Abstract
Purpose
Sleep impairment is reported to be a consequence of overweight and obesity. However, the weight–sleep relationship can alternately be explained by demographics (e.g. age) and covariates (i.e. mood/affect and behaviour in overweight/obese people; e.g. night-eating). Thus, we examined the weight–sleep quality relationship after controlling for the effects of affect and common behaviour (i.e. night-eating, insufficient exercise, alcohol and electronic device use).
Methods
Online questionnaires asked 161 overweight, obese or normal-weight participants about their sleep quality, night-eating, physical activity, alcohol use, electronic device use and anxiety and depression at T0 (baseline) and T1 (3 months later). Height and weight and waist and hip circumference were objectively measured at T0 and T1, and physical activity was assessed over 24 h (using actigraphy) at T0 and T1. Hierarchical multiple regression analyses evaluated whether the weight measures (i.e. body-mass-index [BMI], waist-to-hip ratio [WHR] and obesity category [overweight/obese vs. normal-weight]) predicted sleep quality and its components at T0 and T1, after controlling demographics (at step 1) and covariates (affective distress and behaviour) at step 2, and entering weight measures at step 3; maximum 8 variables in the analyses.
Results
High BMI predicted several aspects of sleep quality after taking into account co-existing behaviour, affect and demographics: sleep disturbances at T0 and lower sleep efficiency at T1. WHR and obesity category did not predict any aspects of sleep quality. Several co-existing behaviour were related to or predicted sleep quality score and aspects of sleep quality including night-eating, alcohol use and electronic device use and affective symptoms (i.e. anxiety, depression).
Conclusion
Results suggest that a person’s weight may impact on their sleep quality above and beyond the effects of their co-existing behaviour and affect, although their co-existing behaviour and affect may also adversely impact on sleep quality.
Level of evidence
Level III, evidence obtained from well-designed cohort.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Overweight and obesity (e.g. as indexed by high body-mass-index [BMI]) has been shown to be associated with impaired sleep in adults [1, 2] in objective and subjective sleep studies. The finding is interpreted as evidence that impaired sleep is the consequence of a person’s weight [2, 3]. However, as detailed below, the relationship between it and sleep quality may be better explained by the affect (e.g. depression) and behaviour (e.g. night-eating) of overweight people, which may alternately contribute to the sleep impairments.
Clinically, obese patients are reported to have longer awake time, less sleep efficiency (SE; i.e. lower percentage of sleep time during the night), and they are sleepier during the day, relative to normal-weight controls [3]. Similarly, in the community setting, high BMI and overweight/obesity are linked to shorter self-reported sleep duration, but, as it is also linked to high emotional stress, it may not indicate true sleep loss [4]. That is to say, obesity and poor sleep quality often co-exist with emotional stress and affective distress (e.g. depression) [5]; for example, anxiety and depression are linked to shorter sleep duration and poor quality sleep [6] and overweight/obese people report higher distress levels than normal-weight people [7]. Thus, affective distress may better explain the documented relationship between weight and sleep.
High BMI has also been shown to be related to excessive daytime sleepiness (EDS) as indexed by objective and subjective sleep tests [5]. In a large obesity study, high BMI, metabolic disturbance (i.e. diabetes, insulin resistance), depression and sleep apnoea all predicted inadequate or disturbed sleep, but so did physical activity levels [8]; suggesting that a sedentary lifestyle and insufficient activity can also impair sleep. Similarly, people who fail to meet the activity guidelines (150 min of moderate-intensity, 75 min of vigorous-intensity activity or combination of both) are more likely to report daytime sleepiness than those who do meet them [9] whereas regular activity improves a person’s sleep (e.g. reduced sleep-onset latency [SOL], longer total sleep time) [10]. However, overweight/obese people are more likely to report insufficient physical activity than normal-weight people [11]. Thus, insufficient physical activity may provide a better explanation of the observed weight–sleep relationship.
Excessive night-eating may also provide a better explanation of the weight–sleep relationship. It can impair multiple aspects of sleep including subjective difficulty initiating and maintaining sleep [12] and objective sleep impairments (e.g. less SE, long SOL, more sleep arousals) [13]. In addition, once a person’s sleep is impaired, they are more likely to experience increased appetite, lower leptin levels (i.e. adipose tissue hormone suppressing appetite) and higher ghrelin levels (i.e. peptide that stimulates appetite) [14, 15]. Moreover, night-eating is highly correlated with weight (e.g. high BMI, obesity category) [16] and it is likely to contribute to weight gain and overweight/obesity over time [17].
Finally, other behaviour may better explain the weight–sleep relationship. For example, heavy alcohol use can interfere with sleep induction and maintenance [18], and it is linked to weight gain and overweight/obesity [19]. Similarly, electronic device use can interfere with sleep quality [20] and it is linked to overweight/obesity [21]. Further, demographics may potentially confound the relationship between weight and sleep including older age which is linked to difficulty initiating and maintaining sleep [22] as well as higher BMI [23] and less physical activity [24], night-eating [25] and electronic device use [26]) whereas female gender is associated with poor sleep quality [27] but less exercise [28] and obesity [29]).
Thus, assorted findings in the literature suggest that the weight–sleep relationship can be better explained by demographics (e.g. gender), affective distress (e.g. depression) and frequent behaviour (e.g. night-eating) in affected individuals. However, it is unclear which of these factors can best account for the weight–sleep relationship as no prior comparison studies have been conducted. Therefore, we examined whether affective distress and frequent behaviour could alternately explain the weight–sleep relationship by examining if the weight–sleep relationship still existed, after controlling for the putative effects of these factors on sleep in the planned analyses.
Specifically, we tested three weight measures (i.e. BMI, waist-to-hip ratio [WHR], obesity category) as predictors of total sleep quality and sleep quality components at baseline (T0) and 3 months later (T1), after controlling for the effects of the aforementioned demographics and covariates (i.e. affective distress and co-existing behaviour). Two measurement time points (T0 and T1) were used to determine whether the observed results were consistent over time; and, a 3-month interval was chosen between T0 and T1 as it permitted sufficient change to occur in the sleep quality components (i.e. dependent variables), which are reported to be labile over short intervals (e.g. 3 months) [30]. However, we did not expect that significant changes will occur in participant’s weight over 3 months [31], although their behaviour and affect may change over 3 months.
Thus, consistent with the prior literature, we expected that the:
-
1.
Measures of weight (BMI, obesity category) at T0 will no longer predict poor total sleep quality at T0 and T1, after controlling for the effects of the aforementioned covariates and demographics; and,
-
2.
Measures of weight (BMI, obesity category) at T0 will no longer predict the components of sleep quality at T0 and T1, after controlling for the effects of the covariates and demographics. Specific hypotheses were not provided for WHR as it has not previously been evaluated in regards to sleep quality. Nonetheless, as it indexes abdominal obesity [32], it permits an examination of whether abdominal obesity is more (or less) strongly related to sleep quality than other measures of weight.
Methods
Participants
Participants were recruited via social networking websites (e.g. Facebook, Gumtree), Australian National University (ANU) Psychology Research Participation Scheme, sleep centres (e.g. Canberra Sleep Clinic), local magazines (e.g. Canberra Weekly), and email snowballing. Criteria for study inclusion were: age of 18–65 years, resided in Canberra, Australia, and BMI ≥ 18.5 (BMI = weight (kg)/height (m) squared; World Health Organisation [WHO]) [33], that is, normal-weight, overweight or obese. The study was granted full approval by the ANU Human Research Ethics Committee (protocol #2015/013).
Potential participants (n = 165) clicked on the URL embedded in the study advertisement and 162 of them completed the T0 questionnaire (participation rate = 98%), but one did not complete the actigraphy phase of the study, leaving 161 participants. Three months later (T1), most of them (n = 155) completed the T1 questionnaire and second actigraphy phase, resulting in an attrition rate of 3.7% (6/161). No remuneration was provided to the participants, but the ANU student participants could receive 3 course credit points for hours of study participation.
Procedure
Interested individuals clicked on the URL imbedded in the advertisement, read the study information page and indicated their consent to participate. They were then asked to provide their height (in metres) and weight (in kg) to calculate their BMI. If their self-reported BMI was < 18.5 they were redirected to the end of the survey and thanked for their time. If their BMI ≥ 18.5, they were asked to complete the T0 questionnaire which asked about demographics, sleep quality, concurrent behaviour and affective distress.
After completing the T0 questionnaire, they were sent an email asking them to organise a time to meet the researcher (SE) face-to-face to fit the physiological monitoring equipment, either in a research room at ANU or in their home. At this meeting, participant’s height, weight, and waist and hip circumferences were objectively measured. Height was measured using a Seca 213 Portable Stadiometer and weight was measured using Seca 750 mechanical floor scales. BMI was calculated according to the formula: BMI = weight (kg)/height2 (m2) [33]. Waist and hip circumference were measured using a 152-cm soft measuring tape wrapped around the narrowest part of the waist and widest part of the hips to calculate WHR: waist circumference (cm)/hip circumference (cm) [34]. Objectively measured BMI and WHR were the predictor variables in this study, along with obesity category (overweight/obese vs. normal-weight), using standard WHO categories: normal-weight (BMI: 18.5–24.9), overweight (BMI: 25–29.9), and obese (BMI ≥ 30) [33].
Participants were then fitted with a non-invasive watch-like monitoring device (Actical®) on the wrist, either left or right, according to preference, and using a disposable sterile wristband, to objectively monitor their physical activity levels over 24 h. The Actical® system’s menu was used to configure the devices for each participant, by inputting their study ID number, age, gender, height and weight. Participants wore the devices continuously for 24 h, after which time, they returned them to the researcher.
Three months later, participants were sent an email with a URL link to the T1 questionnaire which asked them the same questions as the T0 questionnaire. After completing the questionnaire, they were again asked to meet the researcher face-to-face to have their height, weight, and waist and hip circumference objectively measured and the Actical® device fitted. They wore the device for 24 h and then returned it to the researcher. No participants removed their device during the two 24-h monitoring periods.
Apparatus
Physical activity (total number of steps) was assessed over 24 h at T0 and T1 using the Actical® accelerometers (Phillips, Respironics), which tolerate normal daily activities such as showering. The devices recorded activity using a 1-min epoch length. Data held on the devices were downloaded using ActiReader® communications interface software via a wireless link. The devices have been used to monitor physiological [35, 36] and behavioural parameters [37] in humans and they show moderate convergent validity with other objective measures of physical activity [38].
Materials
Sleep quality was assessed using the Pittsburgh Sleep Quality Index (PSQI; 19 items) which assesses total sleep quality, subjective sleep quality (SSQ), sleep-onset latency (SOL), sleep duration, habitual sleep efficiency (SE), sleep disturbances (SD), sleep medication use (SM) and daytime dysfunction (DD). Participants were asked to rate their agreement with each item using 4-point scales ranging from 0 (not in the past month) to 3 (three or four times a month), with high scores indicating poorer sleep. PSQI is a valid measure of sleep quality in clinical and non-clinical samples: it distinguishes between good vs. poor sleepers and it has moderate convergent validity with objective sleep measures [39] and information collated from sleep diaries [40]. PSQI is reported to have high internal consistency with Cronbach’s α (CA) of 0.80 [41]. In this study, PSQI total score had adequate internal consistency at T1 with CA of 0.72 and SD (9 items) had a CA of 0.63. Internal consistency was not calculated for the other PSQI subscales as they were comprised of only one or two items.
Physical activity was assessed using the Rapid Assessment of Physical Activity (RAPA; 9 items) [42] which asks about exercise intensity, using yes/no responses. Participants were provided with a definition of physical activity and examples of light, moderate and vigorous activity to assist in rating their current activity on two scales: physical activity and strength/flexibility. RAPA has adequate test–retest reliability (r = 0.65) [43] and is a valid measure of activity that distinguishes between people who do/do not undertake moderate exercise [44]. In this study, RAPA had adequate test–retest reliability (r = 0.72) over 3 months. Using the RAPA, participants were considered to be sufficiently active [45] if they had spent ≥ 150-min engaged in moderate-intensity activity or 75-min vigorous-intensity activity/week.
Physical activity was also assessed using the Global Physical Activity Questionnaire-Version 2 (GPAQv2) [46, 47] that collects activity information across three domains: occupational, transportation and leisure-time activity. GPAQ asks if a person has engaged in moderate or vigorous-intensity exercise in each activity domain. If they answered yes, they were asked how many days they engaged in the activities in a typical week and how long they spent doing the activities on a typical day. GPAQ data was cleaned and screened using GPAQ guidelines [48] and sub-scores were calculated for each activity domain based on the average number of days, hours and minutes spent doing the activity each week. A measure of total physical activity (GPAQ score) taking into account the intensity of the activities (by way of metabolic equivalents [METs]) was calculated. GPAQ score has adequate test–retest reliability (r = 0.67) [49] and it is a valid measure of physical activity [47]. In this study, GPAQ score had adequate test–retest reliability (r = 0.64) over 3 months and it was more highly correlated with the actigraphy-derived activity data suggesting that it more closely indexed whether participants met the CDC requirements for optimal physical activity [50]. Although RAPA and GPAQ scores were moderately correlated (r = 0.32–0.47), RAPA scores were poorly correlated with the objective activity measures (see supplementary file).
Night-eating was assessed using the Night-Eating Questionnaire (NEQ, 14 items) that detects the presence of night-eating syndrome (NES) in adults across four subscales: morning anorexia, evening hyperphagia, nocturnal ingestions and mood/sleep problems. It also detects night-eating in people without NES (e.g. university students) [51] especially in high stress situations [52]. Night-eating severity was assessed using 5-point scales ranging from 0 (not at all) to 4 (very often), with high scores indicating more night-eating. NEQ score ≥ 25 indicates possible NES. NEQ score has adequate internal consistency with a CA of 0.7 and good convergent validity [53]. In this study, internal consistency of total NEQ score was adequate with a CA of 0.66 at T1. Criteria for NES include: consuming ≥ 50% of daily energy intake after the evening meal, eating after waking from sleep and morning anorexia [54].
Other behaviour (that can disrupt sleep) was examined including alcohol use and electronic device use (including mobile phone and TV use). Participants were asked if they had consumed alcohol or engaged in the activity (yes/no), and if so, the amount (1–5 to > 20 drinks), frequency (every day, 3–4 times per week, twice a week, once a week, once a fortnight, once a month, < once a month, never) and time of day (morning, afternoon, evening, late night) of the use/behaviour. Participants listed all the electronic devices they had used in a typical day along with the number of hours each day and the time/s of day of the use.
Affective distress was assessed using the Depression Anxiety Stress Scales-21 (DASS-21; 21 items) that assesses the presence and severity of depression and anxiety symptoms. Participants were asked to rate each item using 4-point scales ranging from 0 (did not apply to me at all) to 3 (applied to me very much/most of the time), with high scores reflecting more distress (e.g. severe depression (DASS-D > 21) and severe anxiety (DASS-A > 15). The scale has high internal consistency with CAs of 0.84 for anxiety and 0.91 for depression [55] in non-clinical [56] and clinical samples [57]. In this study, its internal consistency was high with CAs of 0.88 for depression and 0.81 for anxiety.
Statistical analyses
An a priori power analysis using G*power (version 3.0.10) estimated that 139 people were required to detect a medium effect size (ƒ2 = 0.15) with alpha set at 0.05, power of 0.8 and using up to 10 predictors in the analyses. Thus, 161 participants were deemed sufficient to conduct the cross-sectional analyses and 155 participants were sufficient for the longitudinal analyses.
Routine statistical analyses were performed using the statistical analysis program SPSS (Version 24). Analysis of Variance (ANOVA) and Chi-square tests evaluated whether the study variables differed between T0 and T1. Hierarchical multiple regression analyses examined the predictors of total sleep quality and PSQI sub-scores (SSQ, SOL, sleep duration, SE, SD, SM, DD) at T0 and T1, after controlling for the effects of demographics (i.e. age, gender) at step 1 of the analysis. Covariates (i.e. behaviour and affective distress) were entered at step 2 and the weight predictor variables (i.e. BMI, WHR, obesity category) were entered at step 3 of the analyses. As a result of the large number of variables in the study, only those that were significantly correlated with the sleep quality variables were included in the analyses, with significance set at p < 0.05.
Results
Means, standard deviations and correlations between the study variables are presented in Tables 1a–i, 2 (see supplementary file for Tables 1a–i).
Sample description
Of the 161 participants, 67 (42%) were male and 94 (58%) were female and the mean age was 26.8-years (range: 18–65 years, SD = 9.45). Repeated-measures ANOVA showed their age did not vary significantly between T0 and T1 (M = 26.8 vs. 26.9 years, SD = 9.5 vs. 9.6, F33, 121 = 0.98, p = 0.52). Chi-square tests showed that they did not differ in terms of gender (χ21 = 0.49, p = 0.48), marital status (χ25 = 4.42, p = 0.49), education (χ24 = 3.05, p = 0.55) or employment (χ26 = 2.57, p = 0.86) between T0 and T1. Nearly two-thirds of them were single (62%, N = 100), 24% (N = 38) were married and the rest were divorced/separated (N = 5), lived with someone (N = 12) or did not want to say (N = 6). More than one-half had finished an undergraduate (29%, N = 47) or postgraduate (24%, N = 39) degree and the rest had finished a diploma (N = 9) or Year 12 (39%, N = 63) or Year 10 or below at high school (N = 3). More than one-half of them were students (60%, N = 96), 20% worked full-time (N = 32) and the rest worked part-time/casual (16%, N = 26), completed home duties (N = 3), were unemployed (N = 1) or permanently unable to work or ill (N = 2).
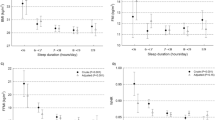
At baseline (T0), in terms of sleep, 83% of them had poor sleep quality (PSQI score ≥ 5; PSQI cutoff score) [39]. One-half of them took longer than 30 min to fall asleep (SOL), nearly 20% had sleep duration < 7 h, 42% had low sleep efficiency (SE < 85%), 98% reported sleep disturbances (SD), 83% had moderate daytime dysfunction (DD), and 17% used sleep medications (SM). In terms of weight, over one-half of them was normal weight, 22% were overweight, and 22% were obese at T0, using WHO weight classifications [33]; see Table 1.
In terms of behaviour and affect, few (14%) reported substantial night-eating (NEQ score > 25) [58] but many of them (70%) were sufficiently active using GPAQ [50] although fewer (29%) were sufficiently active using RAPA cut-off scores [45]; but as the latter figure is closest to the actigraphy-derived data, GPAQ score most likely accurately reflected activity levels. Finally, some participants reported severe depression (19%) and/or anxiety (32%) symptoms relative to DASS-21 cutoff scores [55]. Thus, relative to Australian adult prevalence figures or normed values, fewer of them was overweight/obese [59], physically inactive [45] and clinically significant night-eaters [25] than was expected, but more of them were affectively distressed [60]; see Table 2.
Three months later (baseline [T0] to [T1]), participant’s sleep quality slightly improved (SSQ, SOL, sleep duration, SD, DD) as did their night-eating, anxiety and depression, and their alcohol intake decreased and TV viewing increased; see Table 2. Such small changes are not likely due to demographic changes over time as only six people dropped out of the study before T1. It is likely that the changes were due to normal fluctuations in symptoms and behaviour that are linked to seasonal changes [61, 62] or changing life circumstances (e.g. life-event stress) [63, 64].
Described below are a series of hierarchical multiple regression analyses evaluating whether the weight measures still predicted total sleep quality score and the seven sleep quality components, at T0 and T1, after controlling for the effects of the aforementioned affective and behavioural measures.
Predictors of total PSQI score
Cross-sectional predictors of sleep quality (PSQI score) were examined at T0. Covariates (i.e. depression, anxiety, alcohol intake, work-physical activity, and night-eating) were entered at step 1 of the analysis but as no demographics or weight variables were correlated with it they were not entered into the analysis. In the analysis, anxiety and depression levels, night-eating and alcohol intake were associated with high PSQI score, but not the weight measures; see Table 3.
Longitudinal predictors of sleep quality (PSQI score) were examined at T1. PSQI T0 and age were entered at step 1 and covariates (i.e. depression, anxiety, work-physical activity, and night-eating) were entered at step 2 of the analysis. None of the weight measures was correlated with it so they were not entered into the analysis. In the analysis, none of the variables predicted PSQI score at T1, after controlling for the effects of T0 PSQI score and age; see Table 3.
Predictors of subjective sleep quality
Cross-sectional predictors of SSQ were examined at T0. Demographics (i.e. age) were entered at step 1, covariates (i.e. depression, anxiety, work-physical activity, alcohol intake, and night-eating) were entered at step 2 and the weight variables (i.e. WHR) were entered at step 3 of the analysis. After controlling for the effects of the variables, only depression levels, alcohol intake and night-eating were related to poor SSQ; see Table 4.
Longitudinal predictors of SSQ were examined at T1. T0 SSQ was entered at step 1, covariates (i.e. depression, anxiety, alcohol intake, # steps, night-eating) were entered at step 2. No weight variables were correlated with SSQ so they were not entered in the analysis. After controlling for the effects of T0 SSQ, only alcohol intake predicted SSQ at T1; see Table 4.
Predictors of sleep-onset latency
Cross-sectional predictors of SOL were examined at T0. Demographics (i.e. age) were entered at step 1, covariates (i.e. depression, anxiety, alcohol intake, alcohol frequency, and night-eating) were entered at step 2 but as no weight variables were correlated with it they were not entered into the analysis. After controlling for the effects of the variables, only night-eating was related to longer SOL; see Table 4.
Longitudinal predictors of SOL were examined at T1. T0 SOL and age were entered at step 1 and covariates (i.e. depression, anxiety, alcohol intake, alcohol frequency, and night-eating) were entered at step 2. No weight variables were correlated with it so they were not entered into the analysis. After controlling for the effects of the variables, only frequency of alcohol intake predicted SOL at T1; see Table 4.
Predictors of sleep duration
Cross-sectional predictors of sleep duration were examined at T0. No demographics or weight measures were correlated with it so they were not entered into the analysis and as only anxiety was correlated with it, only anxiety was entered at step 1 of the analysis and only it was related to shorter sleep duration at T0; see Table 5
Longitudinal predictors of sleep duration were examined at T1. T0 sleep duration, age, and gender were entered at step 1 and night-eating was entered at step 2 but no weight variables predicted sleep duration at T1, so they were not entered into the analysis. Only older age was found to predict shorter sleep duration at T1 at step 2; see Table 5.
Predictors of habitual sleep efficacy
Cross-sectional predictors of SE were examined at T0. No demographics or weight measures were correlated with sleep duration so they were not entered into the analysis. Covariates (i.e. depression, anxiety, work-physical activity, and night-eating) were, therefore, entered at step 1 of the analysis. After controlling for the effects of the variables, only night-eating was related to less SE at T0; see Table 5.
Longitudinal predictors of SE were examined at T1. T0 SE and gender were entered at step 1, covariates (i.e. work-physical activity, electronic device use) were entered at step 2, and the weight variables (i.e. BMI) were entered at step 3 of the analysis. After controlling for the effects of the variables, female gender, electronic device use and high BMI predicted lower SE at T1; see Table 5.
Predictors of sleep disturbances
Cross-sectional predictors of SD were examined at T0. No demographics were correlated with it so they were not entered into the analysis. Covariates (i.e. depression, anxiety, and night-eating) were entered at step 1 and the weight variables (i.e. BMI) were entered at step 2 of the analysis. After controlling for the effects of the variables, higher depression levels, night-eating and high BMI were related to more SD at T0; see Table 5.
Longitudinal predictors of SD were examined at T1. T0 SD was entered at step 1 and covariates (i.e. depression, anxiety, work-physical activity, and night-eating) were entered at step 2 but as no demographics or weight measures were correlated with it at T1 they were not entered into the analysis. After controlling for the effects of the variables, none of the variables predicted SD at T1; see Table 5.
Predictors of sleep medication use
Cross-sectional predictors of SM were examined at T0. No demographics were correlated with SD at T1 so they were not entered into the analysis. Covariates (i.e. depression, anxiety, work-physical activity, electronic device use, and night-eating) were entered at step 1 and the weight measures (i.e. obesity category) were entered at step 2 of the analysis. After controlling for the effects of the variables, only electronic device use was related to less SM use; see Table 6.
Longitudinal predictors of SM were examined at T1. SM T0 and age were entered at step 1, covariates (i.e. depression, work-physical activity, and device use) were entered at step 2, and the weight variables (i.e. obesity category) were entered at step 3 of the analysis. After controlling for the effects of the variables, none of the variables predicted SM use; see Table 6.
Predictors of daytime dysfunction
Cross-sectional predictors of DD were examined at T0. Age was entered at step 1, covariates (i.e. depression, anxiety, alcohol intake, and night-eating) were entered at step 2, and the weight variables (i.e. obesity category and WHR) were entered at step 3 of the analysis. After controlling for the effects of the variables, only depression, alcohol intake and night-eating were related to more DD at T0; see Table 6.
Longitudinal predictors of DD were examined at T1. T0 DD was entered at step 1 and covariates (i.e. depression, anxiety, physical activity-RAPA, # steps, work-physical activity, leisure time-physical activity, and night-eating) were entered at step 2 but as no demographics or weight measures were correlated with it so they were not entered into the analysis. After controlling for the effects of the variables, no variables predicted DD at T1; see Table 6.
Discussion
Impaired sleep is reported to be a consequence of overweight/obesity in adults [1, 2], but their behaviour and affective distress (e.g. anxiety, depression) may provide a better explanation of the weight–sleep relationship. That is, certain behaviour (e.g. night-eating, insufficient physical activity, heavy alcohol and electronic device use) are practised more often by overweight/obese people than normal-weight people [11, 16, 19, 21], and the behaviour (and affect) are independent risk factors for impaired sleep [13, 63, 65,66,67]. Thus, an overweight person’s behaviour and affect may adversely impact on their sleep quality more so than their weight. We, therefore, evaluated if weight (i.e. BMI, WHR, obesity category) still predicted aspects of sleep quality after controlling for the effects of demographics, co-existing behaviour and affective symptoms (i.e. covariates). Hypothesis 1 evaluated weight predictors of total sleep quality score and hypothesis 2 examined the predictors of the seven sleep quality components.
Hypothesis 1
examined the weight measures as predictors of total sleep quality score. No weight measures predicted it at T0 or T1 after controlling for the effects of co-existing behaviour, affect and demographics, supporting the null hypothesis. Results indicated that the weight measures failed to predict sleep quality score at baseline and 3 months later, after taking into account the participant’s co-existing behaviour, affect and demographics.
Hypothesis 2
examined the weight measures as predictors of the sleep quality components (SSQ, SOL, sleep duration, SE, SD, DD and SM) at T0 and T1, after controlling for the effects of co-existing behaviour, affect and demographics. Contrary to expectation, some weight measures still predicted sleep quality after taking into account multiple co-existing behaviour, affect and demographics: higher BMI still predicted more sleep disturbances (SD) at T0 and lower sleep efficiency (SE) at T1, but WHR and obesity category did not predict aspects of sleep quality. Results suggest that a person’s weight may impact on their sleep quality above and beyond the effects of their co-existing behaviour and affect. However, abdominal obesity (as indexed by WHR) [32] was unrelated to sleep quality, suggesting that it was not a specific risk factor for impaired sleep in overweight/obese individuals.
Finally, regarding the covariates, at T0, night-eating was related to sleep quality score and five out of seven of the sleep quality components (SSQ, SOL, SE, SD, and DD), whereas other covariates were linked to fewer deficits: depression was related to sleep quality score, SSQ, SD and DD at T0; anxiety was related to sleep quality score and sleep duration at T0; alcohol use (or frequency) was related to sleep quality score, SSQ and DD at T0 and SSQ and SOL at T1; and, electronic device use predicted SM use at T0 and SE at T1. Results suggest that participant’s co-existing behaviour (i.e. night-eating, electronic device and alcohol use) and their affect (i.e. anxiety/depression) may have adversely impacted on their sleep quality, aside from the putative effects of body weight on sleep quality.
Taken together, the study results suggest that a person’s weight may still impact on their sleep quality even after taking into account the effects of co-existing behaviour and affect, but their affect and co-existing behaviour may also have adversely impacted on their sleep quality. Results are consistent with prior research showing that impaired sleep (e.g. short sleep duration) is related to measures of weight (e.g. high BMI, obesity category), [14, 68], behaviour including night-eating (i.e. long SOL, less SE, sleep maintenance) [12, 13, 51], excessive alcohol use [64] and electronic device use [69] and affective symptoms such as anxiety [70] and depression [71, 72], but we did not find any relationship between sleep and physical activity as have other studies [73]. However, the results were different to those obtained using the same sample and weight measures in an objective sleep study [74], in which weight (and night-eating) failed to predict objective sleep. Instead, it was predicted by a combination of other behaviour (i.e. alcohol use, watching TV, and physical inactivity) and affect (i.e. depression). Thus, taken together, the results suggest that a different profile of behaviour may interfere with objective vs. subjective sleep; for example, night-eating, weight and affect may interfere with subjective sleep whereas affect and other behaviour (e.g. watching TV) may interfere with objective sleep; aside from affirming that subjective sleep quality does not provide reliable information about objective sleep [75].
Clinically, the results suggest that a person’s weight may still impact on their sleep quality (e.g. less sleep efficiency and more sleep disturbances) above and beyond the effects of their co-existing behaviour and affect. However, many participants were distressed and they engaged in multiple potentially sleep-disruptive behaviour [6], in particular, the people who reported more night-eating were most likely to report multiple aspects of poor sleep quality as well as being more likely to be overweight/obese [16].
Nonetheless, the study results need to be interpreted in light of several study limitations. First, the sample was relatively small but an a priori power analysis showed that it was sufficient to detect expected medium effect sizes. Second, sleep was assessed using a self-report scale rather than objective sleep tests, which can result in over- or under-reporting [76], but PSQI has good psychometric properties [39], and we examined objective sleep in this sample in another study [74]. Third, the NEQ had barely adequate internal consistency in this study suggesting the results for night-eating should be interpreted with caution. Further, NEQ score includes a sub-score for mood/sleep problems [53] potentially resulting in the double counting of sleep deficits in the participants (i.e. PSQI and NEQ), which in turn, may have exaggerated the strength of the observed relationship. Fourth, alcohol and electronic device use were examined using an un-validated instrument, but they were only covariates (not predictors) in the planned analyses. Finally, the use of an online survey platforms to deliver questionnaires can attract younger and better educated adults as participants [77] which may have reduced the generalisability of the results to older and less educated people.
Conclusions
Measures of weight (i.e. BMI, WHR, and obesity category) were examined as predictors of sleep quality at two time-points 3 months apart (T0 and T1) in a community sample of normal-weight and overweight or obese adults, after controlling for the effects of co-existing behaviour, affect (i.e. covariates; e.g. night-eating, insufficient physical activity, alcohol use, electronic device use, and anxiety/depression symptoms) and demographics, all of which co-exist with overweight/obesity and independently predict sleep outcomes. Results showed that high BMI predicted several aspects of sleep quality: sleep disturbances at T0 and low sleep efficiency at T1, after taking into account co-existing behaviour, affect and demographics. WHR and obesity category failed to predict aspects of sleep quality. Several co-existing behaviour were associated with or predicted sleep quality score and aspects of sleep quality including night-eating, alcohol use, electronic device use and affective symptoms (i.e. anxiety, depression). Results suggest that a person’s weight may still impact on their sleep quality above and beyond the effects of co-existing behaviour and affect, although co-existing behaviour and affect may also adversely impact on their sleep quality. Clinically, the results suggest that multiple potential risk factors for impaired sleep should be examined when investigating the sleep disturbance in overweight clients as they may provide a better explanation of the impaired sleep than their weight.
-
1.
What is already known on this subject?
Impaired sleep is reported to be a consequence of overweight/obesity. However, the behaviour that overweight people engage in (e.g. night-eating, insufficient physical activity) and affective distress (e.g. anxiety, depression) are independent risk factors for impaired sleep which might alternately explain the observed weight–sleep quality relationship.
Thus, it is unclear whether body weight still explains the observation of impaired sleep in overweight/obese people after taking into account their co-existing behaviour and affect.
-
2.
What does this study add?
Relationships between weight measures (i.e. BMI, WHR, and obesity category) and the components of sleep quality were examined in normal-weight, overweight and obese participants, after taking into account their affect (e.g. depression) and co-existing behaviour (e.g. night-eating, insufficient exercise). Results indicate that body weight (i.e. high BMI) was still linked to aspects of poor sleep quality (i.e. sleep disturbances and low sleep efficiency) even after taking into account the effects of multiple co-existing sleep-disrupting behaviour and affect. The results suggest that overweight/obese people are more likely than normal-weight people to have poor sleep quality but their tendency to engage in sleep-disruptive behaviour (i.e. night-eating) and experience distress may partly explain the relationship between overweight/obesity and impaired sleep.
-
3.
You might use the last sentence to summarise any implications for practice, research, policy, or public health.
You might use the last sentence to summarise any implications for practice, research, policy, or public health.
When examining the relationship between body weight and sleep, researchers should seek to control for the effects of common co-existing behaviour (e.g. night-eating) which might alternately explain the observed relationship between body weight and sleep quality.
Data availability statement
The data that support the findings of this study are available from the corresponding author, [S.E], upon reasonable request.
Abbreviations
- BMI:
-
Body mass index
- WHR:
-
Waist to hip ratio
- REM:
-
Rapid eye movement
- NES:
-
Night-eating syndrome
- NEQ:
-
Night-Eating Questionnaire
- SSQ:
-
Subjective sleep quality
- SOL:
-
Sleep-onset latency
- SE:
-
Sleep efficiency
- SD:
-
Sleep disturbances
- SM:
-
Sleep medication use
- DD:
-
Daytime dysfunction
References
Elder S et al (2012) Impact of sleep, screen time, depression, and stress on weight change in the intensive weight loss phase of the LIFE study. Int J Obes (2005) 36(1):86
Patel SR et al. (2008) The association between sleep duration and obesity in older adults. Int J Obes (2005) 32(12):1825
Vgontzas AN et al (1998) Obesity without sleep apnea is associated with daytime sleepiness. Arch Intern Med 158(12):1333–1337
Punjabi NM, Bandeen-Roche K, Young T (2003) Predictors of objective sleep tendency in the general population. Sleep 26(6):678–683
Vgontzas AN et al (2008) Obesity and sleep disturbances: meaningful sub-typing of obesity. Arch Physiol Biochem 114(4):224–236
van Mill JG et al. (2010) Insomnia and sleep duration in a large cohort of patients with major depressive disorder and anxiety disorders. J Clin Psychiatry 71(3): 0–0
Brown R, Umar Y (2020) Overweight/obesity and concurrent disorders, symptoms, behaviour, and body temperature, in Comorbidity. Springer. 43–77
Bixler EO et al (2005) Excessive daytime sleepiness in a general population sample: the role of sleep apnea, age, obesity, diabetes, and depression. J Clin Endocrinol Metab 90(8):4510–4515
Loprinzi PD, Cardinal BJ (2011) Association between objectively-measured physical activity and sleep, NHANES 2005–2006. Ment Health Phys Act 4(2):65–69
Montgomery and Dennis, Physical exercise for sleep problems in adults aged 60+. The Cochrane Library, 2002.
Chan YY et al (2017) Physical activity and overweight/obesity among Malaysian adults: findings from the 2015 National Health and morbidity survey (NHMS). BMC Public Health 17(1):1–12
Aronoff NJ, Geliebter A, Zammit G (2001) Gender and body mass index as related to the night-eating syndrome in obese outpatients. J Acad Nutr Diet 101(1):102
Crispim CA et al (2011) Relationship between food intake and sleep pattern in healthy individuals. J Clin Sleep Med 7(06):659–664
Taheri S et al (2004) Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med 1(3):e62
Spiegel, Penev, Cauter (2004) Brief Communication: Sleep Curtailment in Healthy Young Men Is Associated with Decreased Leptin Levels, Elevated Ghrelin Levels, and Increased Hunger and Appetite. Ann Internal Med. 141(11): p. 846
Meule A et al (2014) The association between night eating and body mass depends on age. Eat Behav 15(4):683–685
Yoshida J et al (2018) Association of night eating habits with metabolic syndrome and its components: a longitudinal study. BMC Public Health 18(1):1366
Roehrs T, Roth (2017) Medication and Substance Abuse. 1380–1389.e4.
Traversy G, Chaput J-P (2015) Alcohol consumption and obesity: an update. Curr Obes Rep 4(1):122–130
White AG, Buboltz W, Igou F (2011) Mobile phone use and sleep quality and length in college students. Int J Humanit Soc Sci 1(18):51–58
Banks E et al (2011) Screen-time, obesity, ageing and disability: findings from 91 266 participants in the 45 and Up Study. Public Health Nutr 14(1):34–43
Goldman SE et al (2007) Poor sleep is associated with poorer physical performance and greater functional limitations in older women. Sleep 30(10):1317–1324
Villareal DT et al (2005) Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Am J Clin Nutr 82(5):923–934
Sun F, Norman IJ, While AE (2013) Physical activity in older people: a systematic review. BMC Public Health 13(1):1–17
Striegel-Moore RH et al (2006) Night eating: prevalence and demographic correlates. Obesity 14(1):139–147
Zickuhr K, Madden M (2012) Older adults and internet use. Pew Internet Am Life Project 6:1–23
Zhang B, Wing Y-K (2006) Sex differences in insomnia: a meta-analysis. Sleep 29(1):85–93
Azevedo MR et al (2007) Gender differences in leisure-time physical activity. Int J Public Health 52(1):8–15
Kanter R, Caballero B (2012) Global gender disparities in obesity: a review. Adv Nutr 3(4):491–498
Sivertsen B et al (2019) Sleep patterns and insomnia in young adults: a national survey of Norwegian university students. J Sleep Res 28(2):e12790
Van Staveren WA et al (1986) Seasonal variation in food intake, pattern of physical activity and change in body weight in a group of young adult Dutch women consuming self-selected diets. Int J Obes 10(2):133–145
Ahmad N et al (2016) Abdominal obesity indicators:waist circumference or waist-to-hip ratio in Malaysian adults population. Int J Prevent Med 7
WHO (2013) BMI Classification. http://apps.who.int/bmi/index.jsp?introPage=intro_3.html. Accessed 8 Apr 2018
Organization WH (2011) Waist circumference and waist-hip ratio: report of a WHO expert consultation, Geneva, 8-11 December 2008
Jones DJH, Evershed K (2005) Actigraphic assessment of circadian activity and sleep patterns in bipolar disorder. Bipolar Disord 7(2):176–186
Yang C-C, Hsu Y-L (2010) A review of accelerometry-based wearable motion detectors for physical activity monitoring. Sensors 10(8):7772–7788
Kaplan KA et al (2012) Evaluating sleep in bipolar disorder: comparison between actigraphy, polysomnography, and sleep diary. Bipolar Disord 14(8):870–879
Noah A et al (2013) Comparison of steps and energy expenditure assessment in adults of Fitbit Tracker and Ultra to the Actical and indirect calorimetry. J Med Eng Technol 37(7):456–462
Buysse DJ et al (1989) The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res 28(2):193–213
Buysse DJ et al (2008) Relationships Between the Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS), and Clinical/Polysomnographic Measures in a Community Sample. J Clin Sleep Med 4(6):563–571
Carpenter JS, Andrykowski MA (1998) Psychometric evaluation of the pittsburgh sleep quality index. J Psychosom Res 45(1):5–13
Health Promotion Research Centre. RAPA; 9-item. 2006. https://depts.washington.edu/hprc/resources/products-tools/rapa/
Vega-López S et al (2014) Validity and reliability of two brief physical activity questionnaires among Spanish-speaking individuals of Mexican descent. BMC Res Notes 7 (1):29
Topolski et al. (2006) Peer reviewed: the Rapid Assessment of Physical Activity (RAPA) among older adults. Prevent Chronic Dis 3(4)
Brown et al (2013) Development of evidence-based physical activity recommendations for adults (18–64 years): report prepared for the Australian Government Department of Health, August 2012
WHO (2018) Global Physical Activity Questionnaire version 2. 2012 April 2018. http://www.who.int/chp/steps/GPAQ
Armstrong T, Bull F (2006) Development of the world health organization global physical activity questionnaire (GPAQ). J Public Health 14(2):66–70
WHO (2012) Global physical activity questionnaire (GPAQ) analysis guide. http://www.who.int/ncds/surveillance/steps/resources/GPAQ_Analysis_Guide.pdf
Bull FC, Maslin TS, Armstrong T (2009) Global physical activity questionnaire (GPAQ): nine country reliability and validity study. J Phys Act Health 6(6):790–804
U.S. Department of Health and Human Services (2018) Physical Activity Guidelines for Americans, 2nd edition. https://health.gov/paguidelines/second-edition/pdf/Physical_Activity_Guidelines_2nd_edition.pdf
Yeh S-SS, Brown RF (2014) Disordered eating partly mediates the relationship between poor sleep quality and high body mass index. Eat Behav 15(2):291–297
Wichianson JR et al (2009) Perceived stress, coping and night-eating in college students. Stress Health J Int Soc Investig Stress 25(3):235–240
Allison et al (2008) The Night Eating Questionnaire (NEQ): psychometric properties of a measure of severity of the Night Eating Syndrome. Eat Behav 9(1):62–72
O’Reardon JP, Peshek A, Allison KC (2005) Night Eating Syndrome. CNS Drugs 19(12):997–1008
Lovibond PF, Lovibond SH (1995) The structure of negative emotional states: Comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav Res Ther 33(3):335–343
Crawford JR, Henry JD (2003) The Depression Anxiety Stress Scales (DASS): Normative data and latent structure in a large non-clinical sample. Br J Clin Psychol 42(2):111–131
Page AC, Hooke GR, Morrison DL (2007) Psychometric properties of the Depression Anxiety Stress Scales (DASS) in depressed clinical samples. Br J Clin Psychol 46(3):283–297
Allison et al (2010) Proposed diagnostic criteria for night eating syndrome. Int J Eat Disord 43(3):241–247
Australian Institute of Health & Welfare Overweight and obesity: an interactive insight. 2019
Australian Bureau of Statistics. National Health Survey: First Results, 2014–15 First results 2015. http://www.abs.gov.au/ausstats/abs@.nsf/PrimaryMainFeatures/4364.0.55.001?OpenDocument
Muzet, Libert, Candas (1984) Ambient temperature and human sleep. Experientia 40(5):425–429
Brobeck JR (1948) Food intake as a mechanism of temperature regulation. Yale J Biol Med 20(6):545
Âkerstedt T (2006) Psychosocial stress and impaired sleep. Scand J Work Environ Health 493–501
Roehrs T, Roth T (2001) Sleep, sleepiness, sleep disorders and alcohol use and abuse. Sleep Med Rev 5(4):287–297
Marshall NS, Glozier N, Grunstein RR (2008) Is sleep duration related to obesity? A critical review of the epidemiological evidence. Sleep Med Rev 12(4):289–298
Gay CL, Lee KA, Lee S-Y (2004) Sleep patterns and fatigue in new mothers and fathers. Biol Res Nurs 5(4):311–318
Chang A-M et al (2015) Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proc Natl Acad Sci 112(4):1232–1237
Janson et al (2001) Insomnia in men—a 10-year prospective population based study. Sleep 24(4):425–430
Fossum IN et al (2014) The association between use of electronic media in bed before going to sleep and insomnia symptoms, daytime sleepiness, morningness, and chronotype. Behav Sleep Med 12(5):343–357
Arriaga F et al (1996) The sleep of non-depressed patients with panic disorder: a comparison with normal controls. Acta Psychiatr Scand 93(3):191–194
Jansson-Fröjmark M, Lindblom K (2008) A bidirectional relationship between anxiety and depression, and insomnia? A prospective study in the general population. J Psychosom Res 64(4):443–449
Roberts RE, Duong HT (2014) The prospective association between sleep deprivation and depression among adolescents. Sleep 37(2):239–244
Sherrill DL, Kotchou K, Quan SF (1998) Association of physical activity and human sleep disorders. Arch Intern Med 158(17):1894–1898
Eid SW et al (2021) A comparison of physical activity levels, sleep disrupting behavior, and stress/affective distress as predictors of sleep as indexed by actigraphy. J Phys Act Health 1:1–12
Baker FC, Maloney S, Driver HS (1999) A comparison of subjective estimates of sleep with objective polysomnographic data in healthy men and women. J Psychosom Res 47(4):335–341
de Mortel V (2008) Faking it: social desirability response bias in self-report research. Aust J Adv Nurs 25(4):40
Howard, Rainie, Jones (2001) Days and nights on the internet: the impact of a diffusing technology. Am Behav Sci 45(3):383–404
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflict of interest. This manuscript has not been previously published, and is not presently under consideration by another journal, and will not be submitted to another journal before a final editorial decision from Eating and Weight Disorders is rendered. No funds, grants, or other support was received.
Ethics approval
The questionnaire and methodology for this study was approved by the Human Research Ethics committee of the Australian National University (Ethics approval number: #2015/013.
Consent to participate and consent to publish
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eid, S.W., Brown, R.F., Maloney, S.K. et al. Can the relationship between overweight/obesity and sleep quality be explained by affect and behaviour?. Eat Weight Disord 27, 2821–2834 (2022). https://doi.org/10.1007/s40519-022-01435-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40519-022-01435-1