Abstract
Innate immunity is the first line of the host immune system to fight against infections. Natural killer cells are the innate immunity lymphocytes responsible for fighting against virus-infected and cancerous cells. They have various mechanisms to suppress viral infections. On the other hand, viruses have evolved to utilize different ways to evade NK cell-mediated responses. Viruses can balance the response by regulating the cytokine release pattern and changing the proportion of activating and inhibitory receptors on the surface of NK cells. Exosomes are a subtype of extracellular vesicles that are involved in intercellular communication. Most cell populations can release these nano-sized vesicles, and it was shown that these vesicles produce identical outcomes to the originating cell from which they are released. In recent years, the role of NK cell-derived exosomes in various diseases including viral infections has been highlighted, drawing attention to utilizing the therapeutic potential of these nanoparticles. In this article, the role of NK cells in various viral infections and the mechanisms used by viruses to evade these important immune system cells are initially examined. Subsequently, the role of NK cell exosomes in controlling various viral infections is discussed. Finally, the current position of these cells in the treatment of viral infections and the therapeutic potential of their exosomes are reviewed.
Video Abstract
Similar content being viewed by others
Introduction
Viral infections cause millions of morbidities and mortalities yearly [1]. A recent estimate shows 291,243 to 645,832 influenza-associated deaths occur annually [2]. Also, viruses can increase the mortality rate in special groups of patients. For example, the hepatitis C virus (HCV) is a risk factor for cardiovascular and liver disease-related mortality in patients on maintenance dialysis [3]. Viruses are also known as significant causes of cancer. They can play their tumorigenic role via different mechanisms. Approximately 20 percent of human cancers are related to viruses, and 15 percent of cancer mortality is attributed to virus-associated cancers [4, 5]. Furthermore, dynamic balances and imbalances in the global ecosystem and relation between humans and other animals can lead to the emergence of new viral agents like the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and following epidemics and pandemics that highlight the importance of viral infections [6,7,8].
The possibility of immune system preponderance and successful viral clearance depends on effective coordination between two arms of the immune system named innate and adaptive immunity [9]. Viruses use several mechanisms to bypass or dysregulate the immune system [10]. For example, the severity of liver damage in hepatitis B virus (HBV) infection depends on the immune reaction against the infected hepatocytes [11, 12]. The other strategy of some viruses, such as human immunodeficiency virus (HIV) and Ebola virus (EBOV), is suppressing the immune system to pave the way for infection progression [13, 14].
Before adaptive immune responses are formed, the innate immune system is responsible for defending against many microorganisms [15]. Natural killer (NK) cells are lymphocytes of the innate immune system and play an essential role in the fight against cancerous and virus-infected cells [16]. Human mature NK cells were traditionally known as CD3−CD56+ lymphocytes that comprise a homogenous group of cells. However, it is well-defined that these cells undergo a differentiation process that involves functional and phenotypical changes [17]. It has been shown that patients with NK cell deficiencies are susceptible to viral infections [18]. NK cells use various mechanisms to destroy the infected cells. NK cells express multiple extracellular ligands, including the Fas ligand (FasL) and the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), to induce cytolysis of the target cell using surface death receptors [19]. Interaction of FasL and TRAIL with death receptors of virus-infected cells results in apoptosis of the target cells.
Moreover, NK cells possess granules containing perforin and granzymes that can enter the target cell and commence caspase-mediated apoptosis [16]. This case removes improper cells and helps cellular homeostasis [17]. Besides the cytotoxicity, NK cells participate in antiviral defense through releasing proinflammatory cytokines or exosomes [16, 20]. Exosomes, as a group of extracellular vesicles, can transfer cytoplasmic and membrane contents such as proteins, mRNA, and miRNA from NK cells to the virus-infected cell, exerting effects similar to those of NK cells. Therefore, they can be considered as a therapeutic target that is cell-free based.
In this review, we summarize the interactions and mutual effects of NK cells and viruses, as well as discuss the potential roles of NK cell-derived exosomes in viral infections and their potential use in antiviral therapies.
NK cell biology
NK cells are part of the innate immune system and represent up to 20% of circulating lymphocytes in healthy humans. These cells are found in different parts of the human body, such as peripheral blood, bone marrow (BM), spleen, and non-lymphoid organs [21, 22]. NK cells are known and distinguished by CD16 (an activating Fc receptor) and CD56 (neural cell adhesion molecule (NCAM)) along with lacking a specific T cells activating receptor (TCR) and its signal-transducing adaptor, CD3ε [23]. NK cells are major histocompatibility (MHC)-independent and can kill infected or malignant cells without prior sensitization. NK cells are most similar to T cytotoxic cells and also to innate lymphoid cells (ILCs) [24].
Recent advances in NK cell biology revealed other places as a niche for NK cells, including tonsils, spleen, and (lymph nodes) LNs [25, 26]. For example, the liver is the main site for generating NK cells during fetal life from CD127+ CD117+ common lymphoid progenitors (CLP), and it has been revealed that NK cells can be generated from thymic precursors in the presence of interleukin (IL)-2 and IL-15 [27, 28]. Lin− CD34+ CD133+ CD244+ hematopoietic stem cells (HSC) must first be differentiated into CD45RA+ lymphoid-primed multipotential progenitor (LMPP) to generate NK cells. LMPP differentiates into CLP by expressing CD7, CD38, CD127 (IL-7Rα), and CD10. CLPs are committed to producing Pro-B, Pre-T, natural killer cell progenitors (NKPs), and innate lymphoid cells (ILCs). To produce NK lineage, CLP expresses the IL-15 receptor β chain (CD122) as a sign of becoming NKPs, not other lineages. It has been shown that IL-15 is associated with NK cells survival, maturation, and differentiation. Transcription factors such as T-bet, Id2, Tox, Eomes, Nfil3, Ets1, PU.1, and Tcf1 are involved in NK cell maturation and development. Nfil3 and PU.1 are critical for the early stage of NK cell development. However, Eomes and T-bet are required for the later maturation stages [26, 29,30,31,32]. Through the down-regulation of CD3ε and the expression of CD7+ CD127+ NKP, they enter stage 2 of pre-NK. Based on lacking or expressing IL-1R1, stage 2 of pre-NK is divided into stages 2a and 2b, respectively. The transition to Stage 3 immature NK cells (iNK) comes with the expression of activating receptors such as NKG2D, NKp30, NKp46, and CD161. Immature NK cells develop to stage 4a CD56bright NK cell, and then to stage 4b by expressing NKp80 [33,34,35,36,37,38]. CD56bright NK cells are the less matured NK cell. After that, NK cells start to up-regulation of CD16 and CD94/NKG2C along with downregulation of CD56 and c-Kit (CD117) and CD94/NKG2A to become more mature (stage 6) cell (CD56dim NK cell). The final stage (stage 7) of CD56dim NK cells maturation is the expression of CD57 (HNK-1, Leu-7) and killer cell immunoglobulin-like receptors (KIR). CD56bright NK cells are a smaller population and are mostly related to secreting inflammatory cytokine. They are predominantly found in secondary lymphoid tissues; however, CD56dim NK cells constitute a significant population in the peripheral blood and are primarily associated with cytotoxic functions [30, 33, 39, 40].
NK cell self-tolerance
Similar to other immune cells, NK cells must learn not to attack self-cells, which is known as the education process. The interaction between inhibitory Natural Killer cell receptor (iNKRs) and their ligand on self-cells leads to acquiring tolerance to self. This process helps the NK cells distinguish self-cells from infected or transformed cells, reducing their MHC class I, known as the "missing-self" hypothesis. NK cells are educated if they have an iNKR to interact with an inhibitory ligand on the self-cells [41,42,43]. Without inhibitory receptor-ligand interaction with self-cells, NK cells become hyporesponsive to avoid harming bodies during immune responses [41].
NK cell receptors
NK cell receptors are mainly categorized into three subgroups. Group one is KIR which are composed of inhibitory KIRs (iKIRs) and activating KIRs (aKIRs) [44]. These highly polymorphic receptors are encoded on human chromosome 19q13.4 [45]. Inhibitory receptors are generally more important than activating receptors and play a vital role in education and missing self. KIR2DL1 (ligand HLA-C2), KIR2DL2/L3 (ligand HLA-C1), KIR2DL4 (ligand HLA-G), KIR3DL1 (ligand HLA-A and HLA-B) and KIR3DL2 (HLA-A * 03-A * 11 and HLA-F) are critical iKIR [44]. It has been shown that after influenza infection, the binding of KIR2DL1 to its ligand increased [46]. A recent study has stated a protective role of lacking KIR3DL in individuals infected with HIV and homozygous for Bw4 [47]. The well-known aKIR is KIR2DS1, which recognizes HLA-C2 as its ligand. KIR2DS1 shares high homology with KIR2DL1 [48, 49]. Although KIR2DS1 is one of the well-known activating receptors, there is a lack of evidence for its protective function in viral infection. For example, a study showed KIR2DS1-related fatal outcomes in EBOV infection [50]. However, the protective role of KIR2DS1 has been confirmed in placental human cytomegalovirus (HCMV) infection [51]. Another receptor is KIR2DS2, which recognizes HLA-C1 and has high homology with KIR2DL2/L3 [48]. This receptor protects against HCV and primary HCMV infection after kidney or bone marrow transplantation [52,53,54]. The adverse protective effect of KIR2DS2 has been shown in cases of Herpes simplex virus type 1 (HSV-1) infection [55]. KIR2DS3 is expressed at low levels on the NK cell surface and has no known ligand [48]. It has been shown that this receptor is related to the unsuccessful clearance of hepatitis B and C viruses [56,57,58]. KIR2DS4 has various ligands such as HLA-C1 and HLA-C2 alleles, HLA-A*11:02, and HLA-F [48, 59]. This receptor is associated with an increased susceptibility to HIV infection [60, 61]. KIR2DS5 is a lesser-known receptor which assumed to bind to HLA-C2 [48, 62]. It has been shown that KIR2DS5 is related to an elevated risk of symptomatic HCMV infection [63]. The last aKIR is KIR3DS1, which has been demonstrated that recognize HLA-BBw4 and HLA-F as the ligand [48, 64, 65]. The protective role of KIR3DS1 in viral infections is well described. It is clear that KIR3DS1 is associated with better outcomes in HBV, HIV, and HCV infection [66,67,68].
The second group is Killer cell C-type lectin receptors, composed of inhibitory and activating receptors. NKG2A/CD94 is an inhibitory receptor that recognizes HLA-E as its ligand [44]. A study demonstrated that NKG2A is required to control poxvirus infection through CD8+ T cells [69]. It has been shown that NKG2A expression increased on the surface of NK cells by HCV and HBV infection, which can restrain immune response [70, 71]. NKG2C/CD94 is an activating C-type lectin receptor that knows HLA-E as the ligand [44]. It has been reported that NKG2C+ NK cells were inversely correlated with HIV viral load and exhibited anti-HIV activity [72]. Moreover, the NKG2C- NK cell frequency was higher in patients living with HIV than in HIV-exposed seronegative subjects [73]. However, a study on Brazilian individuals infected with HIV showed no association between complete deletion of NKG2C and susceptibility to HIV infection [74]. NKG2D is another activating receptor related to C-type lectin receptors. Its ligands are composed of MICA, MICB, and ULBP, which are up-regulated on the surface of infected cells [44]. It has been shown that in HCV infection, NS5A protein can stimulate monocytes through toll-like receptor (TLR) 4, which results in an increase in IL-10 production. In turn, IL-10 decreases the NKG2D expression, leading to the impairments of NK cell antiviral response [75]. HVC virus also can downregulate the expression of NKG2D ligands to avoid immune response [76]. HIV can also evade the immune response by downregulating the NKG2D ligand through its NEF protein [77].
The third comprises the natural cytotoxicity receptors (NCR), mainly categorized into NKP30, NKP44, NKP46, and NP80 [44]. NKP30 and NKP46 are expressed on NK cells before activation; however, NKP44 is expressed only after activation [44]. These activating receptors help NK cells lyse the cells expressing their ligands. The ligands for the NCRs have not been fully recognized. However, BAT-3 and B7-H6 for NKP30, PCNA, NIDOGEN-1, PDGF-DD for NKP44, and the complement factor P for NKP46 have been identified [44]. It has been shown that NKP44 and NKP46, but not NKP30, play a vital role in the lysis of influenza-infected cells through binding to hemagglutinin (HA) [78]. The previous data has shown that HCMV, via its pp65 protein, inhibits the functional activity of NKP30, resulting in NK cell inhibition [79]. NKP30 expression has been related to protection against HCV infection based on a recent study [80]. However, HCV-infected cells have been demonstrated to inhibit ex-vivo NK cell functions and downregulate NKP30 on the surface of NK cells [81]. NKP80 is less known than other receptors and participates in NK cell cytotoxicity by binding to its ligand, activation-induced C-type lectin (AICL) [82].
DNAX accessory molecule-1 (DNAM-1), or CD226, is an activating receptor that improves NK cell cytotoxicity by interacting with its ligands, CD155 and CD112 [83]. Lysosomal-associated membrane protein-1 (LAMP-1 or CD107) is an NK cell-related receptor that is upregulated on NK cells after cytokine secretion and NK cell-mediated lysis of target cells [84]. 2B4 and NTB-A belong to the Signaling Lymphocyte Activating Molecule (SLAM) family expressed on NK cells' surface and assist NK cell-related cytotoxicity. 2B4 ligand is CD48, and NTB-A recognizes NTB-A by homophilic interactions [85].
NK cell in viral infections
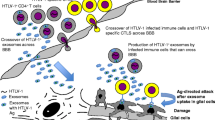
NK cells are one of the first barriers against viral infections. Their different methods to detect and annihilate the infected cells have been discussed previously. Conversely, viruses have evolved to develop different mechanisms to evade the NK cell response (summary in Fig. 1 and with more details in Table 1). This section describes the interaction between different viral families and NK cells.
Viral infections have different ways of evading the immune response by the NK cells. A They can alter the proportion of ligands on the cell surface of infected cells to change the NK cell response after interacting with the infected cells from activation to inhibition. B Also, they can use cytokines and viral proteins to send inhibitory signals to the NK cells. C Some viruses can also directly infect the NK cells and induce apoptosis
Parvoviridae
Members of the Parvoviridae family are non-enveloped viruses with a linear single-stranded DNA genome that can infect a diverse array of animals, from arthropods to vertebrates [123]. B19 is the most well-known member of this family because of its involvement in various diseases such as hydrops fetalis, aplastic anemia, erythema infectiosum, myocarditis, and rheumatologic diseases [124,125,126]. Another member of this family is the Human bocavirus (HBoV), which is assumed to contribute to respiratory and gastrointestinal complications [127].
Alteration of host cytokine status after B19 infection affects the NK cells. Infection of monocytes by B19 results in decreased expression of tumor necrosis factor (TNF)-α mRNA [86]. TNF-α enhances IL-2 effects to cause NK cell differentiation, activation, and cytotoxicity [128]. Infection of human airway epithelial cells by HBoV-1 increases the release of IL-18 [129]. IL-18 promotes NK cell proliferation [130] and provokes antiviral activity of NK cells by inducing the production of IFN-γ [131]. Additionally, Parvovirus infection may affect the number of NK cells. A comparison of B19-infected renal transplant recipients with non-infected recipients indicated a considerably lower number of lymphocytes, including NK cells [132]. Moreover, a significant decrease in the number of NK cells, as well as T and B lymphocytes, was observed in a patient with B19-related hepatitis negative for hepatitis A, B, and C viruses [133].
Polyomaviridae
The Polyomaviridae family is a group of non-enveloped viruses with a circular double-stranded DNA genome that is only 5.5 kbp in size. The capsid is icosahedrally shaped and is constructed of 72 pentameric capsomers. The Polyomaviridae family consists of 5 genera and 37 species, which are infectious to avian and mammalian species [134]. Their genome encompasses three parts: (I) The regulatory region contains promoters and the origin of replication that regulates the expression of the other parts. (II) The early region encodes early proteins, including large and small T antigens. (III) The late region is expressed from the complementary region and encodes capsid and agnoprotein [135]. To date, 14 human polyomaviruses (HPyVs) have been identified. Some of them are known as human pathogens [134]. For instance, the BK virus is involved in nephritis pathogenesis, JC virus in progressive multifocal leukoencephalopathy, MCPyV in Merkel cell carcinoma, and TSPyV in Trichodysplasia spinulosa. HPyVs 6 and 7 are also suggested to be potential contributors in pruritic rashes [136].
A correlation was observed between the genotype of activating NK cell immunoglobulin-like receptors and controlling BK virus infection in renal transplant recipients. Upregulation of HLA-F expression has led to enhanced binding of KIR3DS1 to BK virus-infected cells and, thus, activation of KIR3DS1+ cells [137]. In patients with BK virus-associated nephritis, NK cells possessed lower KIR3DS1 activating receptors than a control group of recipients negative for BK virus infection. Researchers also found that the telomeric part of KIR haplotypes is associated with infection, suggesting an NK cell genetic predisposition to BKV infection [87]. Solenocytes of Polyomavirus-infected mice with severe combined immunodeficiency (SCID) showed many MHC-1 molecules that can mitigate NK cell activity. These mice finally developed an NK cell-resistant lymphoproliferative disease [138]. Infection of NK cell-deficient mice resulted in faster tumor development than TCRβ × δ KO mice. The established cell line from the developed expressed Rae-1, a cellular stress molecule that acts as an NKG2D ligand, resulting in the efficient destruction of these cells by NK cells [139].
Papillomaviridae
The Papillomaviridae is a family of dsDNA viruses with a non-enveloped icosahedral virion. They are classified based on the genetic similarity of the major capsid protein (L1) [140]. Human Papillomaviruses (HPVs) are well-known for their ability to cause different malignancies in humans, especially anogenital cancers. For example, almost all cervical cancers are HPV + [141]. The main transmission route is through sexual activity, but the viruses can spread via skin-skin contact. Up to now, more than 200 HPV types have been detected, of which at least 14 high-risk genotypes have been recognized. Interestingly, about 80% of people contract the virus in their lifetime [142].
Despite the efforts of NK cells to recognize and kill the cancerous cells, HPV can modulate that. For instance, a comparison of the NK cell count in tissues of women with cervical cancer showed that patients with higher HPV viral load had lower numbers of NK cells while patients with lower viral load had higher numbers of NK cells [143]. HPV oncoproteins E6 and E7 can downregulate the transcription of type 1 IFNs. E6 is also shown to downregulate the expression of IFN-κ, a member of type 1 IFNs [88]. They can also suppress the IL-18-induced IFN-γ by binding to the IL-18 receptor [89]. Viral oncoproteins also can inhibit inflammasome activation to avoid NK cell activity by cytokines. E6 degrades the p53 to inhibit the transcription of Interferon Regulatory Factor 6 (IRF6) and thereby decreasing the IL-1β [90]. E7 interaction with Interferon Gamma Inducible Protein 16 (IFI16) and TRIM21 and recruitment of the E3 ligase TRIM21 to degrade the IFI16 inflammasome by ubiquitination leads to reduced IL-18 [91]. A remarkable downregulation of NK cell activating receptors NKp30, NKp46, and NKG2D in the high-grade squamous intraepithelial lesion and cervical cancer was observed [92], and NK cells in HPV infected cervical cancer microenvironment show inadequate cytotoxicity [93]. Moreover, HPV seems to induce carcinogenesis by stimulating inflammatory reactions by increasing KIR2DS5 activating receptor that is shown to be associated with cancers [144].
Herpesviridae
The family Herpesviridae is an extensive group of double-stranded DNA, enveloped viruses able to establish persistent infection in the immune competent hosts. This family is further classified into three main branches according to genome organization, biological characteristics, and cellular tropism: Alpha-, Beta-, and Gammaherpesviruses [145]. HSV1 and HSV2 are the most well-known members of this family, known for their role in various complications such as cold sores, herpetic keratitis, and encephalitis. The other member, which infects more than 90% of the world population, is the Epstein-Barr virus (EBV) [146], a lymphotropic virus that causes infectious mononucleosis. EBV was first discovered in cultured African endemic Burkitt’s lymphoma cells [147]. To date, its association with different types of cancer, such as nasopharyngeal and gastric cancer, has been found [148]. HCMV is the other important and prevalent herpesvirus that poses a dangerous threat to immunocompromised individuals by causing various clinical manifestations, including retinitis, gastroenteritis, hepatitis, pneumonitis, transplant rejection, atherosclerosis and damaging fetus [149].
In 1989, Biron et al. reported a 13-years-old girl with NK cell deficiency that had severe infection with HSV, Varicella Zoster virus (VZV), and HCMV [150]. Another study on children with herpetic encephalitis found that all patients had NK deficiencies [151]. Mutations in GATA-2, which is required for NK cell maturation [152], hinder early differentiation of CD56bright NK cells, which predisposes individuals to herpesvirus infections and mutations in minichromosome maintenance complex component 4 (MCM4) or IRF8 inhibits the maturation of CD56dim NK cells, which makes individuals prone to EBV-associated lymphoproliferative diseases [153]. Herpesviruses developed several mechanisms to evade NK cells. It has been observed that patients with NK cell deficiencies are prone to life-threatening infections by different Herpesviruses. MICA, MICB, and ULBP1 are upregulated in HCMV-infected cells. Placement of these proteins on the surface of infected cells triggers NK cells via binding to NKG2D [105]. UL16 can bind to ULBP1 and MICB to neutralize their effects, but not MICA due to extracellular α2 domain differences [94]. The other HCMV glycoprotein UL142 is able to inhibit MICA and ULBP3 [105]. UL148A has recently been found to downregulate MICA through lysosomal degradation. However, it cannot solely act, and an unknown viral factor seems essential for its proper function [101]. US18 and US20 are other HCMV-coded proteins that target MICA and the NKp30 ligand B7-H6, which induces MICA lysosomal degradation [102, 105]. HCMV US9 can selectively target MICA*008 to its proteasomal degradation [104]. A new family of HCMV genes called the US12 gene family was introduced recently that targets NK cell ligands, adhesion molecules, and cytokine receptors [105]. HSV-1 infection decreases the cell surface levels of MICA, ULBP1, ULBP2, and ULBP3 in a non-elucidated process [105]. The expression of MICA and MICB is decreased in HHV-7-infected astrocytoma cells. ULBP1 was transferred by HHV-7 to a lysosomal compartment, which resulted in its degradation. [107]. DNAM-1 ligands are the other targets of Herpesviruses. UL141 is reported to downregulate cell surface expression of DNAM-1 ligands CD112 and CD155 [105]. It has been observed that infection with laboratory HCMV strains AD169 and Towne that faced deletion mutations in the UL141 gene results in higher sensitivity to NK cells compared with infection with clinically isolated strains [154]. Moreover, UL141 downregulates surface TRAIL death receptors required for TRAIL-induced NK cell-mediated apoptosis [99]. In the case of Alphaherpesviruses, gD stimulates the degradation of CD112, and strains lacking gD failed to evade recognition by DNAM-1 [155]. Interaction of the HCMV major structural protein pp65 with NKp30 disrupts the CD3ζ signaling pathway and enfeebles the NK cell cytotoxicity [79]. Also, It has been seen that the ORF54-encoded protein of Kaposi sarcoma herpesvirus (KSHV) downregulates an unknown ligand of NKp44 [108]. Herpesviruses encode CD16 (FcγIII receptor) analogs to impede the antibody-dependent cellular cytotoxicity (ADCC). These proteins are the gE-gI complex of HSV and VZV, gp34, gp95, gpRL13, and gp68 of HCMV [105]. EBV BCRF1 encodes IL-10 to hamper NK cell activation [156]. KSHV inhibits NK cell migration by encoding a viral chemokine, vMIP-II (vCCL2), which acts as an antagonist for chemokine receptors of Fractalkine and RANTES [105, 109]. The other KSV protein, K5, retains the NKG2D and NKp80 ligands MICA and AICL within the cell to avoid their presence on the cell surface [110].
To combat MHC-I-dependent activity, herpesviruses developed various strategies. For instance, the HCMV UL18 protein binds to leukocyte immunoglobulin-like receptor 1 (LIR-1), an MHC-I inhibitory receptor. Furthermore, HCMV UL40 upregulates the expression of the nonclassical MHC-I molecule HLA-E to protect the infected cell from the activation of CD94/NKG2A+ NK cells [105]. Polymorphism in the UL40 sequence affects the HLA-E/NKG2 binding affinity since the UL40 protein with polymorphism showed less affinity to NKG2 receptors [157].
Coronaviridae
The Coronaviridae family consists of enveloped viruses characterized by single-stranded positive-sense RNA, capable of infecting both humans and animals [158]. This family is divided into four subfamilies, alpha (α), beta (β), gamma (γ), and delta (δ) coronaviruses (CoVs). Seven members of this family are known to infect humans: NL63 and 229E in the alpha-CoV and HKU1, OC43, SARS-CoV-1, Middle East respiratory syndrome, (MERS-CoV), and SARS-CoV-2 in the beta CoV. Thus far, three pandemics have occurred among the members of this family, SARS-CoV-1, MERS-CoV, and SARS-CoV-2; the latter is more infectious and contagious and is responsible for the latest pandemic, named Coronavirus disease (COVID-19), leading to the death of millions of people worldwide [159].
Patients infected with SARS-CoV-2 had low numbers of CD56dim and CD56bright NK cells that might result from trafficking to the lungs via CXCR3, CXCR6, and CCR5 chemokines [160, 161]. NK cells in the lung are active and proliferative, especially the CD56bright subset, and thus, they appear to be cytokine-derived since CD56bright cells respond more vigorously to cytokines [160]. The activation of these cells appears to be triggered by IL-6, IL-6R, and IL-18 [162]. Moreover, prolonged activation of NK cells can contribute to their dysfunction. It has been suggested that prolonged stimulation of NK cells by IL-15 may cause NK cell dysfunction, probably by epigenetic reprogramming. In addition, surface expression of HLA-E is elevated in patients infected with SARS-CoV-2 [160]. This is attributed to the Spike 1 (S1) protein [111]. On the other hand, viral Non-structural protein 13 (Nsp13) encodes an HLA-presented peptide that hampers the inhibition of NKG2A+ NK cells and, thus, helps the NK cells to restrain the infection [163]. Antibodies against SARS-CoV-2 trigger ADCC of infected cells by NK cells, and this was even higher in infected patients than in those who received an S1 coding vaccine. Cross-reactive antibodies in sera collected before the pandemic also could stimulate ADCC [164].
The immunophenotype of NK cells might affect the severity of SARS-CoV-2 infection. Maruthamuthu et al. have linked the absence of KIR3DL1+HLA-Bw4+ and KIR3DL2+HLA-A3/11+ and the plethora of KIR2DS1+KIR2DS5+ to the severe infection [165]. Another study reported more KIR2DS4 in severe and KIR3DS1+HLA-B*15:01+ in mild patients compared with controls [166]. Furthermore, KIR2DS2 and HLA-C1 complex exhibited potent effects against COVID-19 adverse effects [167]. A study of hospitalized and outpatients has suggested that NKG2C is a pivotal actor in limiting the severity of COVID-19 since KLRC2del is observed more in hospitalized patients. It also demonstrated that the HLA-E*0101 variant is more prevalent in hospitalized patients [168].
Intriguingly, a high probability of HCMV reactivity in SARS-CoV-2 infected patients was observed. Although the mechanism has not been completely discovered yet, it is suggested that the high proportion of NKG2C−/NKG2A+ cells in patients with COVID-19 is associated with higher HCMV viremia and mortality [169, 170].
Flaviviridae
The Flaviviridae family consists of enveloped single-stranded RNA viruses that are further classified into four genera: Flavivirus, Hepacivirus, Pegivirus, and Pestivirus. Flaviviruses such as Zika virus (ZIKV), West Nile virus (WNV), Dengue virus (DENV), Yellow fever virus (YFV), Japanese encephalitis virus (JEV), Tick-borne encephalitis virus (TBEV) are mosquito-borne and tick-borne viruses that mostly infect humans and cause a wide range of symptoms from mild febrile condition to severe hemorrhagic fever. The only human-infecting member of the Hepacivirus is HCV. Members of the Flaviviridae family are ubiquitous and annually cause millions of new cases [171].
NK cell response to Flavivirus is restricted to less differentiated CD56bright and CD56dim cells since they are more sensitive to IL-12, IL-15, IL-18, and type I IFNs [17]. Utilizing West Nile virus (WNV) like particles and WNV-infected cells indicated that the interaction of Flavivirus envelope (E) protein with NKp44 receptor leads to IFN-γ production and activation of NK cells [172]. The addition of Anti-DENV monoclonal antibodies to DENV-infected Raji cells with reduced MHC-I expression in the presence of PBMCs taken from a patient infected with DENV showed increased cytotoxicity, while the addition of sera from healthy individuals has not shown such result [173]. Similar the SARS-CoV-2, the immunophenotype can also affect NK cell response to Flaviviruses. It has been shown that the inhibitory receptor KIR2DL3 and homozygosity of its ligand HLA-C1 are associated with HCV clearance [17, 174]. While NKG2A+ and CD94+ cells were elevated in acute HCV infection, they were not involved in the anti-viral response. Conversely, NKp30+, NKp46+, CD161+, and NKG2D+ were detected in patients that successfully resolved infection [175]. Another study also showed NKp56high NK cells had a higher cytotoxicity IFN-γ secretion ability than NKp56dim cells [176].
DENV can evade NK cell response by upregulating MHC-I through an unknown mechanism, but the viral non-structural proteins are proposed to be involved in this process. Besides reducing cytotoxicity, aggregation of MHC-I may increase affinity to NK cell inhibitory receptors [112]. Infecting IL-12p40−/− and IL-18−/− mice with DENV decreased IFN-γ release and escalated the disease severity [177]. DENV-infected cells expressed fewer NKp30 and NKp46 ligands to avoid NK cell activation [178]. In the case of HCV, it has been observed that high viral load was associated with NK cell exhaustion through elevating exhaustion marker programmed cell death protein (PD)-1 expression [179]. In addition, cell-to-cell contact between HCV-infected human hepatoma cell line and NK cells reduces degranulating and IFN-γ production ability and downregulates expression of activating receptors such as NKG2D and NKp30 [113].
Orthomyxoviridae
Viruses in the Orthomyxoviridae family have enveloped viral particles that harbor a negative-sense, single-stranded, and segmented RNA genome. This family is classified into seven genera: influenza virus (IV) A, B, and C; Thogotovirus; Isavirus; and a new genus Quaranjavirus. IVs are responsible for numerous endemics and pandemics in history with tremendous mortality [180]. Symptoms can be different from a cold-like disease to lethal pneumonia. These viruses are highly prone to genetic alterations and can evade preexisting immunity in the host’s body [181].
IV uses HA glycoprotein to enter the host cell by attaching to α-2,3 and/or α-2,6 linked terminal sialic acids. IV can infect the NK cells since both sialic acids are present at the surface of NK cells. Also, the activation NKp46 receptor possesses α-2,6 terminal sialic acids. While NK cells can detect IV-infected cells through the attachment of NKp46 to HA and act against them, IV can counterattack the NK cells by infecting them in this way [16]. Mice lacking the NCR1 (NKp46 in humans) gene died after infection by IV A H1N1 while NK cells were accumulated at the site of infection while NCR1+ mice survived, showing the pivotal role of NKp56 in the eradication of IV infection [182]. Interaction of NK cells with intact or free HA leads to the downregulation of NKp30 and NKp46 to mitigate cytotoxicity.
Furthermore, IV induces apoptosis of NK cells that can be prevented by caspase-3 inhibitor (Z-DEVD-FMK) [183], which is supported by evidence noting depletion of peripheral NK cells in IV-infected patients [16]. IV also affects the cytokine and chemokine status of NK cells. It has been shown that IFN-γ, GM-CSF, MIP-1α, MIP-1β, and RANTES are downregulated in IV-infected NK cells [184]. However, data are debating about IFN-γ and TNF-α since their upregulation in the presence of IL-15 is reported [16].
Gene expression and NK cell phenotype affect the anti-influenza response. A cohort study on the expression profile of the KLRD1 gene showed that this gene is downregulated in symptomatic IV-shedders and depicted a negative association with the severity of symptoms. Thus, the expression profile of this gene can be a predictor of influenza severity [185]. CD56brightCD49a+ NK cells exhibited a more efficient response than CD56brightCD49a− against IV infections, as assessed by CD107a expression analysis [186]. Interestingly, the lethal outcome of infecting TLR7-KO mice with IV showed that capacity of NK cells for activation, interferon (IFN)-γ production, and cytotoxicity is related to TLR-7 [187]. Therefore, we suggest the evaluation of TLR-7 variants and mutations to understand their impact in the severity of influenza in patients.
Pneumoviridae
Members of the Pneumoviridae family are a group of large enveloped negative-sense RNA viruses that were previously a subfamily within the Paramyxoviridae family. After reclassification in 2016, they have become an independent family with two genera Orthopneumovirus and Metapneumovirus. These viruses mainly infect mammals, but some can also infect birds [188]. Human respiratory syncytial virus (RSV) is a human pathogen in this family that is notorious for causing the most frequent viral acute lower respiratory tract infections in infants with a high burden of hospitalization, pediatric intensive care unit (PICU) admission, and mortality worldwide [189].
In an in vitro study, RSV infected about 90% of NK cells, showing its ability to infect various subsets of NK cells [190]. In contrast to other immune cells, RSV-infected NK cells do not release viral particles [16]. However, NK cell phenotype can be indirectly affected by RSV. Pre-incubation of NK cells with RSV resulted in the downregulation of NKG2D and NKp44 [16, 114]. RSV infection also upregulates MICA on the lung epithelial cells. This might be due to the increased secretion of IFN-γ that affects MICA expression with a negative impact mechanism that eventually inhibits NK cells [191]. RSV-infected infants expressed LILRB1 higher than controls. It may implicate a high risk of severe RSV infection. RSV infection of lung epithelial cells upregulates the LLT1 gene that stimulates CD161 [114, 192].
Expression of CXCL10 on lung epithelial cells leads to infiltration of NK cells to the lungs and, consequently, reduces the number of NK cells in the peripheral blood [114]. When co-culturing with RSV-infected cells, neonatal NK cells showed decreased perforin secretion compared with adults. However, CD107 expression is upregulated in neonatal and adult RSV-infected NK cells [16]. IFN-γ production negatively impacts antibody production in RSV-infected infants [193]. Blocking IFN-γ production and depletion of NK cells, T cells, or both exhibited a remarkable enhancement of anti-RSV antibody production [114]. It shows the role of IFN-γ secretion by NK and T cells in APC inhibition [114]. Moreover, IFN-γ increased IL-15 expression in epithelial cells that improved CD8+ T cells. In conclusion, IFN-γ secreting NK cells enhance the antigen-specific CD8+ T cells that eventually inhibit NK cell activity [114, 194].
Filoviridae
Viruses in the Filoviridae family are non-segmented negative-stranded famous for causing fatal hemorrhagic fever outbreaks of zoonotic origin [195]. To date, a total of 12 members have been identified within this family. The medically important members are included within the two groups named EBOV and Marburg virus (MARV) [196]. EBOV includes Bundibugyo ebolavirus, Reston ebolavirus, Sudan ebolavirus, Taï Forest ebolavirus, and Zaire Ebolavirus. Only Bundibugyo, Sudan, and Zaire EBOVs were attributed to human infections, with a case fatality rate of 25%, 50%, and 80%, respectively [197]. Filovirus outbreaks have led to the loss of tens of thousands of lives [198].
Host cells infected with the EBOV are reportedly resistant to being destroyed by NK cells [199]. EBOV infection suppresses the release of type I IFNs and IL-12 [200]. The VP24 and VP35 of EBOV disrupt NK cells' maturation and activation [115, 116]. However, EBOV viral-like particles (VLPs) cannot cause the same result, indicating the prominent role of cytokines in NK cell-mediated immunity to EBOV [200]. NK cells use NKp30 and probably NKG2D to recognize EBOV VLPs. In contrast, EBOV downregulates ligands of these receptors to avoid NK cell activation [201]. To counteract NK cell activation via type I IFNs signaling, Marburg virus VP40 hinders the activation and function of Janus kinase 1 (JAK1), which results in the impaired type I IFN-induced phosphorylation of STAT1 and STAT2 [117].
One of the main characteristics of Ebola disease is severe immunosuppression due to lymphocyte apoptosis. NK cells are shown to infiltrate EBOV-infected mice organs and kill infiltrating T cells [202]. Moreover, elevated levels of IFN-γ were observed in patients with hemorrhagic shock in contrast to survivors [200]. In this case, the similarity of mRNA levels of IFN-γ in both groups suggests that the difference in the translation level may contribute to the disease fate [200, 203]. EBOV glycoprotein VP40 triggers NK cells through an IL-12 and IL-18-dependent manner, leading to a higher increase of TNF-α, IFN-γ, perforin, and granzyme B by CD3−CD56+ cells [204].
Retroviridae
Retroviruses are enveloped viruses containing a dimer of single-stranded positive-sense RNA and a reverse transcriptase enzyme. They have a restricted host range and among them, human T-lymphotropic viruses (HTLV), HIV, and simian foamy viruses (SFV) are able to infect humans. According to the epidemiological, phylogenetic, and genomic characteristics investigations, it is assumed that HIV originated from the simian immunodeficiency virus (SIV) [205]. HIV belongs to the genus Lentivirus; it is estimated that 37.9 million people live with HIV infection [206]. HIV is classified into HIV-1 and HIV-2, originating independently from chimpanzee simian immunodeficiency virus (SIV) and Old-World monkey SIV, respectively. HIV poses a considerable threat to the body since it cripples the immune system by triggering pyroptosis through caspase-1 activation in lymphoid CD4 T cells [205].
HIV shows tropism to CD4+ T cells and other immune cells such as dendritic cells and macrophages. HIV has developed various mechanisms to avoid NK cell-induced immune response. NK cells release CCR5 ligands, including β-chemokines, CCL3, CCL4, and CCL5, that hinder the attachment of the virus to the cells [207]. The phenotype variation of NK cells is related to different innate immune conditions in HIV-infected patients. For example, combining HLA-B Bw4-80I KIR3DL1*h or KIR3DS1 improves immune response. In the case of KIR3DS1+ NK cells, an HLA-B Bw4-80I-dependent suppression of viral replication has been observed, showing the important role of NK cells in anti-HIV immunity [17]. Characterizing the KIR and HLA genes DRB1*11 and DRB1*12 has shown that the inhibitory KIR2DL5B and the HLA-DRB1*12 allele may cause protection against HIV-1 in seronegative couples. Also, KIR gene haplotype (AA and Bx) is not associated with infection in serodiscordant partners [208]. While MHC-I downregulation happens in HIV infection, HLA-C and HLA-E85 are exempted from downregulation, resulting in impaired recognition of the infected cells [209]. Furthermore, although NKG2D ligands are upregulated in HIV-infected cells, viral accessory proteins hamper the activation of NK cells [17, 210]. HIV nef protein counteracts the recognition by NK cells via decreasing the cell surface amount of MICA, ULBP1, and ULBP2 [77]. Infection of CD4+ T cells with HIV has resulted in the downregulation of NKp44 ligands via nef-mediated intracellular retention of these ligands [118]. Nef also, alongside vpu, downregulates the expression of NTB-A and PVR (CD155) [119, 120]. HIV-infected cells release soluble ligands for the NKG2D receptor that downregulates and functionally impair NKG2D receptors. Patients receiving highly active antiretroviral therapy have shown improved NKG2D function and lower plasma levels of the soluble ligands for the NKG2D [122].
HIV infection can alter the NK cell receptor functions. For instance, a remarkable loss of CCR7+CD56bright NK cells that results in higher viral load has been shown in treatment-naïve patients [211]. In addition, the loss of CD56dim cells has also been reported [211]. It has been shown that treating HIV-infected patients changes the CD56bright and CD56dim population in the body [212]. The SIV infection in the animal models caused the consolidation of CD56−CD16+ NK cells in lymph nodes [17, 213].
The crosstalk between NK cells and virus-infected cells via exosomes
Exosomes
The cells interact with each other in various ways, such as cell–cell contact, secretion of soluble molecules, and by secreting exosomes. Exosomes are a subtype of extracellular vesicles involved in intercellular communication [214]. Most cell populations can secrete these Nano-sized vesicles, and it was demonstrated that they exert the same effects as the cell from which they are secreted [215]. Exosomes have lipid bilayer structures and can carry various macromolecules such as miRNAs, mRNAs, proteins, etc. In addition, these vesicles can carry some molecules of the origin cell on their surface [216].
The properties of NK cell-derived exosomes
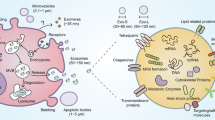
Like other immune cells, NK cells can also secrete exosomes with various cargo and affect the target cells (Fig. 2). Exosomes from different sources have some common characteristics; for example, they contain multivesicular bodies associated proteins such as TSG101 and Alix or some tetraspanin glycoproteins like CD9, CD63, CD81, and CD82. Although definitive biomarkers for NK cell-derived exosomes have not been identified yet, some studies demonstrated that in addition to typical exosome markers, NK cell-derived exosomes exhibit some specific biomarkers such as CD56, DNAM-1, NKp30, NKp44, NKp46, and NKG2D [44].
NK cells can secrete exosomes with various cargo. These exosomes contain TSG101, Alix, CD9, CD63, CD81, and CD82. In addition to typical exosome markers, NK cell-derived exosomes exhibit specific biomarkers such as CD56, DNAM-1, NKp30, NKp44, NKp46, and NKG2D. NK cell-derived exosomes contain perforin and express FasL, which contribute to the cytotoxicity effects of these vesicles. Cytotoxic proteins such as perforin, granzyme A, granzyme B, and granulysin can induce apoptosis in target cells. Since the mechanism of action of NK cells and their exosomes against tumor and virus-infected cells is relatively similar, it seems that NK cell exosomes use the following mechanisms to fight against virus-infected cells. NK cell derived-exosomes can overexpress the NK activating receptors NKp30, NKp44, NKp46, and NKG2D and be able to educate naïve NK cells, which evolve into a memory-like state, with increased cytotoxicity and enhanced tumor-killing capacity. NK cell-derived exosomes were rich in miR-10b-5p, miR-155-5p, or miR-92a-3p. T cell response can be the putative target of these small RNAs. So, NK cell-derived exosomes promote Th1 differentiation and increase IL-2 and IFN-γ production. On the other hand, the polarization of monocyte to monocyte-derived dendritic cells can be affected by NK cell-derived exosomes, and these vesicles increased the expression of MHC-II and CD86 on these cells. NK cell-derived exosomes can shift macrophage polarization to M1. As mentioned, the existence of FasL and TRAIL on the surface of NK cell-derived exosomes and cargo of perforin, granzyme A, granzyme B, and granulysin were confirmed by multiple studies supporting direct cytotoxic effects of NK cell exosomes in the face of target cells. miRNAs are another tools that these valuable exosomes use to inhibit target cells
As mentioned, exosomes exert the effects of the cell they originated, so NK cell-derived exosomes are expected to have cytotoxic effects on target cells. Lugini et al. isolated exosomes from resting and activated NK cells and showed that these exosomes contain perforin and express FasL, which contribute to the cytotoxicity effects of these vesicles [217]. Jong et al. isolated a large quantity of NK cell-derived exosomes and assessed their cytotoxic effects. They demonstrated that NK cell-derived exosomes contain several cytotoxic proteins such as perforin, granzyme A, granzyme B, and granulysin. These cytotoxic proteins can activate the caspase-dependent pathway in target cells and induce apoptosis [218]. Another study introduced other effector factors for NK cell-derived exosomes' cytotoxic functions, such as TRAIL, fibrinogen, and β-actin. Moreover, the proteome analysis in this study confirmed that these exosomes contain NKG2D and FasL, and these molecules can cause cytotoxic effects on target cells [219]. Other studies indicated that NK cell-derived exosomes can carry IFN-γ and TNF-α [220, 221]. Mass spectrometry and cytokine analysis performed by Federici et al. demonstrated that NK cell-derived exosomes express markers that contribute to their cytotoxic effects, trafficking, adhesion, and activation of immune responses. These markers contain NKG2D, CD94, perforin, granzymes and CD40L [222].
Mechanism of action of NK cell-derived exosomes
As mentioned, NK cells have roles in defense against tumors and viral infections. Due to the availability of different cancer cell lines, as well as the fact that cancer is non-contagious and safer than viral infection, most of the studies related to NK cells have focused on their anti-tumor properties. So, the effects of NK cell-derived exosomes were assessed on different cancer cells. Many studies investigated the cytotoxic effects of NK cell-derived exosomes on both hematologic and solid cancer cells (Fig. 2). In a study conducted in 2012, healthy donors' NK cells were purified, and their exosomes were isolated to assess the cytotoxicity of NK cell-derived exosomes against hematologic cell lines (Jurkat and K562) and also cell lines from solid tumors (SKBR3 and DAUDI). This report indicated that NK cell-derived exosomes induced cytotoxicity in hematologic cell lines, while the cell lines deriving from solid tumors appeared to be relatively resistant to NK cell-derived exosomes' cytotoxicity [217]. Another study investigated the cytotoxic effects of NK cell-derived exosomes on MCF-7, SupB15, CHLA-136, CHLA-255, and NALM-6 cell lines. Their results showed that the percentages of dead cells increased after incubation with NK cell-derived exosomes [218]. In addition to these, the cytotoxic effects of NK92MI-derived exosomes on different leukemic cell lines were confirmed by Samara et al. NK cell-derived exosomes also indicated effective therapeutic effects in vivo [223]. After analysis of the tumor-targeted homing ability of NK cell-derived exosomes, high uptake was detected in the tumor site of the hepatocellular carcinoma-bearing mice. In addition, the tumor growth was suppressed by NK cell-derived exosomes. Taken together, the results of different reports exhibit that NK cell-derived exosomes can exert similar effects of NK cells on tumor cells.
Since the mechanism of action of NK cells and their exosomes against tumor and virus-infected cells is relatively similar, it seems that NK cell-derived exosomes use the following mechanisms to fight against virus-infected cells.
NK cell-derived exosome's mechanism of action can be direct or indirect. Indirectly, these exosomes affect other immune cells and activate them to exhibit immune responses against cancer cells or virus-infected cells. Directly, the exosomes elicit cytotoxic effects using the effector molecules that exist in them.
Interestingly, it was shown that NK cell-derived exosomes can affect non-activated NK cells, and these vesicles can activate the NK cells. Shoae-Hassani et al. indicated that NK cell-derived exosomes could overexpress the NK activating receptors NKp30, NKp44, NKp46, and NKG2D and be able to educate naïve NK cells, which evolve into a memory-like state, with increased cytotoxicity and enhanced tumor-killing capacity [224]. Dosil et al. performed next-generation sequencing for analysis of the small RNA content of resting and in vitro-activated NK cells and the exosomes secreted by them. Then, in silico-predicted mRNA targets were done to identify the immune-related molecules that these small RNAs can target. First, it was clear that NK cell-derived exosomes were rich in miR-10b-5p, miR-155-5p, or miR-92a-3p. Since T cell response can be the putative target of these small RNAs, the CD4+ T cells were isolated and treated with NK cell-derived exosomes. The results showed that these exosomes promote Th1 differentiation via Gata3 downmodulation and T-bet de-repression correlating with increased levels of miR-10b-5p and miR-92a-3p. In addition, NK cell-derived exosomes can activate the CD4+ T cells and increase IL-2 and IFN-γ production but not Treg responses.
On the other hand, the polarization of monocyte to monocyte-derived dendritic cells can be affected by NK cell exosomes, and these vesicles increase the expression of MHC-II and CD86 on the surface of these cells. So, the antigen presentation and T cell activation can be improved [225]. NK cell-derived exosomes can shift macrophage polarization to M1. It was shown that the expression of iNOS and arginase-1 were increased and decreased, respectively [226]. NK cell-derived exosomes harbor specific cargo that acts on the TGF-β pathway, relieving immunosuppression [227, 228]. In addition, the exosomes secreted by NK cells can suppress the expression of PD-1 and inhibit the exhaustion of T cells in chronic viral infections and tumors [228, 229].
As mentioned, the existence of FasL and TRAIL on the surface of NK cell-derived exosomes and cargo of perforin, granzyme A, granzyme B, and granulysin were confirmed by multiple studies supporting direct cytotoxic effects of NK cell-derived exosomes in the face of target cells.
Killing the target cells can be performed by caspase-dependent or independent pathways. For example, perforin can induce pores in the target cell membrane and contribute to the lysis of the cell or entrance of the granzymes or granulysin into the cytoplasm of the target cell [44]. Granzyme A can damage DNA by cleavage of the SET protein complex or induction of ROS release. Despite granzyme A, granzyme B can activate caspase-dependent apoptosis by direct activating pro-caspase or releasing cytochrome c from mitochondria [228]. In addition, ER-mediated apoptosis can be induced by granulysin [230]. Despite contradictory reports, it seems that using membrane-bound FasL is another mechanism NK cell-derived exosomes use to kill the target cells. A caspase-dependent apoptosis pathway initiates after FasL binds to the Fas on viral-infected or tumor cells. miRNAs are another tool NK cell-derived exosomes use to inhibit target cells [44, 231].
Effects of exosomes derived from virus-infected cells on NK cells
In addition to immune cells such as NK cells, virus-infected cells can also secrete exosomes that might also affect immune cells. It was shown that these exosomes contain the proteins, nucleic acids, and other components of the original virus. These vesicles can be taken up by other immune or non-immune cells. So they can transfer the virus-related cargo. Some studies reported that exosomes secreted by viruses-infected cells contribute to NK cell-cell dysfunction or activation [232].
Yang et al. isolated exosomes from the serum of chronic hepatitis B (CHB) patients. These exosomes were found to contain HBV DNA, HBV RNA, and HBs Ag. After the isolation and purification of NK cells from the peripheral blood of the healthy donors and CHB patients, they observed that the cytotoxicity of NK cells from CHB patients against K562 cells was decreased compared to the cytotoxicity of healthy donors' NK cells. They also observed reduced CD107a (degranulation molecule) expression on the surface of NK cells. In addition, the secretion of IFN-γ was decreased. So, they concluded that HBV infection suppresses NK cell function. They also assessed whether HBV impaired NK cell function by exosomes. For this purpose, they incubated healthy donor NK cells with exosomes from CHB patients. They observed a decrease in NK cell cytotoxicity, CD107a, IFN-γ, and TNF-α production of NK cells than those incubated with exosomes from healthy donors. This report showed that the expression of the activating and inhibitory receptors on NK cells was decreased and increased, respectively by CHB patients-derived exosomes. Moreover, according to the results of this study, the proliferation of HBV + NK cells was reduced, and they displayed apoptosis [232].
Another study showed that HBV-infected cells release exosomes that contain HBV RNA. These exosomes could upregulate the expression of NKG2D ligands on macrophages by stimulating MyD88, TICAM-1, and MAVS-dependent pathways. Since NKG2D ligands have an essential role in the activation of NK cells, they assessed the function of the hepatic NK cells and indicated that the IFN-γ production of hepatic NK cells in response to HBV only occurs in the presence of hepatic macrophages. So, it concluded that exosomes that are released from HBV-infected cells have an essential role in NK cell activation by increasing the NKG2D ligand expression via macrophages [233].
NK cell-based therapies in viral infection
NK cells have significant roles in the immune response against viral infections that can cause patient morbidity and mortality. Therefore, strengthening the host immune system by therapeutic approaches that augment NK cells’ cytotoxicity and longevity, transferring NK cells, and using NK cell-derived exosomes might be beneficial strategies for infected patients (Fig. 3). Here, we discuss the recent developments in these therapeutic approaches.
Enhancing the proliferation, cytotoxicity, and lifespan of NK cells
Antibody as an NK cell-engager agent
NK cells are the main immune cells that mediate ADCC because the FcγRIII or CD16 is highly expressed on these cells [234]. The use of antibodies against specific antigens is one of the effective approaches in immunotherapy because these antibodies can exploit various arms of the immune system, for example by injection of the antibodies, neutralization, opsonophagocytosis, and ADCC could be occurred that contribute to the clearance of pathogen [234].
The use of broadly neutralizing antibodies against HIV is the potential immunotherapy approach. It was shown that in addition to neutralization, these antibodies could engage activating Fc receptors and enhance protective activity [235]. Passive vaccination by a broadly neutralizing antibody (3BNC117) in mice demonstrated that these antibodies could bind to Fc receptors and induce ADCC; substantially, the clearance of the infected cells was improved [236]. In addition to passive vaccination, active vaccination can induce ADCC-mediating antibodies. Seasonal influenza vaccination of older adults induces ADCC-mediation antibodies that contribute to the augmented ADCC activity against H5 and H7 strains [237]. Another study evaluated the serum samples of people who received a recombinant H7 hemagglutinin vaccine, and serological assays were conducted. It was demonstrated that antibodies induced by H7 vaccination have functional activity in ADCC [238].
NK cell therapy has some limitations; for example, unlike T cells and B cells, NK cells do not have specificity, and the proliferation and viability of these cells are limited in vivo. To overcome these limitations, some interesting immunotherapy approaches were suggested, such as bispecific (BiKEs) and trispecific (TriKEs) killer cell engagers [239]. BiKEs that bind to CD16A on NK cells and HIV-1 envelope glycoprotein-expressing cells could kill the infected cells. Li et al. generated a BiKE that consists of CD16A-binding antibody domains fused with one-domain soluble human CD4. Using this BiKE against HIV-infected cells could activate NK cells and induce cytokine production and degranulation [240].
Preventing the NK cell exhaustion
It was shown that in chronic viral infections or various cancers, the NK cells became exhausted, and the function of these cells was impaired. The exhausted NK cells upregulated the expression of inhibitory receptors and immune checkpoints. It was shown that classical NK cells’ receptors like NKG2A, KIRs, and LIRs were increased in chronic viral infections or cancers. In addition to this, recently, it was demonstrated that the NK cells could express various immune checkpoints such as CTLA-4, PD-1, B7-H3, LAG-3, TIGIT, CD96, TIM-3, Siglec-7/9, CD200, and CD47. Since the immune checkpoint blockade (ICB) indicated significant results in the activation of T cells and control of cancers and infectious diseases, the use of ICB for enhancing the function of NK cells was investigated, and it was shown that the ICBs could augment the cytotoxic function of the NK cells [241]. The antibody known as Monalizumab, which hinders NKG2A, has been shown to have positive impacts in the treatment of various forms of cancer. As NKG2A plays an essential role in the exhaustion of NK cells, it seems that monalizumab can be considered as NK cell ICB [242]. Blocking the NKG2A enhanced the activity of human NK cells and eliminated HBV infection in mice [71]. Additionally, IL-6 plays a crucial role in the exhaustion and dysfunction of NK cells in individuals with COVID-19. Consequently, the utilization of Tocilizumab, an IL-6 inhibitor, can help prevent the exhaustion of NK cells [243, 244].
Cytokines as NK cell activating agents
Activation of the endogenous NK cells by cytokines is one of the challenging approaches in viral infections and cancers. Many cytokines were used for this aim, such as IL-2, IL-12, IL-15, and IFN-α [245]. IL-12 and IL-15 secretion by monocytes was decreased in COVID-19. So, it was shown that the administration of these cytokines can compensate for the NK cell dysfunction resulting from reduced cytokine secretion [246]. Garrido et al. investigated the effects of IL-15 treatment on NK cell stimulation and effector function. ADCC, IFN-γ production, and anti-viral activity of NK cells were improved [247].
Transfer of functional NK cells
Transferring the functional NK cells is one of the promising therapeutic approaches in viral infections. Different sources of NK cells, such as autologous, haploidentical, adoptive, and off-the-shelf cryopreserved NK cell products, were used for this aim. The clinical trials in this field are summarized in Table 2.
Adoptive NK cells
Recently, some evidence has somewhat changed the views regarding the NK cell as one of the components of innate immunity. The first indication referred to the studies that evaluated the CD94+NKG2C+ NK cells in HCMV-infected patients and showed that adaptive-like and memory-like NK cells could respond to CMV infection in mouse models and humans [248]. Indeed, adaptive NK cell refers to a group of NK cells with memory properties such as higher longevity, cytotoxicity, and cytokine secretion. The expression of NKG2C, CD57, and IFN-γ is higher in these cells than in normal NK cells [249]. In a study, the severity of COVID-19 in kidney transplant recipients was compared in recipients infected with SARS-CoV-2 and HCMV, or SARS-CoV-2 alone. It was shown that the severity of COVID-19 is higher in the second group. This report can correlate with the effects of adaptive NK cells on COVID-19 [250]. Adaptive NK cells can be prepared in the laboratory using HCMV-infected fibroblasts. So, these cells might be considered a new weapon for virus eradication or infected cell elimination [251].
Allogenic and haploidentical NK cells
Autologous NK cells or haploidentical healthy donors’ NK cells can be considered for transferring the NK cells in virus-infected patients. One of the major limitations of this aim is the low count of NK cells in peripheral blood [239]. Additionally, the cytotoxicity of these NK cells is low. So, strategies should be applied to overcome this issue. Oyer et al. designed a particle-based method for expanding cytotoxic NK cells from peripheral blood mononuclear cells. These particles are prepared from plasma membranes of K562-mb21-41BBL cells, expressing 41BBL and membrane-bound interleukin-21 (PM21 particles). PM21 could expand NK cells ex vivo and in vivo [252]. Using G-CSF along with optimized cell culture conditions is another suggested method for expanding cytotoxic NK cells [245].
In addition to peripheral blood, NK cells can be obtained from induced pluripotent stem cells, umbilical cord stem cells, and bone marrow [239]. CYNK-001 is a group of NK cells derived from umbilical cord stem cells. These cells have anti-tumor and anti-viral properties. CYNK-001 is a laboratory-produced NK cell that recognizes and eliminates virus-infected cells using NKG2D, DNAM, and NCRs [253, 254]. Clinical trials using CYNK-001 for patients with COVID-19 are currently ongoing (NCT04365101).
Chimeric antigen receptor NK cells
Chimeric antigen receptor (CAR) is a genetically-designed receptor consisting of antigen binding domain, mostly single chain variable fragment (ScFv), a transmembrane region, and a signal transduction domain that is different in each generation of the CARs. The immune cells, mostly T and NK cells, can be genetically modified by CAR transduction and express this receptor on their surface. One of the most significant advantages of CAR NK cells over normal NK cells is their ability for specific recognition of the target cells [255]. In a study, the hematopoietic progenitor cells were transduced with CAR and then differentiated into functional NK and T cells. These differentiated cells prevented HIV infection in humanized mice. It was shown that CAR-NK cells could detect and eliminate mimetic HIV-infected cell lines [256]. Recently, a clinical trial has been underway to treat COVID-19 patients using CAR NK cells that express the angiotensin-converting enzyme 2 (ACE2) receptor on their surface (NCT04324996). Moreover, a modified CAR NK cell was developed to enhance its survival by secreting IL-15 and targeting the S protein of SARS-CoV-2 through the expression of a CAR with an extracellular domain of ACE2. This modified cell has exhibited promising effects against VSV-SARS-CoV-2 chimeric viral particles and the recombinant SARS-CoV-2 spike protein subunit S1. It enhances cytotoxicity and promotes the production of TNF-α and IFN-γ [257].
Therapeutic potential of NK cell-derived exosomes in viral infection
Using exosomes as a treatment option is becoming favorable among scientists. A study by Sengupta et al. aimed to assess the safety and efficacy of utilizing exosomes derived from bone marrow mesenchymal stem cells (MSCs) as a potential treatment for COVID-19-infected patients. Following four days of treatment, significant improvements were observed in both laboratory test results and clinical symptoms, along with elevated lymphocyte count, with no adverse effects reported [258]. Another study showed that Milk exosomes can bind to monocyte-derived dendritic cells (MDDCs) through DC-SIGN (dendritic cell-specific intercellular adhesion molecule-3 grabbing non-integrin), thereby inhibiting HIV-1 infection in MDDCs and preventing the subsequent transfer of the virus to CD4 T cells [259]. Additionally, exosomes released by virus-infected cells may be used to trigger NK cells. For instance, Hepatocytes infected with HBV release exosomes containing HBV nucleic acids. These exosomes can trigger the activation of NK cells by stimulating specific pathways like MyD88, TICAM-1, and MAVS, resulting in the expression of NKG2D ligands on the cell surface [233].
As we mentioned, NK cells can be used as treatments for viral infection; however, the research on the role of NK cell-derived exosome in treating viral infections are rare, and more studies are needed to shed light on this issue. Because these exosomes can exert similar effects of NK cells and have favorable properties for defense against viral infections.
Conclusion
In conclusion, NK cells are essential members of antiviral immunity. Their ability to recognize and destroy infected cells using various mechanisms, including releasing cytotoxic granules and producing cytokines, is essential in fighting against viruses. Recently, studies have delineated that the role of exosomes in the antiviral response of NK cells is essential since they can modulate signaling pathways involved in NK activity. Also, some ncRNAs can enhance NK cells' cytotoxic activity and cytokine production. In addition, NK cell-secreted exosomes can inhibit viral replication and boost the antiviral response of other immune cells, such as T cells.
Therapeutic approaches targeting NK cells are attractive options and have shown promising outcomes in treating viral infections. Immunotherapies relying on NK cells, including the transfer of ex vivo expanded NK cells and using allogenic and haploidentical NK cells, have shown promising results in clinical trials. However, further research is required to investigate the potential side effects of these approaches and optimize them to achieve a higher yield. Also, a comprehensive understanding of interactions between ncRNAs, exosomes, and other factors in regulating NK cells in antiviral immunity seems necessary. Despite these challenges, targeting NK cells remains an engaging approach to alleviating viral infections, especially when currently available treatments have limited efficacy or adverse effects.
Perspective
Activating and inhibitory receptor interactions on NK cells and the function of cytokines and other signaling molecules in controlling NK cell function will be the main topics of future research on NK cells and viral infection. It may be possible to create targeted therapies by identifying particular exosomal cargoes and non-coding RNAs involved in controlling NK cell function during viral infection. Promising research areas include the creation of NK cell-based therapies specifically tailored for each patient and the application of cytokines and exosomes as targeted treatments for viral infections.
Availability of data and materials
Not applicable.
References
Khorramdelazad H, et al. Immunopathological similarities between COVID-19 and influenza: Investigating the consequences of Co-infection. Microb Pathog. 2021;152:104554.
Iuliano AD, et al. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. The Lancet. 2018;391(10127):1285–300.
Fabrizi F, Dixit V, Messa P. Hepatitis C virus and mortality among patients on dialysis: A systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2019;43(3):244–54.
Varn FS, et al. Genomic Characterization of Six Virus-Associated Cancers Identifies Changes in the Tumor Immune Microenvironment and Altered Genetic ProgramsGenomic Characterization of Virus-Associated Cancers. Can Res. 2018;78(22):6413–23.
Sadri Nahand J, et al. Cell death pathways and viruses: Role of microRNAs. Mol Ther Nucleic Acids. 2021;24:487–511.
Morens DM, Fauci AS. Emerging Pandemic Diseases: How We Got to COVID-19. Cell. 2020;182(5):1077–92.
Farnoosh G, et al. Are Iranian sulfur mustard gas-exposed survivors more vulnerable to SARS-CoV-2? Some similarity in their pathogenesis. Disaster Med Public Health Prep. 2020;14(6):826–32.
Ranjbar M, et al. Role of CCL2/CCR2 axis in the pathogenesis of COVID-19 and possible Treatments: All options on the Table. Int Immunopharmacol. 2022;113:109325.
Aghbash PS, et al. The role of Th17 cells in viral infections. Int Immunopharmacol. 2021;91:107331.
Wan Z, et al. Regulatory T cells and T helper 17 cells in viral infection. Scand J Immunol. 2020;91(5):e12873.
Jindal A, Kumar M, Sarin SK. Management of acute hepatitis B and reactivation of hepatitis B. Liver Int. 2013;33:164–75.
Ayoobi F, et al. Reduced expression of TRIF in chronic HBV infected Iranian patients. Clin Res Hepatol Gastroenterol. 2013;37(5):491–5.
Rosenberg ZF, Fauci AS. Immunopathogenic mechanisms of HIV infection: cytokine induction of HIV expression. Immunol Today. 1990;11:176–80.
Zampieri CA, Sullivan NJ, Nabel GJ. Immunopathology of highly virulent pathogens: insights from Ebola virus. Nat Immunol. 2007;8(11):1159–64.
Klotman ME, Chang TL. Defensins in innate antiviral immunity. Nat Rev Immunol. 2006;6(6):447–56.
van Erp EA, et al. Viral infection of human natural killer cells. Viruses. 2019;11(3):243.
Björkström NK, Strunz B, Ljunggren H-G. Natural killer cells in antiviral immunity. Nat Rev Immunol. 2022;22(2):112–23.
Orange JS. Human natural killer cell deficiencies and susceptibility to infection. Microbes Infect. 2002;4(15):1545–58.
Wiley SR, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3(6):673–82.
Miller JS. Biology of Natural Killer Cells in Cancer and Infection. Cancer Invest. 2002;20(3):405–19.
Krasnova Y, et al. Bench to bedside: NK cells and control of metastasis. Clin Immunol. 2017;177:50–9.
Langers I, et al. Natural killer cells: role in local tumor growth and metastasis. Biologics. 2012;6:73.
Caligiuri MA. Human natural killer cells. Blood. 2008;112(3):461–9.
Cherrier DE, Serafini N, Di Santo JP. Innate lymphoid cell development: AT cell perspective. Immunity. 2018;48(6):1091–103.
Scoville SD, Freud AG, Caligiuri MA. Modeling human natural killer cell development in the era of innate lymphoid cells. Front Immunol. 2017;8:360.
Yu J, Freud AG, Caligiuri MA. Location and cellular stages of natural killer cell development. Trends Immunol. 2013;34(12):573–82.
Mebius RE, et al. The fetal liver counterpart of adult common lymphoid progenitors gives rise to all lymphoid lineages, CD45+ CD4+ CD3− cells, as well as macrophages. J Immunol. 2001;166(11):6593–601.
Vargas CL, et al. Development of thymic NK cells from double negative 1 thymocyte precursors. Blood. 2011;118(13):3570–8.
Becknell B, Caligiuri MA. Interleukin-2, interleukin-15, and their roles in human natural killer cells. Adv Immunol. 2005;86:209–39.
Mujal AM, Delconte RB, Sun JC. Natural killer cells: From innate to adaptive features. Annu Rev Immunol. 2021;39:417–47.
Kee BL, Morman RE, Sun M. Transcriptional regulation of natural killer cell development and maturation. Adv Immunol. 2020;146:1–28.
Gordon SM, et al. The transcription factors T-bet and Eomes control key checkpoints of natural killer cell maturation. Immunity. 2012;36(1):55–67.
Abel AM, et al. Natural killer cells: development, maturation, and clinical utilization. Front Immunol. 2018;9:1869.
Luetke-Eversloh M, Killig M, Romagnani C. Signatures of human NK cell development and terminal differentiation. Front Immunol. 2013;4:499.
Renoux VM, et al. Identification of a human natural killer cell lineage-restricted progenitor in fetal and adult tissues. Immunity. 2015;43(2):394–407.
Di Vito C, Mikulak J, Mavilio D. On the way to become a natural killer cell. Front Immunol. 2019;10:1812.
Perera Molligoda Arachchige AS. Human NK cells: From development to effector functions. Innate Immun. 2021;27(3):212–29.
Wang D, Malarkannan S. Transcriptional regulation of natural killer cell development and functions. Cancers. 2020;12(6):1591.
Angelo LS, et al. Practical NK cell phenotyping and variability in healthy adults. Immunol Res. 2015;62(3):341–56.
Michel T, et al. Human CD56bright NK cells: an update. J Immunol. 2016;196(7):2923–31.
Elliott JM, Yokoyama WM. Unifying concepts of MHC-dependent natural killer cell education. Trends Immunol. 2011;32(8):364–72.
Malmberg, K.-J., et al. Natural killer cell-mediated immunosurveillance of human cancer. in Seminars in immunology. 2017. Elsevier.
Nash WT, et al. Know thyself: NK-cell inhibitory receptors prompt self-tolerance, education, and viral control. Front Immunol. 2014;5:175.
Zafarani, A., et al., The Role of NK Cells and Their Exosomes in Graft Versus Host Disease and Graft Versus Leukemia. Stem Cell Reviews and Reports, 2022: p. 1–20.
Rajalingam R. Diversity of killer cell immunoglobulin-like receptors and disease. Clin Lab Med. 2018;38(4):637–53.
Achdout H, Manaster I, Mandelboim O. Influenza virus infection augments NK cell inhibition through reorganization of major histocompatibility complex class I proteins. J Virol. 2008;82(16):8030–7.
Zhang X, et al. KIR3DL1-negative CD8 T cells and KIR3DL1-negative natural killer cells contribute to the advantageous control of early human immunodeficiency virus type 1 infection in HLA-B Bw4 homozygous individuals. Front Immunol. 2018;9:1855.
Blunt MD, Khakoo SI. Activating killer cell immunoglobulin-like receptors: Detection, function and therapeutic use. Int J Immunogenet. 2020;47(1):1–12.
Van der Ploeg K, et al. Modulation of human leukocyte antigen-C by human cytomegalovirus stimulates KIR2DS1 recognition by natural killer cells. Front Immunol. 2017;8:298.
Wauquier N, et al. Association of KIR2DS1 and KIR2DS3 with fatal outcome in Ebola virus infection. Immunogenetics. 2010;62(11):767–71.
Crespo ÂC, Strominger JL, Tilburgs T. Expression of KIR2DS1 by decidual natural killer cells increases their ability to control placental HCMV infection. Proc Natl Acad Sci. 2016;113(52):15072–7.
Deborska-Materkowska D, et al. Killer immunoglobulin-like receptor 2DS2 (KIR2DS2), KIR2DL2-HLA-C1, and KIR2DL3 as genetic markers for stratifying the risk of cytomegalovirus infection in kidney transplant recipients. Int J Mol Sci. 2019;20(3):546.
Naiyer MM, et al. KIR2DS2 recognizes conserved peptides derived from viral helicases in the context of HLA-C. Sci Immunol. 2017;2(15):eaal5296.
Zaia JA, et al. The effect of single and combined activating killer immunoglobulin-like receptor genotypes on cytomegalovirus infection and immunity after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2009;15(3):315–25.
Estefania E, et al. Influence of KIR gene diversity on the course of HSV-1 infection: resistance to the disease is associated with the absence of KIR2DL2 and KIR2DS2. Tissue Antigens. 2007;70(1):34–41.
Gauthiez E, et al. A systematic review and meta-analysis of HCV clearance. Liver Int. 2017;37(10):1431–45.
Podhorzer A, et al. The clinical features of patients with chronic hepatitis C virus infections are associated with killer cell immunoglobulin-like receptor genes and their expression on the surface of natural killer cells. Front Immunol. 2018;8:1912.
Zhuang Y, et al. Association between KIR Genes and Efficacy of Treatment of HBeAg-Positive Chronic Hepatitis B Patients with Entecavir. Iran J Immunol. 2018;15(2):112–21.
Goodridge JP, et al. HLA-F and MHC class I open conformers are ligands for NK cell Ig-like receptors. J Immunol. 2013;191(7):3553–62.
Merino AM, et al. KIR2DS4 promotes HIV-1 pathogenesis: new evidence from analyses of immunogenetic data and natural killer cell function. PLoS ONE. 2014;9(6):e99353.
Olvera A, et al. The HLA-C* 04: 01: KIR2DS4: gene combination and human leukocyte antigen alleles with high population frequency drive rate of HIV disease progression. AIDS. 2015;29(5):507–17.
Blokhuis JH, et al. KIR2DS5 allotypes that recognize the C2 epitope of HLA-C are common among Africans and absent from Europeans. Immun Inflamm Dis. 2017;5(4):461–8.
Di Bona D, et al. Association between γ marker, human leucocyte antigens and killer immunoglobulin-like receptors and the natural course of human cytomegalovirus infection: a pilot study performed in a Sicilian population. Immunology. 2018;153(4):523–31.
Burian A, et al. HLA-F and MHC-I open conformers bind natural killer cell Ig-like receptor KIR3DS1. PLoS ONE. 2016;11(9):e0163297.
Carlomagno S, et al. KIR3DS1-mediated recognition of HLA-* B51: modulation of KIR3DS1 responsiveness by self HLA-B allotypes and effect on NK cell licensing. Front Immunol. 2017;8:581.
Besson C, et al. Association of killer cell immunoglobulin-like receptor genes with Hodgkin’s lymphoma in a familial study. PLoS ONE. 2007;2(5):e406.
Jiang Y, et al. KIR3DS1/L1 and HLA-Bw4-80I are associated with HIV disease progression among HIV typical progressors and long-term nonprogressors. BMC Infect Dis. 2013;13(1):1–11.
López-Vázquez A, et al. Protective effect of the HLA-Bw4I80 epitope and the killer cell immunoglobulin-like receptor 3DS1 gene against the development of hepatocellular carcinoma in patients with hepatitis C virus infection. J Infect Dis. 2005;192(1):162–5.
Rapaport AS, et al. The inhibitory receptor NKG2A sustains virus-specific CD8+ T cells in response to a lethal poxvirus infection. Immunity. 2015;43(6):1112–24.
Harrison R, et al. Association of NKG2A with treatment for chronic hepatitis C virus infection. Clin Exp Immunol. 2010;161(2):306–14.
Li F, et al. Blocking the natural killer cell inhibitory receptor NKG2A increases activity of human natural killer cells and clears hepatitis B virus infection in mice. Gastroenterology. 2013;144(2):392–401.
Ma M, et al. NKG2C+ NKG2A− natural killer cells are associated with a lower viral set point and may predict disease progression in individuals with primary HIV infection. Front Immunol. 2017;8:1176.
Alsulami K, et al. Influence of NKG2C Genotypes on HIV Susceptibility and Viral Load Set Point. J Virol. 2021;95(16):e00417-e421.
Toson, B., et al., Assessment of NKG2C copy number variation in HIV-1 infection susceptibility, and considerations about the potential role of lacking receptors and virus infection. J Hum Genet, 2022: p. 1–5.
Sene D, et al. Hepatitis C virus (HCV) evades NKG2D-dependent NK cell responses through NS5A-mediated imbalance of inflammatory cytokines. PLoS Pathog. 2010;6(11):e1001184.
Wen C, et al. Hepatitis C virus infection downregulates the ligands of the activating receptor NKG2D. Cell Mol Immunol. 2008;5(6):475–8.
Cerboni C, et al. Human immunodeficiency virus 1 Nef protein downmodulates the ligands of the activating receptor NKG2D and inhibits natural killer cell-mediated cytotoxicity. J Gen Virol. 2007;88(1):242–50.
Luczo JM, Ronzulli SL, Tompkins SM. Influenza a virus hemagglutinin and other pathogen glycoprotein interactions with nk cell natural cytotoxicity receptors NKp46, NKp44, and NKp30. Viruses. 2021;13(2):156.
Arnon TI, et al. Inhibition of the NKp30 activating receptor by pp65 of human cytomegalovirus. Nat Immunol. 2005;6(5):515–23.
Golden-Mason L, et al. Increased natural killer cell cytotoxicity and NKp30 expression protects against hepatitis C virus infection in high-risk individuals and inhibits replication in vitro. Hepatology. 2010;52(5):1581–9.
Holder KA, et al. Hepatitis C virus–infected cells downregulate NKp30 and inhibit ex vivo NK cell functions. J Immunol. 2013;191(6):3308–18.
Klimosch SN, et al. Genetically coupled receptor–ligand pair NKp80-AICL enables autonomous control of human NK cell responses. Blood. 2013;122(14):2380–9.
El-Sherbiny YM, et al. The requirement for DNAM-1, NKG2D, and NKp46 in the natural killer cell-mediated killing of myeloma cells. Can Res. 2007;67(18):8444–9.
Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294(1–2):15–22.
Duev-Cohen A, et al. The human 2B4 and NTB-A receptors bind the influenza viral hemagglutinin and co-stimulate NK cell cytotoxicity. Oncotarget. 2016;7(11):13093.
Fujita T, et al. In vitro response of immunoregulatory cytokine expression in human monocytic cells to human parvovirus B19 capsid. Biol Pharm Bull. 2007;30(11):2027–30.
Trydzenskaya H, et al. The genetic predisposition of natural killer cell to BK virus–associated nephropathy in renal transplant patients. Kidney Int. 2013;84(2):359–65.
Sasagawa T, Takagi H, Makinoda S. Immune responses against human papillomavirus (HPV) infection and evasion of host defense in cervical cancer. J Infect Chemother. 2012;18(6):807–15.
Lee S-J, et al. Both E6 and E7 oncoproteins of human papillomavirus 16 inhibit IL-18-induced IFN-γ production in human peripheral blood mononuclear and NK cells. J Immunol. 2001;167(1):497–504.
Ainouze M, et al. Human papillomavirus type 16 antagonizes IRF6 regulation of IL-1β. PLoS Pathog. 2018;14(8):e1007158.
Song Y, et al. HPV E7 inhibits cell pyroptosis by promoting TRIM21-mediated degradation and ubiquitination of the IFI16 inflammasome. Int J Biol Sci. 2020;16(15):2924.
Garcia-Iglesias T, et al. Low NKp30, NKp46 and NKG2D expression and reduced cytotoxic activity on NK cells in cervical cancer and precursor lesions. BMC Cancer. 2009;9(1):1–8.
Zhang J, et al. Human papillomavirus type 16 disables the increased natural killer cells in early lesions of the cervix. J Immunol Res. 2019;2019:9182979.
Spreu J, Stehle T, Steinle A. Human cytomegalovirus-encoded UL16 discriminates MIC molecules by their α2 domains. J Immunol. 2006;177(5):3143–9.
Cosman D, Fanger N, Borges L. Human cytomegalovirus, MHC class I and inhibitory signalling receptors: more questions than answers. Immunol Rev. 1999;168(1):177–85.
Prod’homme V, et al. Human cytomegalovirus UL40 signal peptide regulates cell surface expression of the NK cell ligands HLA-E and gpUL18. J Immunol. 2012;188(6):2794–804.
Prod’homme V, et al. Human cytomegalovirus UL141 promotes efficient downregulation of the natural killer cell activating ligand CD112. J Gen Virol. 2010;91(8):2034–9.
Tomasec P, et al. Downregulation of natural killer cell–activating ligand CD155 by human cytomegalovirus UL141. Nat Immunol. 2005;6(2):181–8.
Smith W, et al. Human cytomegalovirus glycoprotein UL141 targets the TRAIL death receptors to thwart host innate antiviral defenses. Cell Host Microbe. 2013;13(3):324–35.
Ashiru O, et al. NKG2D ligand MICA is retained in the cis-Golgi apparatus by human cytomegalovirus protein UL142. J Virol. 2009;83(23):12345–54.
Dassa L, et al. The human cytomegalovirus protein UL148A downregulates the NK cell-activating ligand MICA to avoid NK cell attack. J Virol. 2018;92(17):e00162-e218.
Charpak-Amikam Y, et al. Human cytomegalovirus escapes immune recognition by NK cells through the downregulation of B7–H6 by the viral genes US18 and US20. Sci Rep. 2017;7(1):1–11.
Fielding CA, et al. Two novel human cytomegalovirus NK cell evasion functions target MICA for lysosomal degradation. PLoS Pathog. 2014;10(5):e1004058.
Seidel E, et al. Dynamic co-evolution of host and pathogen: HCMV downregulates the prevalent allele MICA∗ 008 to escape elimination by NK cells. Cell Rep. 2015;10(6):968–82.
De Pelsmaeker S, et al. Herpesvirus evasion of natural killer cells. J Virol. 2018;92(11):e02105-e2117.
Campadelli-Fiume, G. and L. Menotti, Entry of alphaherpesviruses into the cell. 2011.
Schneider CL, Hudson AW. The human herpesvirus-7 (HHV-7) U21 immunoevasin subverts NK-mediated cytoxicity through modulation of MICA and MICB. PLoS Pathog. 2011;7(11):e1002362.
Madrid AS, Ganem D. Kaposi’s sarcoma-associated herpesvirus ORF54/dUTPase downregulates a ligand for the NK activating receptor NKp44. J Virol. 2012;86(16):8693–704.
Yamin R, et al. The viral KSHV chemokine vMIP-II inhibits the migration of Naive and activated human NK cells by antagonizing two distinct chemokine receptors. PLoS Pathog. 2013;9(8):e1003568.
Thomas M, et al. Down-regulation of NKG2D and NKp80 ligands by Kaposi’s sarcoma-associated herpesvirus K5 protects against NK cell cytotoxicity. Proc Natl Acad Sci. 2008;105(5):1656–61.
Bortolotti D, et al. SARS-CoV-2 spike 1 protein controls natural killer cell activation via the HLA-E/NKG2A pathway. Cells. 2020;9(9):1975.
Petitdemange C, et al. Control of acute dengue virus infection by natural killer cells. Front Immunol. 2014;5:209.
Yoon JC, et al. Cell-to-cell contact with hepatitis C virus-infected cells reduces functional capacity of natural killer cells. J Virol. 2011;85(23):12557–69.
Bhat R, Farrag MA, Almajhdi FN. Double-edged role of natural killer cells during RSV infection. Int Rev Immunol. 2020;39(5):233–44.
Zhang AP, et al. The ebola virus interferon antagonist VP24 directly binds STAT1 and has a novel, pyramidal fold. PLoS Pathog. 2012;8(2):e1002550.
Basler CF, et al. The Ebola virus VP35 protein functions as a type I IFN antagonist. Proc Natl Acad Sci. 2000;97(22):12289–94.
Valmas C, et al. Marburg virus evades interferon responses by a mechanism distinct from ebola virus. PLoS Pathog. 2010;6(1):e1000721.
Fausther-Bovendo H, et al. HIV escape from natural killer cytotoxicity: nef inhibits NKp44L expression on CD4+ T cells. AIDS. 2009;23(9):1077–87.
Sowrirajan B, Barker E. The natural killer cell cytotoxic function is modulated by HIV-1 accessory proteins. Viruses. 2011;3(7):1091–111.
Matusali G, et al. The Human Immunodeficiency Virus Type 1 Nef and Vpu Proteins Downregulate the Natural Killer Cell-Activating Ligand PVR. J Virol. 2012;86(8):4496–504.
Bolduan S, et al. HIV-1 Vpu affects the anterograde transport and the glycosylation pattern of NTB-A. Virology. 2013;440(2):190–203.
Matusali G, et al. Soluble ligands for the NKG2D receptor are released during HIV-1 infection and impair NKG2D expression and cytotoxicity of NK cells. FASEB J. 2013;27(6):2440–50.
Cotmore SF, et al. The family Parvoviridae. Adv Virol. 2014;159(5):1239–47.
Manaresi E, Gallinella G. Advances in the Development of Antiviral Strategies against Parvovirus B19. Viruses. 2019;11(7):659.
Hunter LA, Ayala NK. Parvovirus B19 in pregnancy: a case review. J Midwifery Womens Health. 2021;66(3):385–90.
Khatami, A., et al., Association of parvovirus B19 and myocarditis/dilated cardiomyopathy: A systematic review and meta-analysis. Microb Pathog, 2021: p. 105207.
Polo D, et al. Prevalence of human bocavirus infections in Europe. A systematic review and meta‐analysis. Transbound Emerg Dis. 2022;69(5):2451–61.
Almishri W, et al. TNFα augments cytokine-induced NK Cell IFNγ production through TNFR2. J Innate Immun. 2016;8(6):617–29.
Deng X, et al. Human parvovirus infection of human airway epithelia induces pyroptotic cell death by inhibiting apoptosis. J Virol. 2017;91(24):e01533-e1617.
Senju H, et al. Effect of IL-18 on the expansion and phenotype of human natural killer cells: application to cancer immunotherapy. Int J Biol Sci. 2018;14(3):331.
Wu Y, Tian Z, Wei H. Developmental and functional control of natural killer cells by cytokines. Front Immunol. 2017;8:930.
Zhang Q-Q, et al. Clinical utility of immune function based on IFN-γ monitoring of lymphocyte subsets for parvovirus B19 infection in renal recipients. Infect Genet Evol. 2022;103:105307.
Mogensen TH, et al. Chronic hepatitis caused by persistent parvovirus B19 infection. BMC Infect Dis. 2010;10(1):1–5.
Prado JCM, et al. Human polyomaviruses and cancer: an overview. Clinics. 2018;73:e558s.
Imperiale MJ. Polyomavirus miRNAs: the beginning. Curr Opin Virol. 2014;7:29–32.
Morris-Love J, Atwood WJ. Complexities of JC Polyomavirus Receptor-Dependent and-Independent Mechanisms of Infection. Viruses. 2022;14(6):1130.
Koyro TF, et al. Upregulation of HLA-F expression by BK polyomavirus infection induces immune recognition by KIR3DS1-positive natural killer cells. Kidney Int. 2021;99(5):1140–8.
Szomolanyi-Tsuda E, et al. Acute, lethal, natural killer cell-resistant myeloproliferative disease induced by polyomavirus in severe combined immunodeficient mice. Am J Pathol. 1994;144(2):359.
Mishra R, et al. NK Cells and γδ T Cells Mediate Resistance to Polyomavirus-Induced Tumors. PLoS Pathog. 2010;6(5):e1000924.
Sitarz K, et al. The impact of HPV infection on human glycogen and lipid metabolism–a review. Biochim Biophys Acta Rev Cancer. 2022;1877(1):188646.
Yuan Y, et al. HPV post-infection microenvironment and cervical cancer. Cancer Lett. 2021;497:243–54.
Szymonowicz KA, Chen J. Biological and clinical aspects of HPV-related cancers. Cancer Biol Med. 2020;17(4):864.
Gutiérrez-Hoya A, Soto-Cruz I. NK Cell Regulation in Cervical Cancer and Strategies for Immunotherapy. Cells. 2021;10(11):3104.
Tembhurne, A.K., et al., Killer cell immunoglobulin‐like receptor (KIR) gene contents: Are they associated with cervical cancer? J Med Virol, 2022.
Sehrawat S, Kumar D, Rouse BT. Herpesviruses: harmonious pathogens but relevant cofactors in other diseases? Front Cell Infect Microbiol. 2018;8:177.
Mui UN, et al. Human oncoviruses: Mucocutaneous manifestations, pathogenesis, therapeutics, and prevention: Hepatitis viruses, human T-cell leukemia viruses, herpesviruses, and Epstein-Barr virus. J Am Acad Dermatol. 2019;81(1):23–41.
Bu G-L, et al. How EBV Infects: The Tropism and Underlying Molecular Mechanism for Viral Infection. Viruses. 2022;14(11):2372.
Shechter O, et al. Epstein-Barr Virus (EBV) Epithelial Associated Malignancies: Exploring Pathologies and Current Treatments. Int J Mol Sci. 2022;23(22):14389.
Zuhair M, et al. Estimation of the worldwide seroprevalence of cytomegalovirus: A systematic review and meta-analysis. Rev Med Virol. 2019;29(3):e2034.
Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989;320(26):1731–5.
Almerigogna F, et al. Natural killer cell deficiencies in a consecutive series of children with herpetic encephalitis. Int J Immunopathol Pharmacol. 2011;24(1):231–8.
Mace EM, et al. Mutations in GATA2 cause human NK cell deficiency with specific loss of the CD56bright subset. Blood. 2013;121(14):2669–77.
Münz C. Natural Killer Cell Responses during Human γ-Herpesvirus Infections. Vaccines. 2021;9(6):655.
Cerboni C, et al. Human cytomegalovirus strain-dependent changes in NK cell recognition of infected fibroblasts. J Immunol. 2000;164(9):4775–82.
Grauwet K, et al. Modulation of CD112 by the alphaherpesvirus gD protein suppresses DNAM-1–dependent NK cell-mediated lysis of infected cells. Proc Natl Acad Sci. 2014;111(45):16118–23.
Jochum S, et al. The EBV immunoevasins vIL-10 and BNLF2a protect newly infected B cells from immune recognition and elimination. PLoS Pathog. 2012;8(5):e1002704.
Heatley SL, et al. Polymorphism in human cytomegalovirus UL40 impacts on recognition of human leukocyte antigen-E (HLA-E) by natural killer cells. J Biol Chem. 2013;288(12):8679–90.
Jackson CB, et al. Mechanisms of SARS-CoV-2 entry into cells. Nat Rev Mol Cell Biol. 2022;23(1):3–20.
Dandachi I, Aljabr W. Prognosis of COVID-19 in the middle eastern population, knowns and unknowns. Front Microbiol. 2022;13:974205.
Björkström NK, Ponzetta A. Natural killer cells and unconventional T cells in COVID-19. Curr Opin Virol. 2021;49:176–82.
Brownlie D, et al. Comparison of Lung-Homing Receptor Expression and Activation Profiles on NK Cell and T Cell Subsets in COVID-19 and Influenza. Front Immunol. 2022;13:834862.
Koutsakos M, et al. Integrated immune dynamics define correlates of COVID-19 severity and antibody responses. Cell Reports Medicine. 2021;2(3):100208.
Hammer Q, et al. SARS-CoV-2 Nsp13 encodes for an HLA-E-stabilizing peptide that abrogates inhibition of NKG2A-expressing NK cells. Cell Rep. 2022;38(10):110503.
Hagemann K, et al. Natural killer cell‐mediated ADCC in SARS‐CoV‐2‐infected individuals and vaccine recipients. Eur J Immunol. 2022;52:1297–307.
Maruthamuthu S, et al. Individualized Constellation of Killer Cell Immunoglobulin-Like Receptors and Cognate HLA Class I Ligands That Controls Natural Killer Cell Antiviral Immunity Predisposes COVID-19. Front Genet. 2022;13:845474.
Bernal E, et al. Activating killer-cell immunoglobulin-like receptors are associated with the severity of Coronavirus Disease 2019. J Infect Dis. 2021;224(2):229–40.
Littera R, et al. Natural killer-cell immunoglobulin-like receptors trigger differences in immune response to SARS-CoV-2 infection. PLoS ONE. 2021;16(8):e0255608.
Vietzen H, et al. Deletion of the NKG2C receptor encoding KLRC2 gene and HLA-E variants are risk factors for severe COVID-19. Genet Med. 2021;23(5):963–7.
Söderberg-Nauclér C. Does reactivation of cytomegalovirus contribute to severe COVID-19 disease? Immunity & Ageing. 2021;18(1):1–7.
Jaiswal SR, et al. Impact of adaptive natural killer cells, KLRC2 genotype and cytomegalovirus reactivation on late mortality in patients with severe COVID-19 lung disease. Clin Transl Immunol. 2022;11(1):e1359.
Latanova A, Starodubova E, Karpov V. Flaviviridae nonstructural proteins: The role in molecular mechanisms of triggering inflammation. Viruses. 2022;14(8):1808.
Hershkovitz O, et al. NKp44 receptor mediates interaction of the envelope glycoproteins from the West Nile and dengue viruses with NK cells. J Immunol. 2009;183(4):2610–21.
Kurane I, et al. Lysis of dengue virus-infected cells by natural cell-mediated cytotoxicity and antibody-dependent cell-mediated cytotoxicity. J Virol. 1984;52(1):223–30.
Khakoo SI, et al. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science. 2004;305(5685):872–4.
Alter G, et al. Reduced frequencies of NKp30+ NKp46+, CD161+, and NKG2D+ NK cells in acute HCV infection may predict viral clearance. J Hepatol. 2011;55(2):278–88.
Krämer B, et al. Natural killer p46High expression defines a natural killer cell subset that is potentially involved in control of hepatitis C virus replication and modulation of liver fibrosis. Hepatology. 2012;56(4):1201–13.
Fagundes CT, et al. IFN-γ production depends on IL-12 and IL-18 combined action and mediates host resistance to dengue virus infection in a nitric oxide-dependent manner. PLoS Negl Trop Dis. 2011;5(12):e1449.
Petitdemange C, et al. Glycogen synthetase kinase 3 inhibition drives MIC-A/B to promote cytokine production by human natural killer cells in Dengue virus type 2 infection. Eur J Immunol. 2020;50(3):342–52.
Collister M, et al. The influence of hepatitis C viral loads on natural killer cell function. Gastroenterology Res. 2019;12(1):8.
Hao S, et al. Eight years’ advances on Bourbon virus, a tick-born Thogotovirus of the Orthomyxovirus family. Zoonoses (Burlingt). 2022;2(1):18.
Krammer F, et al. Influenza. Nat Rev Dis Primers. 2018;4(1):3.
Gazit R, et al. Lethal influenza infection in the absence of the natural killer cell receptor gene Ncr1. Nat Immunol. 2006;7(5):517–23.
Mao H, et al. Influenza virus directly infects human natural killer cells and induces cell apoptosis. J Virol. 2009;83(18):9215–22.
Guo H, et al. The functional impairment of natural killer cells during influenza virus infection. Immunol Cell Biol. 2009;87(8):579–89.
Bongen E, et al. KLRD1-expressing natural killer cells predict influenza susceptibility. Genome medicine. 2018;10(1):1–12.
Cooper GE, et al. Human CD49a+ lung natural killer cell cytotoxicity in response to influenza A virus. Front Immunol. 2018;9:1671.
Stegemann-Koniszewski S, et al. Respiratory influenza A virus infection triggers local and systemic natural killer cell activation via toll-like receptor 7. Front Immunol. 2018;9:245.
Rima B, et al. ICTV virus taxonomy profile: Pneumoviridae. J Gen Virol. 2017;98(12):2912–3.
Mammas IN, et al. Update on current views and advances on RSV infection. Int J Mol Med. 2020;46(2):509–20.
van Erp EA, et al. Respiratory syncytial virus infects primary neonatal and adult natural killer cells and affects their antiviral effector function. J Infect Dis. 2019;219(5):723–33.
Zdrenghea M, et al. RSV infection modulates IL-15 production and MICA levels in respiratory epithelial cells. Eur Respir J. 2012;39(3):712–20.
Noyola DE, et al. NK cell immunophenotypic and genotypic analysis of infants with severe respiratory syncytial virus infection. Microbiol Immunol. 2015;59(7):389–97.
Tregoning JS, et al. Neonatal antibody responses are attenuated by interferon-γ produced by NK and T cells during RSV infection. Proc Natl Acad Sci. 2013;110(14):5576–81.
Leahy TR, et al. Interleukin-15 is associated with disease severity in viral bronchiolitis. Eur Respir J. 2016;47(1):212–22.
Languon S, Quaye O. Impacts of the Filoviridae family. Curr Opin Pharmacol. 2021;60:268–74.
Jacob ST, et al. Ebola virus disease. Nat Rev Dis Primers. 2020;6(1):1–31.
Malvy D, et al. Ebola virus disease. Lancet. 2019;393(10174):936–48.
He FB, et al. Filovirus VP24 proteins differentially regulate RIG-I and MDA5-dependent Type I and III interferon promoter activation. Front Immunol. 2022;12:694105.
Lubaki NM, et al. The Ebola interferon inhibiting domains attenuate and dysregulate cell-mediated immune responses. PLoS Pathog. 2016;12(12): e1006031.
Diaz-Salazar C, Sun JC. Natural killer cell responses to emerging viruses of zoonotic origin. Curr Opin Virol. 2020;44:97–111.
Edri A, et al. The Ebola-glycoprotein modulates the function of natural killer cells. Front Immunol. 2018;9:1428.
Fausther-Bovendo H, et al. NK cells accumulate in infected tissues and contribute to pathogenicity of Ebola virus in mice. J Virol. 2019;93(10):e01703-e1718.
Piersma SJ, et al. Activation receptor–dependent IFN-γ production by NK cells is controlled by transcription, translation, and the proteasome. J Immunol. 2019;203(7):1981–8.
Le H, et al. Ebola virus protein VP40 stimulates IL-12–and IL-18–dependent activation of human natural killer cells. JCI insight. 2022;7(16):e158902.
Katsura Y, Asai S. Evolutionary medicine of retroviruses in the human genome. Am J Med Sci. 2019;358(6):384–8.
Illanes-Álvarez F, et al. Similarities and differences between HIV and SARS-CoV-2. Int J Med Sci. 2021;18(3):846.
Zapata W, et al. Influence of CCR5 and CCR2 genetic variants in the resistance/susceptibility to HIV in serodiscordant couples from Colombia. AIDS Res Hum Retroviruses. 2013;29(12):1594–603.
Lallogo TD, et al. KIR2DL5B and HLA DRB1*12 alleles seems to be associated with protection against HIV-1 in serodiscordant couples in Burkina Faso. J Med Virol. 2022;94(9):4425–32.
Specht A, et al. Selective downmodulation of HLA-A and-B by Nef alleles from different groups of primate lentiviruses. Virology. 2008;373(1):229–37.
Richard J, et al. HIV-1 Vpr up-regulates expression of ligands for the activating NKG2D receptor and promotes NK cell–mediated killing. Blood. 2010;115(7):1354–63.
Hong, H.S., et al., Loss of CCR7 expression on CD56bright NK cells is associated with a CD56dimCD16+ NK cell-like phenotype and correlates with HIV viral load. 2012.
Ripa M, et al. Dynamics of adaptive and innate immunity in patients treated during primary human immunodeficiency virus infection: results from Maraviroc in HIV Acute Infection (MAIN) randomized clinical trial. Clin Microbiol Infect. 2015;21(9):876. e1-876. e4.
Schafer JL, et al. Accumulation of cytotoxic CD16+ NK cells in simian immunodeficiency virus-infected lymph nodes associated with in situ differentiation and functional anergy. J Virol. 2015;89(13):6887–94.
Mahmoudi M, Taghavi Farahabadi M, Hashemi SM. Exosomes: Mediators of immune regulation. Immunoregulation. 2019;2(1):3–8.
Taghavi-Farahabadi M, et al. Improving the function of neutrophils from chronic granulomatous disease patients using mesenchymal stem cells’ exosomes. Hum Immunol. 2020;81(10–11):614–24.
Taghavi-Farahabadi M, et al. Evaluation of the effects of mesenchymal stem cells on neutrophils isolated from severe congenital neutropenia patients. Int Immunopharmacol. 2020;83:106463.
Lugini L, et al. Immune surveillance properties of human NK cell-derived exosomes. J Immunol. 2012;189(6):2833–42.
Jong AY, et al. Large-scale isolation and cytotoxicity of extracellular vesicles derived from activated human natural killer cells. J Extracell Vesicles. 2017;6(1):1294368.
Choi J-W, et al. Proteome analysis of human natural killer cell derived extracellular vesicles for identification of anticancer effectors. Molecules. 2020;25(21):5216.
Zhu L, et al. Exosomes derived from natural killer cells exert therapeutic effect in melanoma. Theranostics. 2017;7(10):2732.
Di Pace AL, et al. Characterization of human NK cell-derived exosomes: role of DNAM1 receptor in exosome-mediated cytotoxicity against tumor. Cancers. 2020;12(3):661.
Federici C, et al. Natural-killer-derived extracellular vesicles: Immune sensors and interactors. Front Immunol. 2020;11:262.
Samara, A., et al., Using Natural Killer Cell‐derived Exosomes as a Cell‐Free Therapy for Leukemia. Hematological Oncology, 2022.
Shoae-Hassani A, et al. K cell–derived exosomes from NK cells previously exposed to neuroblastoma cells augment the antitumor activity of cytokine-activated NK cells. J Immunother. 2017;40:265.
Dosil SG, et al. Natural killer (NK) cell-derived extracellular-vesicle shuttled microRNAs control T cell responses. Elife. 2022;11:e76319.
Jia R, et al. NK cell-derived exosomes improved lung injury in mouse model of Pseudomonas aeruginosa lung infection. J Physiol Sci. 2020;70:1–12.
Wang L, Wang Y, Quan J. Exosomes derived from natural killer cells inhibit hepatic stellate cell activation and liver fibrosis. Hum Cell. 2020;33:582–9.
Wu F, et al. Natural killer cell-derived extracellular vesicles: novel players in cancer immunotherapy. Front Immunol. 2021;12:658698.
Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol. 2018;18(3):153–67.
Saini RV, et al. Granulysin delivered by cytotoxic cells damages endoplasmic reticulum and activates caspase-7 in target cells. J Immunol. 2011;186(6):3497–504.
Riahi Rad Z, et al. MicroRNAs in the interaction between host–bacterial pathogens: A new perspective. J Cell Physiol. 2021;236(9):6249–70.
Yang Y, et al. Exosomes mediate hepatitis B virus (HBV) transmission and NK-cell dysfunction. Cell Mol Immunol. 2017;14(5):465–75.
Kouwaki T, et al. Extracellular vesicles including exosomes regulate innate immune responses to hepatitis B virus infection. Front Immunol. 2016;7:335.
Mandelboim O, et al. Human CD16 as a lysis receptor mediating direct natural killer cell cytotoxicity. Proc Natl Acad Sci. 1999;96(10):5640–4.
Bournazos S, et al. Broadly neutralizing anti-HIV-1 antibodies require Fc effector functions for in vivo activity. Cell. 2014;158(6):1243–53.
Lu C-L, et al. Enhanced clearance of HIV-1–infected cells by broadly neutralizing antibodies against HIV-1 in vivo. Science. 2016;352(6288):1001–4.
Vanderven HA, et al. Antibody-dependent cellular cytotoxicity responses to seasonal influenza vaccination in older adults. J Infect Dis. 2018;217(1):12–23.
Stadlbauer D, et al. Vaccination with a recombinant H7 hemagglutinin-based influenza virus vaccine induces broadly reactive antibodies in humans. Msphere. 2017;2(6):e00502-e517.
Duan S, Liu S. Targeting NK cells for HIV-1 treatment and reservoir clearance. Front Immunol. 2022;13:842746.
Li W, et al. One-domain CD4 fused to human anti-CD16 antibody domain mediates effective killing of HIV-1-infected cells. Sci Rep. 2017;7(1):1–12.
Khan M, Arooj S, Wang H. NK cell-based immune checkpoint inhibition. Front Immunol. 2020;11:167.
Van Hall T, et al. Monalizumab: inhibiting the novel immune checkpoint NKG2A. J Immunother Cancer. 2019;7:1–8.
Lan S-H, et al. Tocilizumab for severe COVID-19: a systematic review and meta-analysis. Int J Antimicrob Agents. 2020;56(3):106103.
Masselli E, et al. NK cells: A double edge sword against SARS-CoV-2. Adv Biol Regul. 2020;77:100737.
Di Vito, C., et al., Natural killer cells in SARS-CoV-2 infection: pathophysiology and therapeutic implications. Frontiers in immunology, 2022: p. 3295.
Osman M, et al. Impaired natural killer cell counts and cytolytic activity in patients with severe COVID-19. Blood Adv. 2020;4(20):5035–9.
Garrido C, et al. Interleukin-15-stimulated natural killer cells clear HIV-1-infected cells following latency reversal ex vivo. J Virol. 2018;92(12):e00235-e318.
Lopez-Vergès S, et al. Expansion of a unique CD57+ NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc Natl Acad Sci. 2011;108(36):14725–32.
Schlums H, et al. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity. 2015;42(3):443–56.
Soleimanian S, Yaghobi R. Harnessing memory NK cell to protect against COVID-19. Front Pharmacol. 2020;11:1309.
Jaiswal S.R, et al. Focusing on a unique innate memory cell population of natural killer cells in the fight against COVID-19: harnessing the ubiquity of cytomegalovirus exposure. Mediterr J Hematol Infect Dis. 2020;12(1):e2020047.
Oyer JL, et al. Natural killer cells stimulated with PM21 particles expand and biodistribute in vivo: clinical implications for cancer treatment. Cytotherapy. 2016;18(5):653–63.
Pashaei M, Rezaei N. Immunotherapy for SARS-CoV-2: potential opportunities. Expert Opin Biol Ther. 2020;20(10):1111–6.
Tay MZ, et al. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363–74.
Marofi, F., et al., CAR-NK cell: a new paradigm in tumor immunotherapy. Frontiers in oncology, 2021: p. 2078.
Zhen A, et al. HIV-specific immunity derived from chimeric antigen receptor-engineered stem cells. Mol Ther. 2015;23(8):1358–67.
Lu T, et al. Off-the-shelf CAR natural killer cells secreting IL-15 target spike in treating COVID-19. Nat Commun. 2022;13(1):2576.
Sengupta V, et al. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells as Treatment for Severe COVID-19. Stem Cells Dev. 2020;29(12):747–54.
Näslund TI, et al. Exosomes from breast milk inhibit HIV-1 infection of dendritic cells and subsequent viral transfer to CD4+ T cells. AIDS. 2014;28(2):171–80.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Supervision and validation: S Minaeian &; M Mahmoudi; Investigation: A Zafarani, MH Razizadeh, and M Taghavi-Farahabadi; Conceptualization: all authors; writing original draft: A Zafarani, MH Razizadeh, M Taghavi-Farahabadi, M Mahmoudi; Figures and Tables: H Khorramdelazad, A Zafarani, MH Razizadeh; Review and editing: H Khorramdelazad, S Minaeian and M Mahmoudi.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Razizadeh, M.H., Zafarani, A., Taghavi-Farahabadi, M. et al. Natural killer cells and their exosomes in viral infections and related therapeutic approaches: where are we?. Cell Commun Signal 21, 261 (2023). https://doi.org/10.1186/s12964-023-01266-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12964-023-01266-2