Abstract
Purpose of Review
The Epstein-Barr virus (EBV) is a common virus around the globe with approximately 98% of adults testing positive against EBV. However, EBV infection typically begins early in the childhood. Owing to the ability to infect various body organ, EBV is linked to a broad spectrum of symptoms, diseases, and inflammatory conditions. Moreover, since EBV exists in both latent and replicating forms in most healthy individuals, any disruption in the balance between the virus and its host can lead to the development of different diseases, including autoimmune disorders and cancer. Given these circumstances, we draw attention to the crucial need for developing prophylactic measures and treatments for EBV and its associated diseases.
Recent Findings
We propose leveraging the advantages of nanomedicine, such as ferritin and iron oxide nanoparticles, for the creation of EBV vaccines. These advancements can also be applied to developing drugs to treat EBV-associated diseases, such as cancer, autoimmune disorders, and cytokine storm syndrome.
Summary
We emphasize the urgency of having accessible EBV vaccines, as well as effective treatments for EBV-related diseases, especially when early diagnosis is involved. This approach, which includes comprehensive cytokine profiling for patients, can significantly enhance the effectiveness of treatment programs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
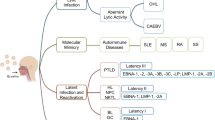
Epstein-Barr virus (EBV), categorized as a gamma herpesvirus, is prevalent worldwide [1]. It has been reported that around 98% of adults are carriers of EBV [2], and this infection typically begins in early childhood [3]. Despite being recognized as the first oncogenic virus, EBV still lacks an available vaccine or a specific treatment option [4]. Some ongoing research efforts have resulted in limited experimental trials addressing this deficiency. Notably, EBV particles were initially observed using electron microscopy in the context of a case of malignant Burkitt's lymphoma in 1964 [5]. Burkitt's lymphoma is a B-cell neoplasm known to appear in atypical anatomical locations, including the mandible, nasopharynx, orbit, kidney, adrenal glands, and ovaries. This virus primarily results in infectious mononucleosis and has close associations with both lymphatic and epithelial malignancies, including Burkitt's lymphoma (BL), Hodgkin's lymphoma (HL), Nasopharyngeal carcinoma (NPC), AIDS-associated B-cell lymphoma, and various lymphoproliferative diseases (LPDs) [6]. EBV can adopt two states, latency and lytic pathways, within its host cell, expressing viral latent or lytic genes to induce tumor cells in EBV-associated lymphoproliferative diseases [7]. The virus can exhibit four different types of latency patterns, namely type I, II, III, and W promoter (Wp) [8].
Virological overview of EBV
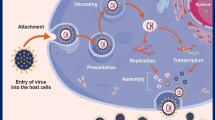
EBV, known as human herpesvirus 4, is composed of double-stranded DNA enclosed by an icosahedral capsid [9], further enveloped by a tegument and a host cell membrane containing integrated glycoproteins (Fig. 1). Infections resulting from EBV can manifest in various ways, ranging from asymptomatic cases to flu-like symptoms, or they can mimic the symptoms of infectious mononucleosis (IM) [10]. IM is a lymphoproliferative disease, typically self-limiting, and characterized by extensive activation of T-cells [11]. Nevertheless, EBV poses a significant life-threatening risk, especially for individuals with compromised immune systems [12].
The Epstein-Barr virus (EBV) typically infects through the mucosal surfaces of the mouth and throat. EBV primarily targets B cells in the immune system; the virus attaches itself to specific receptors on the surface of these cells, allowing it to gain entry. This initial attachment is facilitated by viral envelope glycoproteins interacting with cellular receptors [13]. After attachment, the virus fuses its envelope with the host cell membrane, and releasing its genetic material into the cell. This process allows the viral DNA to enter the host cell's nucleus. Once inside the nucleus, the EBV genome circularizes and starts replicating [13]. The virus utilizes the host cell's machinery to transcribe and translate its genes, producing viral proteins and new viral genomes. EBV can enter a latent phase where it remains in the host cell's nucleus without actively producing new viral particles. During latency, the viral genome persists in the infected cell and can periodically reactivate, leading to the production of new viruses. Infected B cells can release new viral particles, allowing the virus to spread to other cells [13]. In some cases, EBV can also infect epithelial cells in the mouth and throat, contributing to viral shedding and transmission through saliva [13].
Given that EBV can infect virtually any organ system in the body, it is associated with a wide array of diseases (Fig. 2) and inflammatory conditions (Fig. 3). These include autoimmune diseases (Fig. 4), vasculitis [14], organ failure [15], myocarditis [16•], sudden death [17], disturbances in smell, taste, and hearing [18], thrombosis [11], cytokine storm syndrome [19], fatal anomalies [20], abortion [21], acute and chronic skin lesions [22], cancer, hemophagocytic syndrome, and severe coagulopathy [23, 24], chronic fatigue syndrome [25], chronic active EBV [22], ocular manifestations [26], hepatitis, pancreatitis [27], cholestasis, pneumonia, glomerulonephritis [28], enteritis [29], gastritis, cholecystitis [30], cystitis [31], adrenalitis [32], thyroiditis [33], encephalitis, cerebellar ataxia, Alzheimer's disease [34], and sarcoidosis [35].
There is an urgent requirement for the early detection and treatment of illnesses linked to EBV and the development of vaccines to protect against EBV infections. In most healthy individuals, EBV exists in two states: latent and replicating virus. A disturbance in the delicate balance between the virus and its host can lead to the emergence of various diseases [36]. Importantly, the interplay between psychological stressors and cellular factors can trigger the reactivation of EBV [34]. The nature of symptoms resulting from EBV infection varies and depends on factors such as stress levels, the individual's age, environmental conditions, and other pathogens. EBV reactivation can be triggered by specific factors, including immunosuppression, particular cytokines, or steroid hormones [37]. For example, during events like pregnancy or the rapid reduction of steroid use, EBV lytic replication can be facilitated by glucocorticoids, marked by increased expression of the immediate early BZLF1 gene [38, 39].
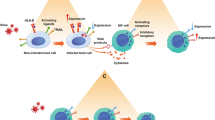
EBV contains genes that code for proteins with both sequence and functional similarity to human proteins, allowing them to regulate EBV-infected cells [22]. Among these proteins, cytokines play a crucial role [40]. While cytokines are essential in facilitating the innate immune response, persistent or dysregulated cytokine activity can potentially lead to pathological conditions, such as severe chronic active EBV development. This highlights the intricate interplay between pro-inflammatory and anti-inflammatory cytokines, a defining aspect of inflammation. Severe chronic active EBV infection results from the clonal expansion of either T- or NK cytotoxic cells, leading to elevated levels of both pro-inflammatory and anti-inflammatory cytokines, including interleukin (IL)-1, interferon-α (IFN-α), IL-13, IL-15, tumor necrosis factor (TNF)-α, and transforming growth factor (TGF)-β [14].
Additionally, EBV-induced myocarditis can lead to congestive heart failure [41]. Thus, we emphasize the critical importance of early diagnosis and treatment of EBV to reduce the risk of mortality. The presence of a cytokine storm is a distinctive feature of hemophagocytic lymphohistiocytosis (HLH), marked by significantly elevated levels of IFN-α, soluble CD25, as well as a marked increase in IL-6, IL-10, and IL-18. Therefore, it is imperative to underscore the significance of evaluating cytokine profiles in patients with EBV-associated diseases [19]. The open reading frame (ORF) of EBV BILF1 promotes immunosuppression and contributes to oncogenic processes [42]. Therefore, compounds that act as inverse agonists for BILF1 have the potential to inhibit cellular transformation and serve as promising candidates for therapeutic intervention [43]. BILF1 encodes a protein responsible for targeting the major histocompatibility complex (MHC) class I molecules, leading to their degradation within lysosomes, which compromises their recognition by immune T-cells [44].
EBV remains the first human virus known to directly contribute to the development of lymphoid and epithelial cancers [45]. Additionally, EBV has been linked to various malignancies, including post-transplant lymphoproliferative disorders, which are the most common forms of cancer arising after organ transplantation [1]. The lifelong presence of EBV in the body during its latent phase explains the prolonged nature of autoimmune diseases, often marked by recurring exacerbations of symptoms [46]. Normally, the immune system works to keep the viral load minimal. However, instances of immunosuppression can trigger EBV reactivation, causing it to transition to the lytic phase [22]. While NK cells typically identify and eliminate virally infected cells, certain EBV proteins can disrupt this mechanism, hindering early viral control and potentially leading to infectious mononucleosis (IM) or severe primary viral infections [47].
Mortality resulting from EBV infection primarily results from the development of hemophagocytic lymphohistiocytosis (HLH), characterized by hypercytokinemia, often referred to as cytokine storm syndrome. Hypercytokinemia leads to cellular damage, organ dysfunction, and ultimately, death [19]. However, the clinical course of HLH varies, ranging from rapid-onset multi-organ failure within hours to prolonged symptoms resembling IM that persist over months [48]. Notably, patients with acute or chronic EBV symptoms exhibit significantly elevated levels of IL-1, IL-2, IL-6, and IFN-α in their serum [19]. Therefore, our objective is to emphasize the importance of both preventive and therapeutic preparedness for EBV and its associated conditions, harnessing the potential benefits of nanomedicine.
Prophylactic and therapeutic aspects of nanomedicine against EBV
Nanomedicine offers a multifaceted approach encompassing both prophylactic and therapeutic dimensions in combating Epstein-Barr virus (EBV) infections [49, 50]. In the prophylactic realm, engineered nanomaterials serve as a potential avenue for vaccine delivery, leveraging their unique properties to enhance vaccine efficacy and immune response. These nanovaccines can be tailored to mimic viral components, stimulating robust immune reactions against EBV before infection [49, 50]. On the therapeutic front, nanomedicine showcases promise in targeted drug delivery, enabling precise transport and release of antiviral agents to infected cells or tissues. Moreover, nanoparticles with surface modifications can specifically recognize and bind to EBV-infected cells, delivering therapeutic payloads such as antivirals or immunomodulators directly to the viral targets. Nanomedicine's ability to enhance drug stability, bioavailability, and target specificity holds substantial potential in shaping both preventative and treatment strategies against EBV infections [49, 50]. Continued research in this domain aims to optimize nanomedicine approaches, offering new avenues to combat EBV and related diseases.
Targeting glycoproteins
A ferritin nanoparticles vaccine was developed to prevent viral infections [51]. This was accomplished by attaching ferritin to a truncated domain from the EBV gp350 protein, containing the crucial receptor-binding site required for B-cell infection [52]. In comparative studies with the soluble gp350 antigen, the nanoparticle-based vaccine demonstrated significant improvement, neutralizing antibody levels 10 to 100 times higher in mice and non-human primates. Additionally, this vaccine conferred protective immunity in a mouse model subjected to a lethal viral challenge [51].
EBV glycoproteins, including gH/gL, gp42, and gB (Fig. 5), have demonstrated higher immunogenicity than the previously favored gp350 antigen [4]. Utilizing distinct EBV antigens, such as gH in combination with gL or with gp42 within a ferritin nanoparticle vaccine (Fig. 6), facilitates the generation of neutralizing antibodies. These antibodies effectively inhibit virus-cell fusion and protect against a broader range of cell types beyond preventing B-cell infection [53]. Clinical trials have recently been initiated to assess the efficacy of an mRNA vaccine known as mRNA-1189. This vaccine comprises four distinct mRNAs encoding gH, gL, gp42, and gp220, along with a ferritin-gp350 nanoparticle vaccine [4].
Schematic illustration showing the Epstein Barr virus envelope glycoproteins (entry complex) and putative host-derived membrane proteins; CD21 (complement receptor type 2), Integrins, ECSP (Epithelial cell surface proteins), Major histocompatibility complex (MHC) molecules, and HLA (Human leukocyte antigen) Class I and II molecules.
SiRNA delivery
Small interfering RNA (siRNA) is a type of RNA molecule involved in gene silencing, acting as a potent tool to regulate gene expression [54]. In the context of treating rheumatoid arthritis (RA), an autoimmune condition linked to Epstein-Barr virus (EBV) infection, siRNA technology holds promise. siRNA works by targeting and binding to specific messenger RNA (mRNA) sequences, leading to mRNA degradation and hindering the translation of proteins essential for disease progression [54]. In RA, siRNA therapy could potentially target genes involved in the inflammatory response, suppressing the overactive immune system characteristic of the disease [54,55,56,57,58]. This approach offers the advantage of precise targeting, potentially reducing off-target effects commonly associated with traditional medications. However, challenges persist, such as effective delivery of siRNA to the affected joints and ensuring sustained therapeutic levels [54,55,56,57,58]. Additionally, off-target effects and potential immune responses to the siRNA delivery system are among the drawbacks that need to be addressed for successful clinical application in RA treatment associated with EBV infection [54,55,56,57,58].
The use of nanoparticle as a drug delivery system introduces innovative approaches to address certain medical conditions, including rheumatoid arthritis (RA), an autoimmune disease associated with EBV infection [59, 60]. It is important to note that prolonged use of synthetic and biological DMARDs (disease-modifying anti-inflammatory drugs), NSAIDs (non-steroidal anti-inflammatory drugs), and glucocorticoids can lead to adverse effects affecting gastrointestinal, hepatic, cardiac, and renal functions [59, 61,62,63]. A significant portion of patients do not respond positively to existing RA treatments or may develop a tolerance to these therapies over time. Unlike traditional medications, nanocarriers are intentionally designed to deliver drugs precisely to the site of joint inflammation, thus avoiding systemic side effects [59]. Instances of this approach involve directing therapeutic substances toward cell receptors or employing small interfering RNAs (siRNAs) to reduce the activity of genes associated with particular signaling pathways. [60, 61]. SiRNAs have been used to inhibit EBV replication by selectively targeting the protease (PR) gene [64••]. The EBV PR is encoded by the BVRF2 gene and plays a crucial role in viral capsid maturation and packaging of viral DNA in the later stages of the EBV lytic cycle.
Surface-coated nanoparticles
Surface-modified nanoparticles present an innovative approach for delivering cytokines in combating Epstein-Barr virus (EBV) infections. These nanoparticles undergo specific surface alterations, empowering them to effectively transport and release cytokines, vital immune signaling molecules, to targeted sites [49, 50, 65]. In the context of EBV, these modified nanoparticles can be engineered with surface ligands or antibodies that recognize and bind to receptors present on immune cells implicated in combating the virus. By encapsulating cytokines within these nanoparticles, their controlled and targeted release at infection sites can modulate the immune response against EBV [49, 50, 65]. This precision delivery enhances the efficacy of the cytokines, potentially mitigating EBV infection severity and associated symptoms [49, 50, 65]. Nevertheless, challenges regarding nanoparticle stability, ensuring compatibility within the body, and precise targeting of infected cells remain pivotal for the successful application of surface-modified nanoparticles in delivering cytokines against EBV infections. Ongoing research endeavors aim to refine and optimize these nanoparticles, holding promise for augmenting therapeutic strategies against EBV.
Surface-coated nanoparticles (Fig. 7) have been employed, similar to those used for delivering RA medications, by influencing immune cells like macrophages in inflamed joints [66, 67]. This therapeutic approach is used to manage conditions characterized by excessive cytokine production, particularly IL-6. Notably, EBV viremia levels are correlated with IL-6 levels [27]. IL-6 receptor monoclonal antibody obstructs IL-6 binding to its receptor, thus disrupting IL-6's activities. This intervention leads to the improvement of immunological and hematological abnormalities, as well as the relief of systemic inflammatory symptoms, including cachexia. Importantly, it demonstrates potent therapeutic efficacy while maintaining high safety and general tolerability [68].
Targeting human Epidermal Growth Factor Receptor (EGFR)
The human epidermal growth factor receptor (EGFR) is overexpressed in around one-third of epithelial malignancies, spanning various types such as head and neck, colorectal, breast, ovarian, prostate, bladder, and lung cancers [69]. Dysregulated and persistent EGFR activation plays a pivotal role in promoting tumor growth and progression. This includes fostering heightened cell proliferation, facilitating cell cycle advancement, promoting tumor angiogenesis, facilitating invasion and metastasis, as well as hindering the programmed cell death process [70,71,72]. Superparamagnetic iron oxide nanoparticles (SPIONs) have been employed to leverage these insights for therapeutic purposes. They are conjugated with various substances, notably by conjugating the anti-EGFR monoclonal antibody cetuximab to dextran coated SPIONs using periodate oxidation. Cetuximab blocks ligand binding to EGFR, inhibiting ligand-induced phosphorylation and activating the EGFR tyrosine kinase. Notably, SPIONs have demonstrated superior sensitivity, reduced toxicity, and a longer plasma half-life than paramagnetic gadolinium chelates. Research findings indicate that SPIONs not only retain the therapeutic effects of cetuximab but also have the ability to specifically target tumors expressing EGFR [69]. The therapeutic potential of cetuximab-polyethylene glycol (PEG)-dextran SPIONS (cet-PEG-dex SPIONs) for addressing EGFR-expressing tumors was explored in vitro. This included Western blot analysis, evaluating the reduction in surface EGFR levels, assessing apoptosis, and conducting an antibody-dependent cell-mediated cytotoxicity (ADCC) assay [73].
Conclusion and future prospective
EBV is a ubiquitous herpesvirus associated with several diseases, including infectious mononucleosis and various cancers. EBV is known for its global prevalence, with nearly 98% of adults exhibiting serological positivity, and the infection typically commences during early childhood. Furthermore, EBV is distinguished by its latency and ability to persist in our bodies throughout our lifetime. Additionally, its capacity to infect various organs, systems, and tissues makes it associated with a wide array of diseases. The need for available vaccines against EBV infection and treatments for the associated diseases is pressing, especially when considering early diagnosis. A comprehensive understanding of patients' cytokine profiles can significantly enhance treatment programs' effectiveness. Nanomedicine offers a versatile platform for developing novel and highly targeted antiviral approaches against EBV infection. These approaches may significantly improve treatment outcomes, reduce side effects, and offer curative options for EBV-associated diseases. Nanoparticles can be designed to deliver antiviral drugs specifically to the infected cells by encapsulating or attaching antiviral agents to enhance drug delivery and stability and reduce systemic toxicity.
In addition, nanoparticles can be engineered to serve as carriers for antigens, enhancing the efficiency of vaccines (nano-vaccines) against EBV, which can elicit a stronger and longer-lasting immune response. By utilizing nanoparticles to deliver siRNA or microRNA inhibitors that can specifically target and silence viral genes and hinder the replication and gene expression of EBV. Meanwhile, nanoparticles can be used to deliver CRISPR-Cas9 or other gene-editing tools to directly modify viral genes within the infected cells, potentially providing a curative approach to EBV-associated diseases. Importantly, research into nanoparticles that can target and disrupt the latent EBV reservoirs may provide a means of preventing viral reactivation and associated diseases like lymphomas and nasopharyngeal carcinoma.
Data availability
No datasets were generated or analysed during the current study.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Smatti MK, Al-Sadeq DW, Ali NH, et al. Epstein-Barr virus epidemiology, serology, and genetic variability of LMP-1 oncogene among healthy population: an update. Front Oncol. 2018. https://doi.org/10.3389/fonc.2018.00211.
Smatti MK, Yassine HM, AbuOdeh R, et al. Prevalence and molecular profiling of Epstein Barr virus (EBV) among healthy blood donors from different nationalities in Qatar. PLoS ONE. 2017. https://doi.org/10.3389/fonc.2018.00211.
Papesch M, Watkins R. Epstein-Barr virus infectious mononucleosis. Clin Otolaryngol Allied Sci. 2021. https://doi.org/10.1046/j.1365-2273.2001.00431.x.
Zhong L, Krummenacher C, Zhang W, et al. Urgency and necessity of Epstein-Barr virus prophylactic vaccines. NPJ Vaccines. 2022. https://doi.org/10.1038/s41541-022-00587-6.
Epstein MA, Achong BG, Barr YM. Virus particles in IN cultured lymphoblasts from Burkitt’s lymphoma. Lancet. 1964. https://doi.org/10.1016/s0140-6736(64)91524-7.
Dunmire SK, Verghese PS, Balfour HH Jr. Primary epstein-barr virus infection. J Clin Virol. 2018;102:84–92.
Houldcroft CJ, Kellam P. Host genetics of Epstein-Barr virus infection, latency and disease. Rev Med Virol. 2015;25:71–84.
Kang M-S, Kieff E. Epstein-Barr virus latent genes. Exp Mol Med. 2015;47:e131–e131.
Farrell PJ. Epstein-Barr virus. The B95–8 strain map. Methods Mol Biol. 2001. https://doi.org/10.1385/1-59259-227-9:3.
Mashav N, Saar N, Chundadze T, Steinvil A, Justo D. Epstein-Barr virus-associated venous thromboembolism: a case report and review of the literature. Thromb Res. 2008. https://doi.org/10.1016/j.thromres.2008.03.005.
Sutkowski N, Palkama T, Ciurli C, et al. An Epstein-Barr virus-associated superantigen. J Exp Med. 1996. https://doi.org/10.1084/jem.184.3.971.
Houen G, Trier NH, Frederiksen JL. Epstein-Barr Virus and multiple sclerosis. Front Immunol. 2020. https://doi.org/10.3389/fimmu.2020.587078.
Bu GL, Xie C, Kang YF, Zeng MS, Sun C. How EBV infects: the tropism and underlying molecular mechanism for viral infection. Viruses. 2022;14(11):2372. https://doi.org/10.3390/v14112372.
Lee MS, Hwang SK, Kim YE, et al. Central nervous system vasculitis from Epstein-Barr virus-associated T/natural killer-cell lymphoproliferative disorder in children: a case report. Brain Dev. 2019. https://doi.org/10.1016/j.braindev.2019.05.009.
Cohen JI. Optimal treatment for chronic active Epstein-Barr virus disease. Pediatr Transplant. 2009. https://doi.org/10.1111/j.1399-3046.2008.01095.x.
• Sahu S, Giri S, Malik S, Gupta N. Unusual infectious mononucleosis complicated by vasculitis. Med J DY Patil Univ. 2016. https://doi.org/10.4103/0975-2870.167969. This study highlights that EBV can cause Infectious mononucleosis (IM) that range from acute myocarditis and later with large-vessels arteritis.
Ishikawa T, Zhu BL, Li DR, Zhao D, Maeda H. Epstein-Barr virus myocarditis as a cause of sudden death: two autopsy cases. Int J Legal Med. 2005. https://doi.org/10.1007/s00414-005-0540-1.
Alagha AK, Hirsch AR. Association for Chemoreception Sciences (AChemS), 37th Annual Meeting, Bonita Springs, Florida. Chem Senses. 2015. https://doi.org/10.1093/chemse/bjv029.
Verbist KC, Nichols KE. Cytokine storm syndromes associated with Epstein-Barr virus. In: Cron R, Behrens E, editors. Cytokine storm syndrome. Cham: Springer; 2019.
Ornoy A, Dudai M, Sadovsky E. Placental and fetal pathology in infectious mononucleosis: a possible indicator for Epstein-Barr virus teratogenicity. Diagn Gynecol Obstet. 1982. Spring 4(1):11–6.
Khashman BM, Hussein AA. Eptein-Barr virus infection and related with expression of fibronectin among aborted women in Baqubah city. Biochem Cell Arch. 2018;18(2):2203–2207.
Sangueza-Acosta M, Sandoval-Romero E. Epstein-Barr virus and skin. An Bras Dermatol. 2018. https://doi.org/10.1590/abd1806-4841.20187021.
Hsieh WC, Chang Y, Hsu MC, et al. Emergence of anti-red blood cell antibodies triggers red cell phagocytosis by activated macrophages in a rabbit model of Epstein-Barr virus-associated hemophagocytic syndrome. Am J Pathol. 2007. https://doi.org/10.2353/ajpath.2007.060772.
Altemimi H, Jones R. Uncommon complication of Epstein-Barr virus in a young patient. Eur J Intern Med. 2013. https://doi.org/10.1016/j.ejim.2013.08.429.
Jovanović J, Cvjetković D, Brkić S, Madle-Samardzija N. Epstein-Barrov virus i sindrom hronicnog umora [The Epstein-Barr virus and chronic fatigue syndrome]. Med Pregl. 1995;48(11–12):391–3.
Victor AA. Ocular manifestations in Epstein Barr virus infection. In: Epstein-Barr Virus-New Trends. IntechOpen, 2020. https://doi.org/10.5772/intechopen.93721.
Lehner GF, Klein SJ, Zoller H, Peer A, Bellmann R, Joannidis M. Correlation of interleukin-6 with Epstein-Barr virus levels in COVID-19. Crit Care. 2020. https://doi.org/10.1186/s13054-020-03384-6.
Mansour C, Dang P. Painless Jaundice with high ferritin level: a rare presentation of infectious mononucleosis. LLUSJ. 2020. https://scholarsrepository.llu.edu/llu-student-journal/vol4/iss1/7.
Watanabe H, Yamazaki Y, Fujishima F, et al. Epstein-Barr virus-associated enteritis with multiple ulcers: the first autopsy case. Pathol Int. 2020. https://doi.org/10.1111/pin.13013.
Kim JM, Song CW, Song KS, Kim JY. Acute gastritis associated with Epstein-Barr virus infection in a child. Korean J Pediatr. 2016. https://doi.org/10.3345/kjp.2016.59.11.S68.
Jhang JF, Hsu YH, Peng CW, et al. Epstein-Barr virus as a potential etiology of persistent bladder inflammation in human interstitial cystitis/bladder pain syndrome. J Urol. 2018. https://doi.org/10.1016/j.juro.2018.03.133.
Hertel NT, Jacobsen BB, Pedersen FK, Heilmann C. Adrenocortical insufficiency associated with Epstein-Barr virus infection in a patient with the Wiskott-Aldrich syndrome. Eur J Pediatr. 1987. https://doi.org/10.1007/BF02467365.
Vrbikova J, Janatkova I, Zamrazil V, Tomiska F, Fucikova T. Epstein-Barr virus serology in patients with autoimmune thyroiditis. Exp Clin Endocrinol Diabetes. 1996. https://doi.org/10.1055/s-0029-1211428.
Sausen DG, Bhutta MS, Gallo ES, Dahari H, Borenstein R. Stress-induced Epstein-Barr virus reactivation. Biomolecules. 2021. https://doi.org/10.3390/biom11091380.
Theate I, Michaux L, Dardenne S, et al. Epstein-Barr virus-associated lymphoproliferative disease occurring in a patient with sarcoidosis treated by methotrexate and methylprednisolone. Eur J Haematol. 2002. https://doi.org/10.1034/j.1600-0609.2002.02748.x.
Linde A. Diagnosis of Epstein-Barr virus-related diseases. Scand J Infect Dis Suppl. 1996;100:83–8.
Bocian J, Januszkiewicz-Lewandowska D. Zakażenia EBV – cykl życiowy, metody diagnostyki, chorobotwórczość. Postepy Hig Med Dosw (online). 2011. https://doi.org/10.5604/17322693.943104
Jiyad Z, Moriarty B, Creamer D, Higgins E. Generalized pustular psoriasis associated with Epstein-Barr virus. Clin Exp Dermatol. 2015. https://doi.org/10.1111/ced.12493.
Yang EV, Webster Marketon JI, Chen M, Lo KW, Kim SJ, Glaser R. Glucocorticoids activate Epstein Barr virus lytic replication through the upregulation of immediate early BZLF1 gene expression. Brain Behav Immun. 2010. https://doi.org/10.1016/j.bbi.2010.04.013.
Cohen JI. Epstein-Barr virus infection. N Engl J Med. 2000. https://doi.org/10.1056/NEJM200008173430707.
Aknouk M, Choe S, Osborn H, et al. Recognizing rare sequelae of Epstein-Barr virus myocarditis leading to dilated cardiomyopathy and acute congestive heart failure with multivalvular regurgitation. Cureus. 2022. https://doi.org/10.7759/cureus.2150.
Tsutsumi N, Qu Q, Mavri M, et al. Structural basis for the constitutive activity and immunomodulatory properties of the Epstein-Barr virus-encoded G protein-coupled receptor BILF1. Immunity. 2021. https://doi.org/10.1016/j.immuni.2021.06.001.
Lyngaa R, Norregaard K, Kristensen M, et al. Cell transformation mediated by the Epstein-Barr virus G protein-coupled receptor BILF1 is dependent on constitutive signaling. Oncogene. 2010. https://doi.org/10.1038/onc.2010.
Zuo J, Currin A, Griffin BD, et al. The Epstein-Barr virus G-protein-coupled receptor contributes to immune evasion by targeting MHC class I molecules for degradation. PLoS Pathog. 2009. https://doi.org/10.1038/onc.2010.173.
Gequelin LC, Riediger IN, Nakatani SM, Biondo AW, Bonfim CM. Epstein-Barr virus: general factors, virus-related diseases and measurement of viral load after transplant. Rev Bras Hematol Hemoter. 2011. https://doi.org/10.5581/1516-8484.20110103.
Toussirot E, Roudier J. Pathophysiological links between rheumatoid arthritis and the Epstein-Barr virus: an update. Joint Bone Spine. 2007. https://doi.org/10.1016/j.jbspin.2007.05.001.
Quinn LL, Zuo J, Abbott RJ, et al. Cooperation between Epstein-Barr virus immune evasion proteins spreads protection from CD8+ T cell recognition across all three phases of the lytic cycle. PLoS Pathog. 2014. https://doi.org/10.1371/journal.ppat.1004322.
Bar-Or A, Pender MP, Khanna R, et al. Epstein-Barr virus in multiple sclerosis: theory and emerging immunotherapies. Trends Mol Med. 2020. https://doi.org/10.1016/j.molmed.2019.11.003.
Shah S, Chougule MB, Kotha AK, et al. Nanomedicine based approaches for combating viral infections. J Control Release. 2021. https://doi.org/10.1016/j.jconrel.2021.08.011.
Granato M. Nanotechnology Frontiers in γ-herpesviruses treatments. Int J Mol Sci. 2021;22(21):11407. https://doi.org/10.3390/ijms222111407.
Jackman JA, Yoon KB, Lei Ouyang L, et al. Biomimetic nanomaterial strategies for virus targeting: antiviral therapies and vaccines. Adv Funct Mater. 2021. https://doi.org/10.1002/adfm.202008352.
Kanekiyo M, Bu W, Joyce MG, et al. Rational design of an Epstein-Barr virus vaccine targeting the receptor-binding site. Cell. 2015. https://doi.org/10.1016/j.cell.2015.07.043.
Bu W, Joyce MG, Nguyen H, et al. Immunization with components of the viral fusion apparatus elicits antibodies that neutralize Epstein-Barr virus in B cells and epithelial cells. Immunity. 2019. https://doi.org/10.1016/j.immuni.2019.03.010.
Alshaer W, Zureigat H, Al Karaki A, et al. siRNA: mechanism of action, challenges, and therapeutic approaches. Eur J Pharmacol. 2021. https://doi.org/10.1016/j.ejphar.2021.174178.
Pauley KM, Cha S. RNAi therapeutics in autoimmune disease. Pharmaceuticals (Basel). 2013. https://doi.org/10.3390/ph6030287.
Dana H, Chalbatani GM, Mahmoodzadeh H, et al. Molecular mechanisms and biological functions of siRNA. Int J Biomed Sci. 2017;13(2):48–57. http://www.ijbs.org/.
Sargazi S, Arshad R, Ghamari R, et al. siRNA-based nanotherapeutics as emerging modalities for immune-mediated diseases: a preliminary review. Cell Biol Int. 2022. https://doi.org/10.1002/cbin.11841.
Kumari A, Kaur A, Aggarwal G. The emerging potential of siRNA nanotherapeutics in treatment of arthritis. Asian J Pharm Sci. 2023. https://doi.org/10.1016/j.ajps.2023.100845.
Nasra S, Bhatia D, Kumar A. Recent advances in nanoparticle-based drug delivery systems for rheumatoid arthritis treatment. Nanoscale Adv. 2022. https://doi.org/10.1039/D2NA00229A.
Kanegane H, Wakiguchi H, Kanegane C, Kurashige T, Tosato G. Viral interleukin-10 in chronic active Epstein-Barr virus infection. J Infect Dis. 1997. https://doi.org/10.1086/517260.
Oray M, Abu Samra K, Ebrahimiadib N, Meese H, Foster CS. Long-term side effects of glucocorticoids. Expert Opin Drug Saf. 2016. https://doi.org/10.1517/14740338.2016.1140743.
Schett G, Emery P, Tanaka Y, et al. Tapering biologic and conventional DMARD therapy in rheumatoid arthritis: current evidence and future directions. Ann Rheum Dis. 2016. https://doi.org/10.1136/annrheumdis-2016-209201.
Buttgereit F. Views on glucocorticoid therapy in rheumatology: the age of convergence. Nat Rev Rheumatol. 2020. https://doi.org/10.1038/s41584-020-0370-z.
•• Larrat S, Morand P, Bas A, et al. Inhibition of Epstein-Barr virus replication by small interfering RNA targeting the Epstein-Barr virus protease gene. Antivir Ther. 2009;14(5):655–662. This study highlights the siRNA which specificially target p38 or c-myc genes can inhibit EBV.
Delshadi R, Bahrami A, McClements DJ, et al. Development of nanoparticle-delivery systems for antiviral agents: a review. J Control Release. 2021. https://doi.org/10.1016/j.jconrel.2021.01.017.
Dolati S, Sadreddini S, Rostamzadeh D, et al. Utilization of nanoparticle technology in rheumatoid arthritis treatment. Biomed Pharmacother. 2016. https://doi.org/10.1016/j.biopha.2016.03.004.
Xiao S, Tang Y, Lv Z, Lin Y, Chen L. Nanomedicine - advantages for their use in rheumatoid arthritis theranostics. J Control Release. 2019. https://doi.org/10.1016/j.jconrel.2019.11.008.
Nakahara H, Nishimoto N. Anti-interleukin-6 receptor antibody therapy in rheumatic diseases. Endocr Metab Immune Disord Drug Targets. 2006. https://doi.org/10.2174/187153006779025694.
Grandis JR, Sok JC. Signaling through the epidermal growth factor receptor during the development of malignancy. Pharmacol Ther. 2004. https://doi.org/10.1016/j.pharmthera.2004.01.002.
Harris AL, Nicholson S, Sainsbury R, Wright C, Farndon J. Epidermal growth factor receptor and other oncogenes as prognostic markers. J Natl Cancer Inst Monogr. 1992;11:181–7 (Erratum in: J Natl Cancer Inst Monogr. 2014).
Ang KK, Berkey BA, Tu X, et al. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002;62(24):7350–6.
Rubin Grandis J, Melhem MF, Gooding WE, e al. Levels of TGF-alpha and EGFR protein in head and neck squamous cell carcinoma and patient survival. J Natl Cancer Inst. 1998. https://doi.org/10.1093/jnci/90.11.824.
Tseng SH, Chou MY, Chu IM. Cetuximab-conjugated iron oxide nanoparticles for cancer imaging and therapy. Int J Nanomed. 2015. https://doi.org/10.2147/IJN.S80134.
Author information
Authors and Affiliations
Contributions
M.A., C.A.C.A., A.F.A, R.F. E.N., M.A.R., M.M.: conceptualization and design. M.A., C.A.C.A., A.F.A, R.F. E.N., M.A.R.: literature search, data extraction and analysis. M.A., C.A.C.A., I.M.B, A.M.A.R.: original manuscript writing. All authors contributed to revision and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Viral Infections
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdelmonem, M., Abdullah, C.A.C., Bastawecy, I.M. et al. Antiviral Nanomedicine-Based Approaches against Epstein-Barr Virus Infection. Curr Treat Options Infect Dis 16, 58–71 (2024). https://doi.org/10.1007/s40506-024-00271-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40506-024-00271-4