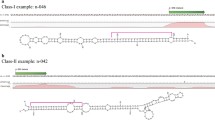
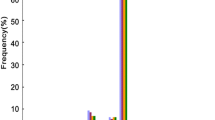Abstract
Flag leaf is the last leaf to senesce and its life span plays a critical role in determining grain quality and yield in rice. Little is known about the molecular changes that occur and their regulation in time-dependent manner during flag leaf senescence in rice. Several studies have explored different aspects of miRNA functions in plant development; however, a diminutive account is available about their role in flag leaf senescence. With an aim to unravel the role of miRNAs in ageing of flag leaf in rice, four small RNA libraries were prepared from three stages of senescence and sequenced by Illumina deep sequencing technology. Thirty-eight known and 494 novel miRNAs were identified in the senescing flag leaves. Digital expression analysis revealed that 21 known and 116 novel miRNAs were differentially expressed during senescence. Family member(s) of miR156, miR159, miR160, miR164, miR169, miR171, miR393, miR396, miR535, miR827, miR1428, miR1432 and miR1861 were differentially expressed in at least one stage of flag leaf senescence. The present study has generated a repository of senescence-related miRNAs that can be utilized to contemplate molecular approaches for manipulating the timing of flag leaf senescence in rice and other related crops.








Similar content being viewed by others
References
Alonso, J. M., Hirayama, T., Roman, G., Nourizadeh, S., & Ecker, J. R. (1999). EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science, 284, 2148–2152.
Aya, K., Ueguchi-Tanaka, M., Kondo, M., Hamada, K., Yano, K., Nishimura, M., et al. (2009). Gibberellin modulates anther development in rice via the transcriptional regulation of GAMYB. Plant Cell, 21, 1453–1472.
Breeze, E., et al. (2011). High-resolution temporal profiling of transcripts during Arabidopsis leaf senescence reveals a distinct chronology of processes and regulation. Plant Cell, 23, 873–894.
Buchanan-Wollaston, V., Earl, S., Harrison, E., Mathas, E., Navabpour, S., Page, T., et al. (2003). The molecular analysis of leaf senescence–a genomics approach. Plant Biotechnology Journal, 1, 3–22.
Burman, N., Bhatnagar, A., & Khurana, J. P. (2018). OsbZIP48, a HY5 transcription factor ortholog, exerts pleiotropic effects in light-regulated development. Plant Physiology, 176, 1262–1285.
Chen, M., Maodzeka, A., Zhou, L., Ali, E., Wang, Z., & Jiang, L. (2014). Removal of DELLA repression promotes leaf senescence in Arabidopsis. Plant Science, 219–220, 26–34.
Chomzynski, P., & Sacchi, N. (1987). Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Analytical Biochemistry, 162, 156–159.
Dhondt, S., Coppens, F., De Winter, F., Swarup, K., Merks, R. M. H., Inze, D., et al. (2010). SHORT-ROOT and SCARECROW regulate leaf growth in Arabidopsis by stimulating S-phase progression of the cell cycle. Plant Physiology, 154, 1183–1195.
Djami-Tchatchou, A. T., Sanan-Mishra, N., Ntushelo, K., & Dubery, I. A. (2017). Functional roles of microRNAs in Agronomically important plants—potential as targets for crop improvement and protection. Frontiers in Plant Science, 8, 378.
Goodstein, D. M., Shu, S., Howson, R., Neupane, R., Hayes, R. D., Fazo, J., et al. (2012). Phytozome: A comparative platform for green plant genomics. Nucleic Acids Research, 40, 1178–1186.
Grbic, V., & Bleecker, A. B. (1995). Ethylene regulates the timing of leaf senescence in Arabidopsis. Plant J., 8, 595–602.
Guo, Y., Cai, Z., & Gan, S. (2004). Transcriptome of Arabidopsis leaf senescence. Plant, Cell and Environment, 27, 521–549.
Guo, Y., & Gan, S. (2005). Leaf senescence: Signals, execution, and regulation. Current Topics in Developmental Biology, 71, 83–112.
Huang, C. K., Lo, P. C., Huang, L. F., Wu, S. J., Yeh, C. H., & Lu, C. A. (2015). A single-repeat MYB transcription repressor, MYBH, participates in regulation of leaf senescence in Arabidopsis. Plant Molecular Biology, 88, 269–286.
Jibran, R., Hunter, D. A., & Dijkwel, P. P. (2013). Hormonal regulation of leaf senescence through integration of developmental and stress signals. Plant Molecular Biology, 82, 547–561.
Kam, J., Gresshoff, P., Shorter, R., & Xue, G. P. (2007). Expression analysis of RING zinc finger genes from Triticum aestivum and identification of TaRZF70 that contains four RING-H2 domains and differentially responds to water deficit between leaf and root. Plant Science, 173, 650–659.
Kanneganti, V., & Gupta, A. K. (2008). Wall associated kinases from plants—an overview. Physiology and Molecular Biology of Plants, 14, 109–118.
Kawahara, Y., et al. (2013). Improvement of the Oryza sativa Nipponbare reference genome using next generation sequence and optical map data. Rice, 6, 1–10.
Kim, J., Chang, C., & Tucker, M. L. (2015). To grow old: Regulatory role of ethylene and jasmonic acid in senescence. Frontiers in Plant Science, 6, 1–7.
Kim, H., Kim, H. J., Vu, Q. T., Jung, S., McClung, C. R., Hong, S., et al. (2018). Circadian control of ORE1 by PRR9 positively regulates leaf senescence in Arabidopsis. Proceedings of the National Academy of Sciences, 115, 8448–8453.
Kim, J. I., Murphy, A. S., Baek, D., Lee, S. W., Yun, D. J., Bressan, R. A., et al. (2011). YUCCA6 over-expression demonstrates auxin function in delaying leaf senescence in Arabidopsis thaliana. Journal of Experimental Botany, 62, 3981–3992.
Kim, J. H., Woo, H. R., Kim, J., Lim, P. O., Lee, I. C., Choi, S. H., et al. (2009). Trifurcate feed-forward regulation of age-dependent cell death involving miR164 in Arabidopsis. Science, 323, 1053–1057.
Koyama, T. (2014). The roles of ethylene and transcription factors in the regulation of onset of leaf senescence. Frontiers in Plant Science, 5, 650.
Koyama, T., Nii, H., Mitsuda, N., Ohta, M., Kitajima, S., Ohme-Takagi, M., et al. (2013). A regulatory cascade involving class II ETHYLENE RESPONSE FACTOR transcriptional repressors operates in the progression of leaf senescence. Plant Physiology, 162, 991–1005.
Leng, Y., Ye, G., & Zeng, D. (2017). Genetic dissection of leaf senescence in rice. International Journal of Molecular Sciences, 18, 2686.
Lim, P. O., Kim, H. J., & Gil Nam, H. (2007). Leaf senescence. Annual Review of Plant Biology, 58, 115–136.
Lim, P. O., Lee, I. C., Kim, J., Kim, H. J., Ryu, J. S., Woo, H. R., et al. (2010). Auxin response factor 2 (ARF2) plays a major role in regulating auxin-mediated leaf longevity. Journal of Experimental Botany, 61, 1419–1430.
Lin, M., Pang, C., Fan, S., Song, M., Wei, H., & Yu, S. (2015). Global analysis of the Gossypium hirsutum L. Transcriptome during leaf senescence by RNA-Seq. BMC Plant Biology, 15, 43.
Liu, M., Yu, H., Zhao, G., Huang, Q., Lu, Y., & Ouyang, B. (2017). Profiling of drought-responsive microRNA and mRNA in tomato using high-throughput sequencing. BMC Genomics, 18, 1–18.
Liu, L., Zhou, E. Y., Zhou, E. G., Ye, R., Zhao, E. L., Li, X., et al. (2008). Identification of early senescence-associated genes in rice flag leaves. Plant Molecular Biology, 67, 37–55.
Luquez, V. M., & Guiamét, J. J. (2001). Effects of the “stay green” genotype GGd1d1d2d2 on leaf gas exchange, dry matter accumulation and seed yield in soybean (Glycine max L. Merr.). Annals of Botany, 87, 313–318.
Mannai, Y., Akabane, K., Hiratsu, K., Satoh-Nagasawa, N., & Wabiko, H. (2017). The NAC transcription factor gene OsY37 (ONAC011) promotes leaf senescence and accelerates heading time in rice. International Journal of Molecular Sciences, 18, 2165.
Mao, C., Lu, S., Lv, B., Zhang, B., Shen, J., He, J., et al. (2017). A rice NAC transcription factor promotes leaf senescence via ABA biosynthesis. Plant Physiology, 174, 1747–1763.
Meyers, B. C., et al. (2008). Criteria for annotation of plant MicroRNAs. Plant Cell, 20, 3186–3190.
Moore, B., Zhou, L., Rolland, F., Hall, Q., Cheng, W.-H., Liu, Y.-X., et al. (2003). Role of the Arabidopsis glucose sensor HXK1 in nutrient, light, and hormonal signaling. Science, 300, 332–336.
Morris, K., MacKerness, S. A., Page, T., John, C. F., Murphy, A. M., Carr, J. P., et al. (2000). Salicylic acid has a role in regulating gene expression during leaf senescence. Plant Journal, 23, 677–685.
Park, S.-Y., et al. (2007). The senescence-induced staygreen protein regulates chlorophyll degradation. Plant Cell, 19, 1649–1664.
Pourtau, N., Mares, M., Purdy, S., Quentin, N., Ruel, A., & Wingler, A. (2004). Interactions of abscisic acid and sugar signalling in the regulation of leaf senescence. Planta, 219, 765–772.
Rajagopalan, R., Vaucheret, H., Trejo, J., & Bartel, D. P. (2006). A diverse and evolutionarily fluid set of microRNAs in Arabidopsis thaliana. Genes & Development, 20, 3407–3425.
Rampino, P., Pataleo, S., Gerardi, C., Mita, G., & Perrotta, C. (2006). Drought stress response in wheat: Physiological and molecular analysis of resistant and sensitive genotypes. Plant, Cell and Environment, 29, 2143–2152.
Sakuraba, Y., Jeong, J., Kang, M.-Y., Kim, J., Paek, N.-C., & Choi, G. (2014). Phytochrome-interacting transcription factors PIF4 and PIF5 induce leaf senescence in Arabidopsis. Nature Communications, 5, 4636.
Schippers, J. H. M., Jing, H.-C., Hille, J., & Dijkwel, P. P. (2007). Developmental and hormonal control of leaf senescence. In S. Gan (Ed.), Senescence processes in plants (pp. 145–170). Oxford: Blackwell Publishing Ltd.
Schommer, C., Palatnik, J. F., Aggarwal, P., Chételat, A., Cubas, P., Farmer, E. E., et al. (2008). Control of jasmonate biosynthesis and senescence by miR319 targets. PLoS Biology, 6, e230.
Shriram, V., Kumar, V., Devarumath, R. M., Khare, T. S., & Wani, S. H. (2016). MicroRNAs as potential targets for abiotic stress tolerance in plants. Frontiers in Plant Science, 7, 1–18.
Stefanowicz, K., Lannoo, N., & Van Damme, E. J. M. (2015). Plant F-box proteins—judges between life and death. Critical Review Plant Sciences, 34, 523–552.
Stocks, M. B., Moxon, S., Mapleson, D., Woolfenden, H. C., Mohorianu, I., Folkes, L., et al. (2012). The UEA sRNA workbench: a suite of tools for analysing and visualizing next generation sequencing microRNA and small RNA datasets. Bioinformatics, 28, 2059–2061.
Veljović-Jovanović, S., Kukavica, B., Stevanović, B., & Navari-Izzo, F. (2006). Senescence- and drought-related changes in peroxidase and superoxide dismutase isoforms in leaves of Ramonda serbica. Journal of Experimental Botany, 57, 1759–1768.
Wu, X., Ding, D., Shi, C., Xue, Y., Zhang, Z., Tang, G., et al. (2016). microRNA-dependent gene regulatory networks in maize leaf senescence. BMC Plant Biology, 16, 73.
Xiao, D., Cui, Y., Xu, F., Xu, X., Gao, G., Wang, Y., et al. (2015). SENESCENCE-SUPPRESSED PROTEIN PHOSPHATASE directly interacts with the cytoplasmic domain of SENESCENCE-ASSOCIATED RECEPTOR-LIKE KINASE and negatively regulates leaf senescence in Arabidopsis. Plant Physiology, 169, 1275–1291.
Xie, Z., Johansen, L. K., Gustafson, A. M., Kasschau, K. D., Lellis, A. D., Zilberman, D., et al. (2004). Genetic and functional diversification of small RNA pathways in plants. PLoS Biology, 2, e104.
Xiong, Y. Y., Ma, J., He, Y. H., Lin, Z., Li, X., Yu, S. M., et al. (2018). High-throughput sequencing analysis revealed the regulation patterns of small RNAs on the development of A. comosus var. bracteatus leaves. Scientific Reports, 8, 1–11.
Xu, X., Bai, H., Liu, C., Chen, E., Chen, Q., Zhuang, J., et al. (2014). Genome-wide analysis of microRNAs and their target genes related to leaf senescence of rice. PLoS ONE, 9, e114313.
Zeng, S., Liu, Y., Pan, L., Hayward, A., & Wang, Y. (2015). Identification and characterization of miRNAs in ripening fruit of Lycium barbarum L. using high-throughput sequencing. Frontiers in Plant Science, 6, 1–15.
Zhang, X., Ju, H. W., Chung, M. S., Huang, P., Ahn, S. J., & Kim, C. S. (2011). The R-R-type MYB-like transcription factor, AtMYBL, is involved in promoting leaf senescence and modulates an abiotic stress response in Arabidopsis. Plant and Cell Physiology, 52, 138–148.
Zhang, W. Y., Xu, Y. C., Li, W. L., Yang, L., Yue, X., Zhang, X. S., et al. (2014). Transcriptional analyses of natural leaf senescence in maize. PLoS ONE, 9, e115617.
Zhou, X., Jiang, Y., & Yu, D. (2011). WRKY22 transcription factor mediates dark-induced leaf senescence in Arabidopsis. Molecular Cell, 31, 303–313.
Zhu, Q.-H., Spriggs, A., Matthew, L., Fan, L., Kennedy, G., Gubler, F., et al. (2008). A diverse set of microRNAs and microRNA-like small RNAs in developing rice grains. Genome Research, 18, 1456–1465.
Zou, C., Sun, K., Mackaluso, J. D., Seddon, A. E., Jin, R., Thomashow, M. F., et al. (2011). Cis-regulatory code of stress-responsive transcription in Arabidopsis thaliana. Proceedings of National Academy of Sciences, 108, 14992–14997.
Acknowledgements
JMS and CVK are thankful for research fellowships from University Grants Commission (UGC), India. R&D grants from DBT, DST-PURSE and University of Delhi are acknowledged. We thank Gopal Joshi for helping with submission of small RNA sequencing data to SRA database. Deep sequencing was carried out by DBT-funded High-Throughput Sequencing Facility at University of Delhi South Campus, New Delhi, India.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sasi, J.M., Kumar, C.V., Mani, B. et al. Identification and characterization of miRNAs during flag leaf senescence in rice by high-throughput sequencing. Plant Physiol. Rep. 24, 1–14 (2019). https://doi.org/10.1007/s40502-019-0436-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40502-019-0436-6