Abstract
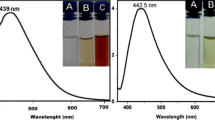
Nanoparticles are particles between 1 and 100 nm in size that has the ability to modify their physio-chemical properties compared to other material. In the present study, leaves of Stevia rebaudiana B. were used to extracts to synthesize Ag nanoparticles from AgNO3 (1 mM concentration) and then studied the effects of these commercial and synthesized Ag nanoparticles on biochemical (chlorophyll content, anthocyanin, flavonoid, carbohydrate, protein and DPPH) and growth characteristics of Stevia at different concentrations (0, 10, 20, 40 mM). UV–visible spectroscopy was used to identify the formation of Ag and analyzed synthesized Ag nanoparticles at 300–700 nm. Absorption maxima at 435 nm wavelength confirmed their synthesis. Examining synthesized AgNPs from S. rebaudiana extract using SEM micrograph showed a 25 nm dimension and spherical shape. Application of synthesized and commercial AgNPs at various concentrations on Stevia showed a dependency on concentrations that at 10 and 20 mM of AgNP, an increase in leaf area, shoot height and dry and fresh weight was recorded. At the biochemical level, applying 40 mM of synthesized AgNP resulted in increased chlorophyll content which led to accumulation of biomass besides soluble anthocyanin, flavonoid and carbohydrate and also total protein and DPPH, compared to plants treated with commercial AgNP. Moreover, the results demonstrated that increasing AgNP concentration enhances glycoside content in both treatments. Based on our findings, synthesized AgNP is more effective in accelerating the growth and improve the quality of natural product in Stevia plants than commercial AgNP.











Similar content being viewed by others
References
Abdul Rahuman, A., Jayaseelan, C., Ramkumar, R., & Perumal, P. (2013). Green synthesis of gold nanoparticles using seed aqueous extract of Abelmoschu sesculentus and its antifungal activity. Industrial Crops and Products, 45, 423–429.
Albrecht, M. A. (2006). Green chemistry and the health implications of nanoparticles. Evans C Raston C. Green Chemistry, 8, 417–432.
Arase, F., Arase, H., Nishitani, M., Egusa, N., Nishimoto, S., Sakurai, N., et al. (2012). AA8 involved in lateral root formation interacts with the TIR1 auxin receptor and ARF transcription factors in Arabidopsis. PLoS ONE, 7, 43–49.
Arnon, D. I. (1949). Copper enzymes in isolated chloroplasts. Polyphenoloxidasein beta vulgaris. Plant Physiology, 24, 1–15.
Asharani, P. V., Wu, Y. L., Gong, Z., & Valiyaveettil, S. (2008). Toxicity of silver nanoparticles in zebra fish models. Nanotechnology, 19, 255102.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Briskin, D. P. (2000). Medicinal plants and phytomedicines. Linking plant biochemistry and physiology to human health. Plant Physiology, 124, 507–514.
Bujak, T., Nizioł-Łukaszewska, Z., Gaweł-Bęben, K., Seweryn, A., Kucharek, M., Rybczyńska-Tkaczyk, K., et al. (2015). The application of different Stevia rebaudiana leaf extracts in the “green synthesis” of AgNPs. Green Chemistry Letters and Reviews, 8, 3–4.
Comotto, M., Casazza, A. A., Aliakbarian, B., Caratto, V., Ferretti, M., & Perego, P. (2014). Influence of TiO2 nanoparticles on growth and phenolic compounds production in photosynthetic microorganisms. Scientific World Journal, 9, 324–333.
Daayf, F., Ongena, M., Boulanger, R., Hadrami, I. E., & Belanger, R. R. (2000). Induction of phenolic compounds in two cultivars of cucumber by treatment of healthy and powdery mildew-infected plants with extracts of Reynoutria sachalinensis. Journal of Chemical Ecology, 26, 1579–1593.
El-Temsah, Y. S., & Joner, E. J. (2012). Impact of Fe and Ag nanoparticles on seed germination and differences in bioavailability during exposure in aqueous suspension and soil. Environmental Toxicology, 27, 42–49.
Fuleki, T., & Francis, F. J. (1968). Quantitative methods for anthocyanins: Extraction and determination of total anthocyanin in cranberries. Journal of Food Science, 33, 72–77.
Garcia-Sanchez, S., Bernales, I., & Cristobal, S. (2015). Early response to nanoparticles in the Arabidopsis transcriptome compromises plant defence and root-hair development through salicylic acid signalling. BMC Genomics, 16, 341–349.
Ghorbanpour, M., & Hadian, J. (2015). Multi-walled carbon nanotubes stimulate callus induction, secondary metabolites biosynthesis and antioxidant capacity in medicinal plant Saturejakhuzestanica grown in vitro. Carbon, 94, 749–759.
Govorov, A. O., & Carmeli, I. (2007). Hybrid structures composed of photosynthetic system and metal nanoparticles: Plasmon enhancement effect. Nano Letters, 7(3), 620–625.
Huang, H., & Yang, X. (2004). Synthesis of polysaccharide-stabilized gold and silver nanoparticles: a green method. Carbohydrate Research, 339, 2627–2631.
Jasim, B., Thomas, R., Mathew, J., & Radhakrishnan, E. K. (2017). Plant growth and diosgenin enhancement effect of silver nanoparticles in Fenugreek (Trigonella foenum-graecum L.). Saudi Pharmaceutical Journal, 25, 443–447.
Kanipandian, N., Kannan, S., Ramesh, R., Subramanian, P., & Thirumurugan, R. (2014). Characterization, antioxidant and cytotoxicity evaluation of green synthesized silver nanoparticles using Cleistanthus collinus extract as surface modifier. Materials Research Bulletin, 49, 494–502.
Khan, M. N., Mobin, M., Abbas, Z. K., Almutairi, K. A., & Siddiqui, Z. H. (2017). Role of nanomaterials in plants under challenging environments. Plant Physiology and Biochemistry, 110, 194–209.
Kim, J. S., Kuk, E., & Yu, K. N. (2007). Antimicrobial effects of silver nanoparticles. Nanomedicine, 3, 95–101.
Kohan-Baghkheirati, E., & Geisler-Lee, J. (2015). Gene expression, protein function and pathways of Arabidopsis thaliana responding to silver nanoparticles in comparison to silver ions, cold, salt, drought, and heat. Nanomaterials, 5, 436–467.
Krishnaraj, C., Jagan, E. G., Ramachandran, R., Abirami, S. M., Mohan, N., & Kalaichelvan, P. T. (2012). Effect of biologically synthesized silver nanoparticles on Bacopa monnieri (Linn.) Wettst. Plant Growth Metabolism, Process Biochemistry, 47, 651–658.
Kumar, A., & Nirmala, V. (2004). Gastric antiulcer activity of the leaves of Caesalpinia pulcherrima. Indian Journal of Pharmaceutical Sciences, 66(5), 676–678.
Kumari, M., Mukherjee, A., & Chandrasekaran, N. (2010). Cytogenetic and genotoxic effects of zinc oxide nanoparticles on root cells of Allium cepa. Science of the Total Environment, 407, 5243.
Larcher, W. (2000). Expression of ascorbic acid oxidase in zucchini squash (Cucurbita pepo L.). Plant Physiology, 96, 159–165.
Li, W. R., Xie, X. B., Shi, Q. S., Duan, S. S., Ouyang, Y. S., & Chen, Y. B. (2011). Antibacterial effect of silver nanoparticles on Staphylococcus aureus. BioMetals, 24, 135–141.
Lim, D., Roh, J. Y., Eom, H. J., Choi, J. Y., Hyun, J., & Choi, J. (2012). Oxidative stress-related PMK-1 P38 MAPK activation as a mechanism for toxicity of silver nanoparticles to reproduction in the nematode Caenorhabditi selegans. Environmental Toxicology and Chemistry, 31, 585–592.
Majlesi, Z., Ramezani, M., & Gerami, M. (2018). Investigation on some main glycosides content of Stevia rebaudian B. under different concentration of commercial and synthesized silver nanoparticles. PBR, 4(1), 1–10.
Masarovicova, E., & Kralova, K. (2013). Metal nanoparticles and plants. Ecological Chemistry and Engineering S, 20, 9–22.
Mazumdar, H., & Ahmed, G. U. (2011). Synthesis of silver nanoparticles and its adverse effect germination. The International Journal of Advanced Biotechnology Research, 2, 404–413.
Mirzajani, F., Askari, H., Hamzelou, S., Schober, Y., Rompp, A., & Ghassempour, A. (2014). Proteomics study of silver nanoparticles toxicity on Oryza sativa L. Ecotoxicology and Environmental Safety, 108, 335–339.
Mohanpuria, P., Rana, N. K., & Yadav, S. K. J. (2008). Bio-synthesis of nanoparticles: Technological concepts and future applications. Nanoparticle Research, 10, 507–517.
Navarro, E., Baun, A., & Behra, R. (2008). Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology, 17, 372–386.
Padalia, H., Jadeja, R., & Chanda, S. (2016). Review: Screening of silver nanoparticle synthetic efficacy of some medicinal plants of Saurashtra region. In V. K. Gupta (Ed.), Natural products: Research review (Vol. 3, pp. 63–83). New Delhi: Daya Publishing House.
Park, Y., Hong, Y., Weyers, A., Kim, Y., & Linhardt, R. (2011). Polysaccharides and phytochemicals: A natural reservoir for the green synthesis of gold and silver nanoparticles. IRT Nano Biotechnology, 5, 69–78.
Perreault, F., Samadani, M., & Dewez, D. (2014). Effect of soluble copper released from copper oxide nanoparticles solubilisation on growth and photosynthetic processes of Lemnagibba L. Nanotoxicology, 8, 374–382.
Phukan, U. J., Jeena, G. S., & Shukla, R. K. (2016). WRKY transcription factors: Molecular regulation and stress responses in plants. Frontiers in Plant Science, 7, 760–767.
Pitta-Alvarez, S. I., Spollansky, T. C., & Giulietti, A. M. (2000). ‘The influence of different biotic and abiotic elicitors on the production and profile of tropane alkaloids in hairy root cultures of Brugmansia candida. Enzyme and Microbial Technology, 26, 491–504.
Qi, M., Liu, Y., & Li, T. (2013). Nano-TiO2improves the photosynthesis of tomato leaves under mild heat stress. Biological Trace Element Research, 156, 323–328.
Qian, H., Peng, X., Han, X., Ren, J., Sun, L., & Fu, Z. (2013). Comparison of the toxicity of silver nanoparticles and silver ions on the growth of terrestrial plant model Arabidopsis thaliana. Journal of Environmental Science, 25, 1947–1955.
Ramezani, M., Rahmani, F., & Dehestani, A. (2017). Study of physio-biochemical responses elicited by potassium phosphite in downy mildew-infected cucumber plants. Arch Phytopathology Plant Protect, 50, 540–554.
Rashmezad, M. A., Asgary, E. A., Tafvizi, F., & Mirzaie, A. (2015). Comparative study on cytotoxicity effect of biological and commercial synthesized nano silver on human gastric carcinoma and normal lung fibroblast cell lines. Tehran University Medical Journal, 72, 799–807.
Raveendran, P., Fu, J., & Wallen, S. L. (2005). A simple and “green” method for the synthesis of Au, Ag, and Au–Ag alloy nanoparticles. Green Chemistry, 8, 34–38.
Rezvani, N., Sorooshzadeh, A., & Farhadi, N. (2012). Effect of nano-silver on growth of saffron in flooding stress. International Journal of Biological, Biomolecular, Agricultural, Food and Biotechnological Engineering, 6, 11–16.
Salama, H. M. H. (2012). Effects of silver nanoparticles in some crop plants, common bean (Phaseolus vulgaris L.) and corn (Zea mays L.). International Research Journal of Biotechnology, 3, 190–197.
Savithramma, N., Ankanna, S., & Bhumi, G. (2012). Effect of nanoparticles on seed germination and seedling growth of Boswellia Ovalifoliolata—An endemic and endangered medicinal tree taxon. Nano Vision, 2, 61–68.
Schluttenhofer, C., & Yuan, L. (2015). Regulation of specialized metabolism by WRKY transcription factors. Plant Physiology, 167, 295–306.
Shah, V., & Belozerova, I. (2009). Influence of metal nanoparticles on the soil microbial community and germination of lettuce seeds. Water Air and Soil Pollution, 197, 143–148.
Sharma, P., Bhatt, D., Zaidi, M. G. H., Pardha Saradhi, P., Khanna, P. K., & Arora, S. (2012). Silver nanoparticle-mediated enhancement in growth and antioxidant status of Brassica juncea. Applied Biochemistry and Biotechnology, 167, 2225–2233.
Shimada, K., Fujikawa, K., Yahara, K., & Nakamura, T. (1992). Antioxidative properties of xanthone on the auto oxidation of soybean in Cylcodextrin emulsion. Journal of Agricultural and Food Chem, 40, 945–948.
Siddiqui, M. H., Al-Whaibi, M. H., Faisal, M., & Al Sahli, A. A. (2014). Nano-silicon dioxide mitigates the adverse effects of salt stress on Cucurbitapepo L. Environmental Toxicology and Chemistry, 33, 2429–2437.
Sivaram, L., & Mukundan, U. (2003). In vitro culture study on Stevia rebaudiana. In Vitro Cellular & Developmental Biology – Plant, 39, 520–523.
Song, J., & Kim, B. (2009). Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess and Biosystems Engineering, 32, 79–84.
Sosan, A., Svistunenko, D., Straltsova, D., Tsiurkina, K., Smolich, I., & Lawson, T. (2016). Engineered silver nanoparticles are sensed at the plasma membrane and dramatically modify the physiology of Arabidopsis thaliana plants. The Plant Journal, 85, 245–257.
Suber, L., Sondi, I., Matijevi, E., & Goia, D. V. J. (2005). Preparation and the mechanisms of formation of silver particles of different morphologies in homogeneous solutions. Journal of Colloid Interface Science, 288, 489–495.
Suriyaprabha, R., Karunakaran, G., Yuvakkumar, R., Rajendran, V., & Kannan, N. (2012). Silica nanoparticles for increased silica availability in maize (Zea mays L) seeds under hydroponic conditions. Current Nanoscience, 8(6), 902–908.
Syu, Y., Hung, J. H., & Chen, J. C. (2014). Impacts of size and shape of silver nanoparticles on Arabidopsis plant growth and gene expression. Plant Physiology and Biochemistry, 83, 57–64.
Tarafdar, J. C., & Raliya, R. (2013). ZnO nanoparticle biosynthesis and its effect on phosphorous-mobilizing enzyme secretion and gum contents in cluster bean (Cyamopsiste tragonoloba L.). Agricultural Sciences, 2, 48–57.
Tiwari, D. K., Dasgupta-Schubert, N., Villaseñor-Cendejas, L. M., Villegas, J., CarretoMontoya, L., & Borjas-García, S. E. (2014). Interfacing carbon nanotubes (CNT) with plants: Enhancement of growth, water and ionic nutrient uptake in maize (Zea mays) and implications for nano agriculture. Applied Nanoscience, 4, 577–591.
Vannini, C., Vannini, G., Domingo, E., Onelli, B., Prinsi, M., Marsoni, L., et al. (2013). Bracale Morphological and proteomic responses of Eruca sativa exposed to silver Nanoparticles or silver nitrate. PLoS ONE, 8, e68752.
Vecerova, K., Vecera, Z., Docekal, B., Oravec, M., Pompeiano, A., & Tríska, J. (2016). Changes of primary and secondary metabolites in barley plants exposed to CdO nanoparticles. Environmental Pollution, 218, 207–218.
Xu, S., Lou, T., Zhao, N., Gao, Y., Dong, L., Jiang, D., et al. (2011). Presoaking with hemin improves salinity tolerance during wheat seed germination. Acta Physiologiae Plantarum, 33, 1173–1183.
Yemm, E. W., & Willis, A. J. (1954). The estimation of carbohydrates in plant extracts by anthrone. Biochemical Journal, 57, 508–514.
Zhishen, J., Mengcheng, T., & Jianming, W. (1999). The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chemistry, 64, 555–559.
Acknowledgements
Authors would like to thank Sana Institute for providing the opportunity to conduct this research. Sari, Iran.
Funding
This paper was supported by Sana Institute, Iran.
Author information
Authors and Affiliations
Contributions
MR: design the experiment, ZM: perform the experiment, MG and MR: analysis the data, MR and MG and ZM: interpret the data and final revision of the manuscript. MR and MG and ZM: approved the final revision of manuscript.
Corresponding author
Ethics declarations
Consent for publication
Not applicable.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ramezani, M., Gerami, M. & Majlesi, Z. Comparison between various concentrations of commercial and synthesized silver nanoparticles on biochemical parameters and growth of Stevia rebaudiana B.. Plant Physiol. Rep. 24, 141–152 (2019). https://doi.org/10.1007/s40502-018-0413-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40502-018-0413-5