cells as well as target organs such as the liver and adipose tissue. Migration of active NF-κB to the nucleus stimulates the production of proinflammatory proteins as well as signalling pathways such as JNK, p38 MAPK, and ERK, which leads to insulin resistance and obesity [166]. Bifidobacterium infantis administration decreased colonic permeability and inflammation in mice, indicating that gut microbial makeup, in addition to food, influences intestinal permeability. High levels of saturated lipid consumption not only increase systemic exposure to potentially proinflammatory-free fatty acids and their derivatives but also enhance the absorption of endotoxins, resulting in greater plasma LPS levels known as “endotoxemia” [8]. Endogenous lipid interaction with cannabinoid receptors (CB1 and CB2) activates adenylate cyclase and also promotes secondary messengers implicated in the MAPK, ERK, and NF-κB pathways, causing inflammation and insulin resistance and eventually contributing to obesity. Additionally, circumstantial evidence suggests that LPS compromises the liver’s critical role in preserving the body’s glucose metabolism. It has been demonstrated that LPS-stimulated macrophages from the gingival sulci release TNF-α, IL -1, and IL-6 in animal models of periodontitis [167]. These cytokines and/or LPS from the gingival sulci may be transported throughout the body and engage TLR-4 receptors on Kupffer cells in the liver to release proinflammatory cytokines, which may lead to insulin resistance and glucose intolerance [168]. Another study concluded that LPS concentrations are an adequate molecular trigger for high fat diet-induced obesity and diabetes. Finally, via regulating insulin sensitivity, the LPS receptor, cluster of differentiation antigen 14 (CD14) determines the cutoff point at which metabolic disorders manifest [169]. These evidence suggest that LPS might contribute to host obesity by modifying intestinal permeability, resulting in endotoxemia, increased calorie supply, and endocannabinoid system (eCB) activation, as well as by modulating lipid metabolism by increasing lipoprotein lipase activity and lipogenesis [8, 170]. However, the molecular details remain to be elucidated, as there is a complex interaction between LPS-induced inflammation and obesity, which needs further research.
Conclusion
The current knowledge and shreds of evidence explaining the current global situation, molecular mechanism inducing obesity, its prominent causes, and the potent role of postbiotics in mitigating obesity. Irregularities in energy homeostasis due to changes in gut microbiota, environment, genetics, and epigenetics are highly linked to obesity. Postbiotics like SCFAs, lipids, and bacteriocins interact with genetics, hormones, nutrition, and certain environmental conditions as potential anti-obesity agents. SCFAs like acetate, propionate, and butyrate have strong capabilities to counteract obesity by regulating metabolic hormones such as GLP-1, and PPY thus reducing feed intake and suppressing appetite. Given the severity and burden of the condition on the healthcare system, the need to identify pharmacological targets for the treatment of obesity and adaptation of smart nutritional strategies are needed to explore which might further overcome this scenario. Further research is needed to explore the exact molecular mechanism of action of postbiotics in mitigating obesity, which might answer most of the questions raised related to this scenario. With the increase in physical activities, the regulated range of energy balance can be achieved, and the internal molecular mechanism to maintain this energy homeostasis can be improved. Moreover, the adoption of a smarter diet will decrease the need for drastic dietary restrictions to avoid abrupt disturbances in the energy system.
Data availability
Not applicable.
References
Morgen CS, Sørensen TIA (2014) Obesity: global trends in the prevalence of overweight and obesity. Nat Rev Endocrinol 10:513–514. https://doi.org/10.1038/nrendo.2014.124
Blüher M (2019) Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol 15:288–298. https://doi.org/10.1038/s41574-019-0176-8
Speakman JR (2013) Evolutionary perspectives on the obesity epidemic: adaptive, maladaptive, and neutral viewpoints. Annu Rev Nutr 33:289–317. https://doi.org/10.1146/annurev-nutr-071811-150711
Deurenberg P, Deurenberg-Yap M (2001) Differences in body-composition assumptions across ethnic groups: practical consequences. Curr Opin Clin Nutr Metab Care 4
Wulan SN, Raza Q, Prasmita HS, Martati E, Maligan JM, Mageshwari U, Fatima I, Plasqui G (2021) Energy metabolism in relation to diet and physical activity: a south Asian perspective. Nutrients 13:1–13. https://doi.org/10.3390/nu13113776
Rohde K, Keller M, la Cour Poulsen L, Blüher M, Kovacs P, Böttcher Y (2019) Genetics and epigenetics in obesity. Metabolism 92:37–50. https://doi.org/10.1016/J.METABOL.2018.10.007
Pigeyre M, Yazdi FT, Kaur Y, Meyre D (2016) Recent progress in genetics, epigenetics and metagenomics unveils the pathophysiology of human obesity. Clin Sci (Lond) 130:943–986. https://doi.org/10.1042/CS20160136
Sivamaruthi BS, Kesika P, Suganthy N, Chaiyasut C (2019) A review on role of microbiome in obesity and antiobesity properties of probiotic supplements. Biomed Res Int. https://doi.org/10.1155/2019/3291367
Wu J, Liu Z, Meng K, Zhang L (2014) Association of adiponectin gene (ADIPOQ) Rs2241766 polymorphism with obesity in adults: a meta-analysis. PLoS ONE. https://doi.org/10.1371/JOURNAL.PONE.0095270
Lenz A, Diamond FB (2008) Obesity: the hormonal milieu. Curr Opin Endocrinol Diabetes Obes 15:9–20. https://doi.org/10.1097/MED.0B013E3282F43A5B
Edelman AB, Jensen JT (2012) Obesity and hormonal contraception: safety and efficacy. Semin Reprod Med 30:479–485. https://doi.org/10.1055/S-0032-1328876
Wernimont SM, Radosevich J, Jackson MI, Ephraim E, Badri DV, MacLeay JM, Jewell DE, Suchodolski JS (2020) The effects of nutrition on the gastrointestinal microbiome of cats and dogs: impact on health and disease. Front Microbiol 11:1–24. https://doi.org/10.3389/fmicb.2020.01266
Ma Y, Sun Y, Sun L, Liu X, Zeng R, Lin X, Li Y (2021) Effects of gut microbiota and fatty acid metabolism on dyslipidemia following weight-loss diets in women: results from a randomized controlled trial. Clin Nutr 40:5511–5520. https://doi.org/10.1016/j.clnu.2021.09.021
Alhabeeb H, Alfaiz A, Kutbi E, Alshahrani D, Alsuhail A, Alrajhi S, Alotaibi N, Alotaibi K, Alamri S, Alghamdi S et al (2021) Gut hormones in health and obesity: the upcoming role of short chain fatty acids. Nutrients 13:481. https://doi.org/10.3390/NU13020481
Moncrieft AE, Llabre MM, McCalla JR, Gutt M, Mendez AJ, Gellman MD, Goldberg RB, Schneiderman N (2016) Effects of a multicomponent life-style intervention on weight, glycemic control, depressive symptoms, and renal function in low-income, minority patients with type 2 diabetes: results of the community approach to lifestyle modification for diabetes rando. Psychosom Med 78:851–860. https://doi.org/10.1097/PSY.0000000000000348
Rastelli M, Knauf C, Cani PD (2018) Gut microbes and health: a focus on the mechanisms linking microbes, obesity, and related disorders. Obesity (Silver Spring) 26:792–800. https://doi.org/10.1002/OBY.22175
Vallianou N, Stratigou T, Christodoulatos GS, Tsigalou C, Dalamaga M (2020) Probiotics, prebiotics, synbiotics, postbiotics, and obesity: current evidence, controversies, and perspectives. Curr Obes Rep 9:179–192. https://doi.org/10.1007/s13679-020-00379-w
Aguilar-Toalá JE, Garcia-Varela R, Garcia HS, Mata-Haro V, González-Córdova AF, Vallejo-Cordoba B, Hernández-Mendoza A (2018) Trends in Food science & technology postbiotics: an evolving term within the functional foods field. Trends Food Sci Technol 75:105–114
Gao J, Li Y, Wan Y, Hu T, Liu L, Yang S, Gong Z, Zeng Q, Wei Y, Yang W et al (2019) A novel postbiotic from lactobacillus rhamnosus GG with a beneficial effect on intestinal barrier function. Front Microbiol 10:1–14. https://doi.org/10.3389/fmicb.2019.00477
Cavallari JF, Fullerton MD, Duggan BM, Foley KP, Denou E, Smith BK, Desjardins EM, Henriksbo BD, Kim KJ, Tuinema BR et al (2017) Muramyl dipeptide-based postbiotics mitigate obesity-induced insulin resistance via IRF4. Cell Metab 25:1063-1074.e3. https://doi.org/10.1016/j.cmet.2017.03.021
Zhong Y, Wang S, Di H, Deng Z, Liu J, Wang H (2022) Gut health benefit and application of postbiotics in animal production. J Anim Sci Biotechnol 13:1–12. https://doi.org/10.1186/s40104-022-00688-1
Umu ÖCO, Rudi K, Diep DB (2017) Modulation of the gut microbiota by prebiotic fibres and bacteriocins. Microb Ecol Health Dis 28:1348886. https://doi.org/10.1080/16512235.2017.1348886
Ferrarese R, Ceresola ER, Preti A, Canducci F (2018) Probiotics, prebiotics and synbiotics for weight loss and metabolic syndrome in the microbiome era. Eur Rev Med Pharmacol Sci 22:7588–7605. https://doi.org/10.26355/eurrev-201811-16301
Faith JJ, Guruge JL, Charbonneau M, Subramanian S, Seedorf H, Goodman AL, Clemente JC, Knight R, Heath AC, Leibel RL et al (2013) The long-term stability of the human gut microbiota. Science. https://doi.org/10.1126/science.1237439
Kolimechkov S, Petrov L (2020) The body mass index: a systematic review. J Exerc Physiol Heal 3:21–27
Abdelaal M, le Roux CW, Docherty NG (2017) Morbidity and mortality associated with obesity. Ann Transl Med. https://doi.org/10.21037/ATM.2017.03.107
Isomaa B, Almgren P, Tuom T (2001) Cardiovascular morbidity and mortality. Diabetes Care 24:683–689
Barazzoni R, Gortan Cappellari G, Ragni M, Nisoli E (2018) Insulin resistance in obesity: an overview of fundamental alterations. Eat Weight Disord 23:149–157. https://doi.org/10.1007/S40519-018-0481-6
Flack KD, Hays HM, Moreland J (2020) The consequences of exercise-induced weight loss on food reinforcement. A randomized controlled trial. PLoS ONE. https://doi.org/10.1371/JOURNAL.PONE.0234692
Romieu I, Dossus L, Barquera S, Blottière HM, Franks PW, Gunter M, Hwalla N, Hursting SD, Leitzmann M, Margetts B et al (2017) Energy balance and obesity: what are the main drivers? Cancer Causes Control 28:247–258. https://doi.org/10.1007/S10552-017-0869-Z
Westerterp KR, Kayser B (2006) Body mass regulation at altitude. Eur J Gastroenterol Hepatol 18:1–3. https://doi.org/10.1097/00042737-200601000-00001
Kayser B, Verges S (2021) Hypoxia, energy balance, and obesity: an update. Obes Rev. https://doi.org/10.1111/obr.13192
Dünnwald T, Gatterer H, Faulhaber M, Arvandi M, Schobersberger W (2019) Body composition and body weight changes at different altitude levels: a systematic review and meta-analysis. Front Physiol. https://doi.org/10.3389/fphys.2019.00430
Hochberg Z (2018) An evolutionary perspective on the obesity epidemic. Trends Endocrinol Metab 29:819–826. https://doi.org/10.1016/J.TEM.2018.09.002
Xiao C, Giacca A, Lewis GF (2008) Oral taurine but Not N-acetylcysteine ameliorates NEFA-induced impairment in insulin sensitivity and beta cell function in obese and overweight, non-diabetic men. Diabetologia 51:139–146. https://doi.org/10.1007/S00125-007-0859-X/FIGURES/4
Yuzefovych L, Wilson G, Rachek L (2010) Different effects of oleate vs. palmitate on mitochondrial function, apoptosis, and insulin signaling in L6 skeletal muscle cells: role of oxidative stress. Am J Physiol Endocrinol Metab. https://doi.org/10.1152/AJPENDO.00238.2010
Gurzov EN, Tran M, Fernandez-Rojo MA, Merry TL, Zhang X, Xu Y, Fukushima A, Waters MJ, Watt MJ, Andrikopoulos S et al (2014) Hepatic oxidative stress promotes insulin-STAT-5 signaling and obesity by inactivating protein tyrosine phosphatase N2. Cell Metab 20:85. https://doi.org/10.1016/J.CMET.2014.05.011
Yokota T, Kinugawa S, Hirabayashi K, Matsushima S, Inoue N, Ohta Y, Hamaguchi S, Sobirin MA, Ono T, Suga T et al (2009) Oxidative stress in skeletal muscle impairs mitochondrial respiration and limits exercise capacity in type 2 diabetic mice. Am J Physiol Heart Circ Physiol. https://doi.org/10.1152/AJPHEART.00267.2009
Satapati S, Sunny NE, Kucejova B, Fu X, He TT, Méndez-Lucas A, Shelton JM, Perales JC, Browning JD, Burgess SC (2012) Elevated TCA cycle function in the pathology of diet-induced hepatic insulin resistance and fatty liver. J Lipid Res 53:1080–1092. https://doi.org/10.1194/JLR.M023382
Bessesen DH (2008) Update on obesity. J Clin Endocrinol Metab 93:2027–2034. https://doi.org/10.1210/JC.2008-0520
Kil DY, Swanson KS (2010) Endocrinology of obesity. Vet Clin North Am Small Anim Pract 40:205–219. https://doi.org/10.1016/J.CVSM.2009.10.004
Kalra S, Kapoor N, Bhattacharya S, Aydin H, Coetzee A (2020) Barocrinology: the endocrinology of obesity from bench to bedside. Med Sci 8:51. https://doi.org/10.3390/MEDSCI8040051
Kaiyala KJ, Woods SC, Schwartz MW (1995) New model for the regulation of energy balance and adiposity by the central nervous system. Am J Clin Nutr. https://doi.org/10.1093/ajcn/62.5.1123S
Schwartz MW, Baskin DG, Kaiyala KJ, Woods SC (1999) Model for the regulation of energy balance and adiposity by the central nervous system. Am J Clin Nutr 69:584–596. https://doi.org/10.1093/AJCN/69.4.584
Murphy KG, Bloom SR (2004) Gut hormones in the control of appetite. Exp Physiol 89:507–516. https://doi.org/10.1113/EXPPHYSIOL.2004.027789
Holzer P, Farzi A (2014) Neuropeptides and the microbiota-gut-brain axis. Adv Exp Med Biol 817:196–219. https://doi.org/10.1007/978-1-4939-0897-4_9
Sun L, Ma L, Ma Y, Zhang F, Zhao C, Nie Y (2018) Insights into the role of gut microbiota in obesity: pathogenesis, mechanisms, and therapeutic perspectives. Protein Cell 9:397–403. https://doi.org/10.1007/S13238-018-0546-3
Petraroli M, Castellone E, Patianna V, Esposito S (2021) Gut microbiota and obesity in adults and children: the state of the art. Front Pediatr 9:657020. https://doi.org/10.3389/FPED.2021.657020
Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S et al (1995) Leptin levels in human and rodent: measurement of plasma leptin and Ob RNA in obese and weight-reduced subjects. Nat Med 1:1155–1161. https://doi.org/10.1038/NM1195-1155
Satoh N, Ogawa Y, Katsuura G, Numata Y, Tsuji T, Hayase M, Ebihara K, Masuzaki H, Hosoda K, Yoshimasa Y et al (1999) Sympathetic activation of leptin via the ventromedial hypothalamus: leptin-induced increase in catecholamine secretion. Diabetes 48:1787–1793. https://doi.org/10.2337/DIABETES.48.9.1787
Eckel LA, Langhans W, Kahler A, Campfield LA, Smith FJ, Geary N (1998) Chronic administration of OB protein decreases food intake by selectively reducing meal size in female rats. Am J Physiol Regul Integr Comp Physiol. https://doi.org/10.1152/AJPREGU.1998.275.1.R186
Farooqi IS, Wangensteen T, Collins S, Kimber W, Matarese G, Keogh JM, Lank E, Bottomley B, Lopez-Fernandez J, Ferraz-Amaro I et al (2007) Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N Engl J Med 356:237–247. https://doi.org/10.1056/NEJMOA063988
Melanocortin-Independent Effects of Leptin on Hepatic Glucose Fluxes | American Diabetes Association Available online: https://professional.diabetes.org/abstract/melanocortin-independent-effects-leptin-hepatic-glucose-fluxes (accessed on 24 December 2021)
Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA et al (2006) Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 49:191–203. https://doi.org/10.1016/J.NEURON.2005.12.021
Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF (1995) A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem 270:26746–26749. https://doi.org/10.1074/JBC.270.45.26746
Hotamisligil GS, Shargill NS, Spiegelman BM (1993) Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259:87–91. https://doi.org/10.1126/SCIENCE.7678183
Saltiel AR (2001) You are what you secrete. Nat Med 7:887–888. https://doi.org/10.1038/90911
Larson DE, Ferraro RT, Robertson DS, Ravussin E (1995) Energy metabolism in weight-stable postobese individuals. Am J Clin Nutr 62:735–739. https://doi.org/10.1093/AJCN/62.4.735
Nawrocki AR, Rajala MW, Tomas E, Pajvani UB, Saha AK, Trumbauer ME, Pang Z, Chen AS, Ruderman NB, Chen H et al (2006) Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J Biol Chem 281:2654–2660. https://doi.org/10.1074/JBC.M505311200
Lee JO, Kim N, Lee HJ, Lee YW, Kim JK, Kim HI, Lee SK, Kim SJ, Park SH, Kim HS (2015) Visfatin, a novel adipokine, stimulates glucose uptake through the Ca2+-dependent AMPK–P38 MAPK pathway in C2C12 skeletal muscle cells. J Mol Endocrinol 54:251–262. https://doi.org/10.1530/JME-14-0274
Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, Matsuki Y, Murakami M, Ichisaka T, Murakami H et al (2005) Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science 307:426–430. https://doi.org/10.1126/SCIENCE.1097243
Arner P (2006) Editorial: visfatin—a true or false trail to type 2 diabetes mellitus. J Clin Endocrinol Metab 91:28–30. https://doi.org/10.1210/JC.2005-2391
Wasim M, Awan FR, Najam SS, Khan AR, Khan HN (2016) Role of leptin deficiency, inefficiency, and leptin receptors in obesity. Biochem Genet 54:565–572. https://doi.org/10.1007/S10528-016-9751-Z
D’Souza AM (2017) The effects of leptin knockout and insulin suppression on glucose metabolism in rodents
Sirico F, Bianco A, D’Alicandro G, Castaldo C, Montagnani S, Spera R, Di Meglio F, Nurzynska D (2018) Effects of physical exercise on adiponectin, leptin, and inflammatory markers in childhood obesity: systematic review and meta-analysis. Child Obes 14:207–217. https://doi.org/10.1089/CHI.2017.0269
Takahashi H, Hosono K, Endo H, Nakajima A (2013) Colon epithelial proliferation and carcinogenesis in diet-induced obesity. J Gastroenterol Hepatol 28(Suppl 4):41–47. https://doi.org/10.1111/JGH.12240
Zhang W, Zhao D, Meng Z, Wang H, Zhao K, Feng X, Li Y, Dun A, Jin X, Hou H (2018) Association between circulating visfatin and gestational diabetes mellitus: a systematic review and meta-analysis. Acta Diabetol 55:1113–1120. https://doi.org/10.1007/S00592-018-1188-X
Imamura F, Micha R, Wu JHY, de Oliveira Otto MC, Otite FO, Abioye AI, Mozaffarian D (2016) Effects of saturated fat, polyunsaturated fat, monounsaturated fat, and carbohydrate on glucose-insulin homeostasis: a systematic review and meta-analysis of randomised controlled feeding trials. PLoS Med. https://doi.org/10.1371/JOURNAL.PMED.1002087
Hernández EA, Kahl S, Seelig A, Begovatz P, Irmler M, Kupriyanova Y, Nowotny B, Nowotny P, Herder C, Barosa C et al (2017) Acute dietary fat intake initiates alterations in energy metabolism and insulin resistance. J Clin Invest 127:695–708. https://doi.org/10.1172/JCI89444
Jepsen SL, Vestergaard ET, Larraufie P, Gribble FM, Reimann F, Lunde Jørgensen JO, Holst JJ, Kuhre RE (2020) Ghrelin does not directly stimulate secretion of glucagon-like peptide-1. J Clin Endocrinol Metab 105:266–275. https://doi.org/10.1210/CLINEM/DGZ046
Monzani A, Perrone M, Prodam F, Moia S, Genoni G, Testa S, Paglialonga F, Rapa A, Bona G, Montini G et al (2018) Unacylated ghrelin and obestatin: promising biomarkers of protein energy wasting in children with chronic kidney disease. Pediatr Nephrol 33:661–672. https://doi.org/10.1007/S00467-017-3840-Z
Rittig N, Svart M, Thomsen HH, Vestergaard ET, Rehfeld JF, Hartmann B, Holst JJ, Johannsen M, Møller N, Jessen N (2020) Oral D/L-3-hydroxybutyrate stimulates cholecystokinin and insulin secretion and slows gastric emptying in healthy males. J Clin Endocrinol Metab. https://doi.org/10.1210/CLINEM/DGAA483
Železná B, Maixnerová J, Matyšková R, Haugvicová R, Blokešová D, Maletínská L (2009) Anorexigenic effect of cholecystokinin is lost but that of CART (Cocaine and Amphetamine Regulated Transcript) peptide is preserved in monosodium glutamate obese mice. Physiol Res 58:717–723. https://doi.org/10.33549/PHYSIOLRES.931511
Sundström L, Myhre S, Sundqvist M, Ahnmark A, McCoull W, Raubo P, Groombridge SD, Polla M, Nyström AC, Kristensson L et al (2017) The acute glucose lowering effect of specific GPR120 activation in mice is mainly driven by glucagon-like peptide 1. PLoS ONE. https://doi.org/10.1371/JOURNAL.PONE.0189060
Grosse J, Heffron H, Burling K, Hossain MA, Habib AM, Rogers GJ, Richards P, Larder R, Rimmington D, Adriaenssens AA et al (2014) Insulin-like peptide 5 is an orexigenic gastrointestinal hormone. Proc Natl Acad Sci U S A 111:11133–11138. https://doi.org/10.1073/PNAS.1411413111
Shimazu-Kuwahara S, Kanemaru Y, Harada N, Ikeguchi E, Ueda Y, Yamane S, Murata Y, Yasoda A, Kieffer TJ, Inagaki N (2019) Glucose-dependent insulinotropic polypeptide deficiency reduced fat accumulation and insulin resistance, but deteriorated bone loss in ovariectomized mice. J Diabetes Investig 10:909. https://doi.org/10.1111/JDI.12978
Christensen M, Calanna S, Sparre-Ulrich AH, Kristensen PL, Rosenkilde MM, Faber J, Purrello F, Van Hall G, Holst JJ, Vilsbøll T et al (2015) Glucose-dependent insulinotropic polypeptide augments glucagon responses to hypoglycemia in type 1 diabetes. Diabetes 64:72–78. https://doi.org/10.2337/DB14-0440
Behary P, Tharakan G, Alexiadou K, Johnson N, Wewer Albrechtsen NJ, Kenkre J, Cuenco J, Hope D, Anyiam O, Choudhury S et al (2019) Combined GLP-1, oxyntomodulin, and peptide YY improves body weight and glycemia in obesity and prediabetes/type 2 diabetes: a randomized, single-blinded placebo-controlled study. Diabetes Care 42:1446–1453. https://doi.org/10.2337/DC19-0449
AhrÉn B (2003) Oxyntomodulin. Encycl Horm. https://doi.org/10.1016/B0-12-341103-3/00227-8
Hu W, Li N, Yang M, Luo X, Ran W, Liu D, Xiong Z, Liu H, Yang G (2013) Circulating Sfrp5 is a signature of obesity-related metabolic disorders and is regulated by glucose and liraglutide in humans. J Clin Endocrinol Metab 98:290–298. https://doi.org/10.1210/JC.2012-2466
Ouchi N, Higuchi A, Ohashi K, Oshima Y, Gokce N, Shibata R, Akasaki Y, Shimono A, Walsh K (2010) Sfrp5 is an anti-inflammatory adipokine that modulates metabolic dysfunction in obesity. Science 329:454. https://doi.org/10.1126/SCIENCE.1188280
Yang J, Bakshi A, Zhu Z, Hemani G, Vinkhuyzen AAE, Nolte IM, van Vliet-Ostaptchouk JV, Snieder H, Esko T, Milani L et al (2015) Genome-wide genetic homogeneity between sexes and populations for human height and body mass index. Hum Mol Genet 24:7445–7449. https://doi.org/10.1093/HMG/DDV443
Yengo L, Sidorenko J, Kemper KE, Zheng Z, Wood AR, Weedon MN, Frayling TM, Hirschhorn J, Yang J, Visscher PM (2018) Meta-analysis of genome-wide association studies for height and body mass index in ∼700000 individuals of European ancestry. Hum Mol Genet 27:3641–3649. https://doi.org/10.1093/HMG/DDY271
Silventoinen K, Jelenkovic A, Sund R, Yokoyama Y, Hur YM, Cozen W, Hwang AE, Mack TM, Honda C, Inui F et al (2017) Differences in genetic and environmental variation in adult BMI by sex, age, time period, and region: an individual-based pooled analysis of 40 twin cohorts. Am J Clin Nutr 106:457–466. https://doi.org/10.3945/AJCN.117.153643
Fairbrother U, Kidd E, Malagamuwa T, Walley A (2018) Genetics of severe obesity. Curr Diab Rep. https://doi.org/10.1007/S11892-018-1053-X
Ke W, Reed JN, Yang C, Higgason N, Rayyan L, Wahlby C, Carpenter AE, Civelek M, O’Rourke EJ (2021) Genes in human obesity loci are causal obesity genes in C. elegans. PLoS Genet. https://doi.org/10.1371/JOURNAL.PGEN.1009736
Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, Powell C, Vedantam S, Buchkovich ML, Yang J et al (2015) Genetic studies of body mass index yield new insights for obesity biology. Nature 518:197. https://doi.org/10.1038/NATURE14177
Gao W, Liu J-L, Lu X, Yang Q (2021) Epigenetic regulation of energy metabolism in obesity. J Mol Cell Biol 13:480–499. https://doi.org/10.1093/JMCB/MJAB043
Yazdi FT, Clee SM, Meyre D (2015) Obesity genetics in mouse and human: back and forth, and back again. PerrJ. https://doi.org/10.7717/peerj.856
Lee AWS, Cox RD (2010) Use of mouse models in studying type 2 diabetes mellitus. Expert Rev Mol Med. https://doi.org/10.1017/S1462399410001729
Rosenthal N, Brown S (2007) The mouse ascending: perspectives for human-disease models. Nat Cell Biol 9:993–999. https://doi.org/10.1038/ncb437
Oliver PL, Bitoun E, Davies KE (2007) Comparative genetic analysis: the utility of mouse genetic systems for studying human monogenic disease. Mamm Genome 18:412–424. https://doi.org/10.1007/S00335-007-9014-8
Toye AA, Moir L, Hugill A, Bentley L, Quarterman J, Mijat V, Hough T, Goldsworthy M, Haynes A, Hunter AJ et al (2004) A new mouse model of type 2 diabetes, produced by N-ethyl-nitrosourea mutagenesis, is the result of a missense mutation in the glucokinase gene. Diabetes 53:1577–1583. https://doi.org/10.2337/DIABETES.53.6.1577
Cefalu WT, Dawes DE, Gavlak G, Goldman D, Herman WH, Van Nuys K, Powers AC, Taylor SI, Yatvin AL (2018) Insulin access and affordability working group: conclusions and recommendations. Diabetes Care 41:1299–1311. https://doi.org/10.2337/DCI18-0019
Clee SM, Attie AD (2007) The genetic landscape of type 2 diabetes in mice. Endocr Rev 28:48–83. https://doi.org/10.1210/ER.2006-0035
Warrington NM, Howe LD, Paternoster L, Kaakinen M, Herrala S, Huikari V, Wu YY, Kemp JP, Timpson NJ, Pourcain BS et al (2015) A genome-wide association study of body mass index across early life and childhood. Int J Epidemiol 44:700–712. https://doi.org/10.1093/IJE/DYV077
Stratigopoulos G, Burnett LC, Rausch R, Gill R, Penn DB, Skowronski AA, LeDuc CA, Lanzano AJ, Zhang P, Storm DR et al (2016) Hypomorphism of fto and rpgrip1l causes obesity in mice. J Clin Invest 126:1897–1910. https://doi.org/10.1172/JCI85526
Yeo GSH (2017) Genetics of obesity: can an old dog teach us new tricks? Diabetologia 60:778. https://doi.org/10.1007/S00125-016-4187-X
Novoselova TV, Chan LF, Clark AJL (2018) Pathophysiology of melanocortin receptors and their accessory proteins. Best Pract Res Clin Endocrinol Metab 32:93–106. https://doi.org/10.1016/J.BEEM.2018.02.002
Ramos-Molina B, Martin MG, Lindberg I (2016) PCSK1 variants and human obesity. Prog Mol Biol Transl Sci 140:47. https://doi.org/10.1016/BS.PMBTS.2015.12.001
Stijnen P, Ramos-Molina B, O’Rahilly S, Creemers JWM (2016) PCSK1 mutations and human endocrinopathies: from obesity to gastrointestinal disorders. Endocr Rev 37:347–371. https://doi.org/10.1210/ER.2015-1117
Han JC (2016) Rare syndromes and common variants of the brain-derived neurotrophic factor gene in human obesity. Prog Mol Biol Transl Sci 140:75–95. https://doi.org/10.1016/BS.PMBTS.2015.12.002
Franco-Robles E, Campos-Cervantes A, Murillo-Ortiz BO, Segovia J, López-Briones S, Vergara P, Pérez-Vázquez V, Solís-Ortiz MS, Ramírez-Emiliano J (2014) Effects of curcumin on brain-derived neurotrophic factor levels and oxidative damage in obesity and diabetes. Appl Physiol Nutr Metab 39:211–218. https://doi.org/10.1139/APNM-2013-0133
Lewis R, Frodyma D, Neilsen B, Costanzo-Garvey D, Fisher K (2017) Coordinating ERK signaling via the molecular scaffold kinase suppressor of ras. F1000Research. https://doi.org/10.12688/F1000RESEARCH.11895.1
Guo L, Costanzo-Garvey DL, Smith DR, Neilsen BK, MacDonald RG, Lewis RE (2016) Kinase suppressor of Ras 2 (KSR2) expression in the brain regulates energy balance and glucose homeostasis. Mol Metab 6:194–205. https://doi.org/10.1016/J.MOLMET.2016.12.004
Anderson EJP, Çakir I, Carrington SJ, Cone RD, Ghamari-Langroudi M, Gillyard T, Gimenez LE, Litt MJ (2016) 60 YEARS OF POMC: regulation of feeding and energy homeostasis by α-MSH. J Mol Endocrinol 56:T157. https://doi.org/10.1530/JME-16-0014
Rubinstein M, Low MJ (2017) Molecular and functional genetics of the proopiomelanocortin gene, food intake regulation and obesity. FEBS Lett 591:2593–2606. https://doi.org/10.1002/1873-3468.12776
Tian Y, Peng B, Fu X (2018) New ADCY3 variants dance in obesity etiology. Trends Endocrinol Metab 29:361–363. https://doi.org/10.1016/J.TEM.2018.02.004
Felix JF, Bradfield JP, Monnereau C, Van Der Valk RJP, Stergiakouli E, Chesi A, Gaillard R, Feenstra B, Thiering E, Kreiner-Møller E et al (2016) Genome-wide association analysis identifies three new susceptibility loci for childhood body mass index. Hum Mol Genet 25:389–403. https://doi.org/10.1093/HMG/DDV472
Wang BF, Wang Y, Ao R, Tong J, Wang BY (2016) AdipoQ T45 G and G276 T polymorphisms and susceptibility to nonalcoholic fatty liver disease among Asian populations: a meta-analysis and meta-regression. J Clin Lab Anal 30:47–57. https://doi.org/10.1002/jcla.21814
Gong Y, Lee JN, Brown MS, Goldstein JL, Ye J (2006) Juxtamembranous aspartic acid in insig-1 and insig-2 is required for cholesterol homeostasis. Proc Natl Acad Sci 103:6154–6159. https://doi.org/10.1073/PNAS.0601923103
Liu FH, Song JY, Shang XR, Meng XR, Ma J, Wang HJ (2014) The gene-gene interaction of INSIG-SCAP-SREBP pathway on the risk of obesity in Chinese children. Biomed Res Int. https://doi.org/10.1155/2014/538564
Tan LJ, Zhu H, He H, Wu KH, Li J, Chen XD, Zhang JG, Shen H, Tian Q, Krousel-Wood M et al (2014) Replication of 6 obesity genes in a meta-analysis of genome-wide association studies from diverse ancestries. PLoS ONE. https://doi.org/10.1371/JOURNAL.PONE.0096149
Li Q, Chen R, Bie L, Zhao D, Huang C, Hong J (2015) Association of the variants in the PPARG gene and serum lipid levels: a meta-analysis of 74 studies. J Cell Mol Med 19:198–209. https://doi.org/10.1111/JCMM.12417
Goni L, Riezu-Boj JI, Milagro FI, Corrales FJ, Ortiz L, Cuervo M, Martínez JA (2018) Interaction between an ADCY3 genetic variant and two weight-lowering diets affecting body fatness and body composition outcomes depending on macronutrient distribution: a randomized trial. Nutrients. https://doi.org/10.3390/NU10060789
Choy RKM, Thomas JH (1999) Fluoxetine-resistant mutants in C. elegans define a novel family of transmembrane proteins. Mol Cell 4:143–152. https://doi.org/10.1016/S1097-2765(00)80362-7
Wu L, Zhou B, Oshiro-Rapley N, Li M, Paulo JA, Webster CM, Mou F, Kacergis MC, Talkowski ME, Carr CE et al (2016) An ancient, unified mechanism for metformin growth inhibition in C. elegans and cancer. Cell 167:1705. https://doi.org/10.1016/J.CELL.2016.11.055
Wang MC, O’Rourke EJ, Ruvkun G (2008) Fat metabolism links germline stem cells and longevity in C. elegans. Science 322:957–960. https://doi.org/10.1126/SCIENCE.1162011
Long X, Spycher C, Han ZS, Rose AM, Müller F, Avruch J (2002) TOR deficiency in C. elegans causes developmental arrest and intestinal atrophy by inhibition of MRNA translation. Curr Biol 12:1448–1461. https://doi.org/10.1016/S0960-9822(02)01091-6
McKay RM, McKay JP, Avery L, Graff JMC (2003) elegans: a model for exploring the genetics of fat storage. Dev Cell 4:131–142. https://doi.org/10.1016/S1534-5807(02)00411-2
Szø JY, Victor M, Loer C, Shi Y, Ruvkun G (2000) Food and metabolic signalling defects in a Caenorhabditis elegans serotonin-synthesis mutant. Nat 403:560–564. https://doi.org/10.1038/35000609
Goh GYS, Winter JJ, Bhanshali F, Doering KRS, Lai R, Lee K, Veal EA, Taubert S (2018) NHR-49/HNF4 integrates regulation of fatty acid metabolism with a protective transcriptional response to oxidative stress and fasting. Aging Cell 17:e12743. https://doi.org/10.1111/ACEL.12743
Rankinen T, Zuberi A, Chagnon YC, Weisnagel SJ, Argyropoulos G, Walts B, Pérusse L, Bouchard C (2006) The human obesity gene map: the 2005 update. Obesity (Silver Spring) 14:529–644. https://doi.org/10.1038/OBY.2006.71
Saunders CL, Chiodini BD, Sham P, Lewis CM, Abkevich V, Adeyemo AA, De Andrade M, Arya R, Berenson GS, Blangero J et al (2007) Meta-analysis of genome-wide linkage studies in BMI and obesity. Obesity (Silver Spring) 15:2263–2275. https://doi.org/10.1038/OBY.2007.269
Hara K, Yamauchi T, Kadowaki T (2005) Adiponectin: an adipokine linking adipocytes and type 2 diabetes in humans. Curr Diabetes Reports 5:136–140. https://doi.org/10.1007/S11892-005-0041-0
Musso G, Gambino R, Cassader M (2010) Obesity, diabetes, and gut microbiota: the hygiene hypothesis expanded? Diabetes Care 33:2277–2284. https://doi.org/10.2337/DC10-0556
de Vadder F, Kovatcheva-datchary P, Goncalves D, Vinera J, Zitoun C, Mithieux G (2013) Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. https://doi.org/10.1016/j.cell.2013.12.016
Neish AS (2009) Microbes in gastrointestinal health and disease. Gastroenterology 136:65. https://doi.org/10.1053/J.GASTRO.2008.10.080
Sanchez M, Panahi S, Tremblay A (2014) Childhood obesity: a role for gut microbiota? Int J Environ Res Public Health 12:162–175. https://doi.org/10.3390/IJERPH120100162
Kim JY, Park YJ, Lee HJ, Park MY, Kwon O (2018) Effect of lactobacillus Gasseri BNR17 on irritable bowel syndrome: a randomized, double-blind, placebo-controlled, dose-finding trial. Food Sci Biotechnol 27:853–857. https://doi.org/10.1007/s10068-017-0296-7
Markowiak P, Ślizewska K (2017) Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 9:1021
Lindsay KL, Kennelly M, Culliton M, Smith T, Maguire OC, Shanahan F, Brennan L, McAuliffe FM (2014) Probiotics in obese pregnancy do not reduce maternal fasting glucose: a double-blind, placebo-controlled, randomized trial (Probiotics in Pregnancy Study). Am J Clin Nutr 99:1432–1439. https://doi.org/10.3945/AJCN.113.079723
Brahe LK, Le Chatelier E, Prifti E, Pons N, Kennedy S, Blædel T, Håkansson J, Dalsgaard TK, Hansen T, Pedersen O et al (2015) Dietary modulation of the gut microbiota–a randomised controlled trial in obese postmenopausal women. Br J Nutr 114:406–417. https://doi.org/10.1017/S0007114515001786
Minami JI, Kondo S, Yanagisawa N, Odamaki T, Xiao JZ, Abe F, Nakajima S, Hamamoto Y, Saitoh S, Shimoda T (2015) Oral administration of bifidobacterium breve B-3 modifies metabolic functions in adults with obese tendencies in a randomised controlled trial. J Nutr Sci 4:e17. https://doi.org/10.1017/JNS.2015.5
Stenman LK, Lehtinen MJ, Meland N, Christensen JE, Yeung N, Saarinen MT, Courtney M, Burcelin R, Lähdeaho ML, Linros J et al (2016) Probiotic with or without fiber controls body fat mass, associated with serum zonulin, in overweight and obese adults-randomized controlled trial. EBioMedicine 13:190–200. https://doi.org/10.1016/J.EBIOM.2016.10.036
Sanchez M, Darimont C, Panahi S, Drapeau V, Marette A, Taylor VH, Doré J, Tremblay A (2017) Effects of a diet-based weight-reducing program with probiotic supplementation on satiety efficiency, eating behaviour traits, and psychosocial behaviours in obese individuals. Nutrients. https://doi.org/10.3390/NU9030284
Kim M, Kim M, Kang M, Yoo HJ, Kim MS, Ahn YT, Sim JH, Jee SH, Lee JH (2017) Effects of weight loss using supplementation with lactobacillus strains on body fat and medium-chain acylcarnitines in overweight individuals. Food Funct 8:250–261. https://doi.org/10.1039/C6FO00993J
Pedret A, Valls RM, Calderón-Pérez L, Llauradó E, Companys J, Pla-Pagà L, Moragas A, Martín-Luján F, Ortega Y, Giralt M et al (2018) Effects of daily consumption of the probiotic bifidobacterium animalis Subsp. lactis CECT 8145 on anthropometric adiposity biomarkers in abdominally obese subjects: a randomized controlled trial. Int J Obes (Lond) 43:1863–1868. https://doi.org/10.1038/S41366-018-0220-0
Khan MJ, Gerasimidis K, Edwards CA, Shaikh MG (2016) Role of gut microbiota in the aetiology of obesity: proposed mechanisms and review of the literature. J Obes. https://doi.org/10.1155/2016/7353642
Johnson CN, Kogut MH, Genovese K, He H, Kazemi S, Arsenault RJ (2019) Administration of a postbiotic causes immunomodulatory responses in broiler gut and reduces disease pathogenesis following challenge. Microorganisms. https://doi.org/10.3390/microorganisms7080268
Abbas Z, Sammad A, Hu L, Fang H, Xu Q, Wang Y (2020) Glucose metabolism and dynamics of facilitative glucose transporters (GLUTs) under the influence of heat stress in dairy cattle. Metabolites 10:312. https://doi.org/10.3390/METABO10080312
Lin HV, Frassetto A, Kowalik EJ, Nawrocki AR, Lu MM, Kosinski JR, Hubert JA, Szeto D, Yao X, Forrest G et al (2012) Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS ONE. https://doi.org/10.1371/JOURNAL.PONE.0035240
Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, Cefalu WT, Ye J (2009) Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 58:1509–1517. https://doi.org/10.2337/DB08-1637
De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, Bäckhed F, Mithieux G (2014) Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 156:84–96. https://doi.org/10.1016/J.CELL.2013.12.016
Li G, Yao W, Jiang H (2014) Short-chain fatty acids enhance adipocyte differentiation in the stromal vascular fraction of porcine adipose tissue. J Nutr 144:1887–1895. https://doi.org/10.3945/jn.114.198531
Den Besten G, Bleeker A, Gerding A, Van Eunen K, Havinga R, Van Dijk TH, Oosterveer MH, Jonker JW, Groen AK, Reijngoud DJ et al (2015) Short-chain fatty acids protect against high-fat diet-induced obesity via a PPARγ-dependent switch from lipogenesis to fat oxidation. Diabetes 64:2398–2408. https://doi.org/10.2337/DB14-1213
Al-Lahham S, Roelofsen H, Rezaee F, Weening D, Hoek A, Vonk R, Venema K (2012) Propionic Acid affects immune status and metabolism in adipose tissue from overweight subjects. Eur J Clin Invest 42:357–364. https://doi.org/10.1111/J.1365-2362.2011.02590.X
Al-Lahham SH, Roelofsen H, Priebe M, Weening D, Dijkstra M, Hoek A, Rezaee F, Venema K, Vonk RJ (2010) Regulation of adipokine production in human adipose tissue by propionic acid. Eur J Clin Invest 40:401–407. https://doi.org/10.1111/J.1365-2362.2010.02278.X
Marzec A, Feleszko W (2020) Postbiotics—a step beyond pre- and probiotics 2. 1–17
do Peluzio M, Martinez JA, Milagro FI (2021) Postbiotics: metabolites and mechanisms involved in microbiota-host interactions. Trends Food Sci Technol 108:11–26. https://doi.org/10.1016/j.tifs.2020.12.004
Moffett JR, Puthillathu N, Vengilote R, Jaworski DM, Namboodiri AM (2020) Acetate revisited: a key biomolecule at the nexus of metabolism, epigenetics, and oncogenesis—part 2: acetate and ACSS2 in health and disease. Front Physiol 11:1451. https://doi.org/10.3389/FPHYS.2020.580171/BIBTEX
Hong YH, Nishimura Y, Hishikawa D, Tsuzuki H, Miyahara H, Gotoh C, Choi KC, Feng DD, Chen C, Lee HG et al (2005) Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology 146:5092–5099. https://doi.org/10.1210/en.2005-0545
Christiansen CB, Gabe MBN, Svendsen B, Dragsted LO, Rosenkilde MM, Holst JJ (2018) The impact of short-chain fatty acids on Glp-1 and Pyy secretion from the isolated perfused rat colon. Am J Physiol Gastrointest Liver Physiol 315:G53–G65. https://doi.org/10.1152/ajpgi.00346.2017
Frost G, Sleeth ML, Sahuri-Arisoylu M, Lizarbe B, Cerdan S, Brody L, Anastasovska J, Ghourab S, Hankir M, Zhang S et al (2014) The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun. https://doi.org/10.1038/NCOMMS4611
Perry RJ, Peng L, Barry NA, Cline GW, Zhang D, Cardone RL, Petersen KF, Kibbey RG, Goodman AL, Shulman GI (2016) Acetate mediates a microbiome-brain-β cell axis promoting metabolic syndrome. Nature 534:213. https://doi.org/10.1038/NATURE18309
Le Poul E, Loison C, Struyf S, Springael JY, Lannoy V, Decobecq ME, Brezillon S, Dupriez V, Vassart G, Van Damme J et al (2003) Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem 278:25481–25489. https://doi.org/10.1074/JBC.M301403200
McNabney SM, Henagan TM (2017) Short chain fatty acids in the colon and peripheral tissues: a focus on butyrate, colon cancer, obesity and insulin resistance. Nutrients. https://doi.org/10.3390/nu9121348
Kasubuchi M, Hasegawa S, Hiramatsu T, Ichimura A, Kimura I (2015) Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients 7:2839–2849. https://doi.org/10.3390/nu7042839
Zhang L, Liu C, Jiang Q, Yin Y (2021) Butyrate in energy metabolism: there is still more to learn. Trends Endocrinol Metab 32:159–169. https://doi.org/10.1016/j.tem.2020.12.003
Thaiss CA, Itav S, Rothschild D, Meijer MT, Levy M, Moresi C, Dohnalová L, Braverman S, Rozin S, Malitsky S et al (2016) Persistent microbiome alterations modulate the rate of post-dieting weight regain. Nature. https://doi.org/10.1038/nature20796
Perry RJ, Peng L, Barry NA, Cline GW, Zhang D, Cardone RL, Petersen KF, Kibbey RG, Goodman AL, Shulman GI (2016) Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature 534:213–217. https://doi.org/10.1038/NATURE18309
Cavallari JF, Barra NG, Foley KP, Lee A, Duggan BM, Henriksbo BD, Anhê FF, Ashkar AA, Schertzer JD (2020) Postbiotics for NOD2 require nonhematopoietic RIPK2 to improve blood glucose and metabolic inflammation in mice. Am J Physiol Endocrinol Metab 318:E579–E585. https://doi.org/10.1152/ajpendo.00033.2020
Rodriguez-Nunez I, Caluag T, Kirby K, Rudick CN, Dziarski R, Gupta D (2017) Nod2 and Nod2-regulated microbiota protect BALB/c mice from diet-induced obesity and metabolic dysfunction. Sci Rep. https://doi.org/10.1038/S41598-017-00484-2
Yan H, Fei N, Wu G, Zhang C, Zhao L, Zhang M (2016) Regulated inflammation and lipid metabolism in colon MRNA expressions of obese germfree mice responding to enterobacter cloacae B29 combined with the high fat diet. Front Microbiol 7:1786. https://doi.org/10.3389/FMICB.2016.01786/BIBTEX
Woting A, Pfeiffer N, Loh G, Klaus S, Blaut M (2014) Clostridium ramosum promotes high-fat diet-induced obesity in gnotobiotic mouse models. MBio 5:1530–1544. https://doi.org/10.1128/MBIO.01530-14/SUPPL_FILE/MBO005142009ST3.DOC
Caesar R, Tremaroli V, Kovatcheva-Datchary P, Cani PD, Bäckhed F (2015) Crosstalk between gut microbiota and dietary lipids aggravates WAT inflammation through TLR signaling. Cell Metab 22:658–668. https://doi.org/10.1016/J.CMET.2015.07.026
Ilievski V, Cho Y, Katwala P, Rodriguez H, Tulowiecka M, Kurian D, Leoni L, Christman JW, Unterman TG, Watanabe K (2015) TLR4 expression by liver resident cells mediates the development of glucose intolerance and insulin resistance in experimental periodontitis. PLoS ONE 10:1–22. https://doi.org/10.1371/journal.pone.0136502
Lackey DE, Olefsky JM (2016) Regulation of metabolism by the innate immune system. Nat Rev Endocrinol 12:15–28. https://doi.org/10.1038/nrendo.2015.189
Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C et al (2007) Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56:1761–1772. https://doi.org/10.2337/db06-1491
Muccioli GG, Naslain D, Bäckhed F, Reigstad CS, Lambert DM, Delzenne NM, Cani PD (2010) The endocannabinoid system links gut microbiota to adipogenesis. Mol Syst Biol. https://doi.org/10.1038/MSB.2010.46
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
ZC and HW conceived the idea and outlined the sketch. WW wrote the initial manuscript. JH, LQ assisted in the literature collection and figures. LC, ZC and HW revised the final draft and made the corrections. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, W., Chen, Z., Han, J. et al. Endocrine, genetic, and microbiome nexus of obesity and potential role of postbiotics: a narrative review. Eat Weight Disord 28, 84 (2023). https://doi.org/10.1007/s40519-023-01593-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40519-023-01593-w