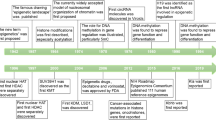
Abstract
Purpose of Review
This review gives an overview of the roles of histone acetylation and methylation in obesity and related metabolic diseases.
Recent Findings
Nutrition can change gene expression via epigenetics such as DNA methylation and post-translational modifications of histones. A growing number of both experimental and clinical studies suggested that histone modifications are very sensitive to changes in nutritional availability and potentially impact the development and progression of metabolic disorders. Recent advances in proteomic studies provided evidence linking histone modifications to over-nutrition and metabolic dysregulation. In this review, we will summarize the recent findings on two classical histone modifications, i.e., acetylation and methylation and the related findings from clinical studies and potential applications.
Summary
The involvement of histone modifications in the progression of metabolic diseases is now widely appreciated. Over the recent years, mass spectrometry-based proteomics approaches discovered and mapped different kind of histone modifications linking obesity and metabolic diseases. The list of these modifications is evergrowing; however, their functions and roles in obesity are not well understood. Same as for the most well studied histone modifications, namely acetylation and methylation. Although much has been learnt from these two modifications, their contributions in regulation of metabolism are still largely unknown. It will be necessary to carry out more studies to further dissect the importance of the availability of substrates and activities of the enzymes for histone acetylation and methylation in the metabolic tissues.
Similar content being viewed by others
References
Romieu I, Dossus L, Barquera S, Blottiere HM, Franks PW, Gunter M, et al. Energy balance and obesity: what are the main drivers? Cancer Causes Control. 2017;28(3):247–58.
Biswas A, Oh PI, Faulkner GE, Bajaj RR, Silver MA, Mitchell MS, et al. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: a systematic review and meta-analysis. Ann Intern Med. 2015;162(2):123–32.
An R, Ji M, Yan H, Guan C. Impact of ambient air pollution on obesity: a systematic review. Int J Obes. 2018;42(6):1112–26.
Austin GL, Ogden LG, Hill JO. Trends in carbohydrate, fat, and protein intakes and association with energy intake in normal-weight, overweight, and obese individuals: 1971-2006. Am J Clin Nutr. 2011;93(4):836–43.
Lucan SC, DiNicolantonio JJ. How calorie-focused thinking about obesity and related diseases may mislead and harm public health. An alternative. Public Health Nutr. 2015;18(4):571–81.
Cheng Z, Zheng L, Almeida FA. Epigenetic reprogramming in metabolic disorders: nutritional factors and beyond. J Nutr Biochem. 2018;54:1–10.
Beckwith J. Fifty years fused to lac. Annu Rev Microbiol. 2013;67:1–19.
Merino E, Jensen RA, Yanofsky C. Evolution of bacterial Trp operons and their regulation. Curr Opin Microbiol. 2008;11(2):78–86.
Pegorier JP, Le May C, Girard J. Control of gene expression by fatty acids. J Nutr. 2004;134(9):2444S–9S.
Filhoulaud G, Guilmeau S, Dentin R, Girard J, Postic C. Novel insights into ChREBP regulation and function. Trends Endocrinol Metab. 2013;24(5):257–68.
Abdul-Wahed A, Guilmeau S, Postic C. Sweet sixteenth for ChREBP: established roles and future goals. Cell Metab. 2017;26(2):324–41.
Guinez C, Filhoulaud G, Rayah-Benhamed F, Marmier S, Dubuquoy C, Dentin R, et al. O-GlcNAcylation increases ChREBP protein content and transcriptional activity in the liver. Diabetes. 2011;60(5):1399–413.
Bond ST, Howlett KF, Kowalski GM, Mason S, Connor T, Cooper A, et al. Lysine post-translational modification of glyceraldehyde-3-phosphate dehydrogenase regulates hepatic and systemic metabolism. FASEB J. 2017;31(6):2592–602.
Seidler NW. GAPDH and intermediary metabolism. Adv Exp Med Biol. 2013;985:37–59.
Choi SW, Claycombe KJ, Martinez JA, Friso S, Schalinske KL. Nutritional epigenomics: a portal to disease prevention. Adv Nutr. 2013;4(5):530–2.
Zhang Y, Kutateladze TG. Diet and the epigenome. Nat Commun. 2018;9(1):3375.
Javaid N, Choi S. Acetylation- and methylation-related epigenetic proteins in the context of their targets. Genes (Basel). 2017;8(8):E196.
Erler J, Zhang R, Petridis L, Cheng X, Smith JC, Langowski J. The role of histone tails in the nucleosome: a computational study. Biophys J. 2014;107(12):2911–22.
Bowman GD, Poirier MG. Post-translational modifications of histones that influence nucleosome dynamics. Chem Rev. 2015;115(6):2274–95.
Shi L, Tu BP. Acetyl-CoA and the regulation of metabolism: mechanisms and consequences. Curr Opin Cell Biol. 2015;33:125–31.
Bender DA. The metabolism of “surplus” amino acids. Br J Nutr. 2012;108(Suppl 2):S113–21.
Taylor EM, Jones AD, Henagan TM. A review of mitochondrial-derived fatty acids in epigenetic regulation of obesity and type 2 diabetes. J Nutrit Health Food Sci. 2014;2(3):1–4.
Lee JV, Shah SA, Wellen KE. Obesity, cancer, and acetyl-CoA metabolism. Drug Discov Today Dis Mech. 2013;10(1–2):e55–61.
Chung MY, Shin EJ, Choi HK, Kim SH, Sung MJ, Park JH, et al. Schisandra chinensis berry extract protects against steatosis by inhibiting histone acetylation in oleic acid-treated HepG2 cells and in the livers of diet-induced obese mice. Nutr Res. 2017;46:1–10.
McDonnell E, Crown SB, Fox DB, Kitir B, Ilkayeva OR, Olsen CA, et al. Lipids reprogram metabolism to become a major carbon source for histone acetylation. Cell Rep. 2016;17(6):1463–72.
Mikula M, Majewska A, Ledwon JK, Dzwonek A, Ostrowski J. Obesity increases histone H3 lysine 9 and 18 acetylation at Tnfa and Ccl2 genes in mouse liver. Int J Mol Med. 2014;34(6):1647–54.
Canani RB, Costanzo MD, Leone L, Pedata M, Meli R, Calignano A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J Gastroenterol. 2011;17(12):1519–28.
Jia Y, Hong J, Li H, Hu Y, Jia L, Cai D, et al. Butyrate stimulates adipose lipolysis and mitochondrial oxidative phosphorylation through histone hyperacetylation-associated beta3-adrenergic receptor activation in high-fat diet-induced obese mice. Exp Physiol. 2017;102(2):273–81.
Gao Z, Yin J, Zhang J, Ward RE, Martin RJ, Lefevre M, et al. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes. 2009;58(7):1509–17.
Li F, Wu R, Cui X, Zha L, Yu L, Shi H, et al. Histone deacetylase 1 (HDAC1) negatively regulates thermogenic program in brown adipocytes via coordinated regulation of histone H3 lysine 27 (H3K27) deacetylation and methylation. J Biol Chem. 2016;291(9):4523–36.
Carrer A, Parris JL, Trefely S, Henry RA, Montgomery DC, Torres A, et al. Impact of a high-fat diet on tissue acyl-CoA and histone acetylation levels. J Biol Chem. 2017;292(8):3312–22.
Gao X, Lin SH, Ren F, Li JT, Chen JJ, Yao CB, et al. Acetate functions as an epigenetic metabolite to promote lipid synthesis under hypoxia. Nat Commun. 2016;7:11960.
Nie L, Shuai L, Zhu M, Liu P, Xie ZF, Jiang S, et al. The landscape of histone modifications in a high-fat diet-induced obese (DIO) mouse model. Mol Cell Proteomics. 2017;16(7):1324–34.
Greer EL, Shi Y. Histone methylation: a dynamic mark in health, disease and inheritance. Nat Rev Genet. 2012;13(5):343–57.
Obeid R. The metabolic burden of methyl donor deficiency with focus on the betaine homocysteine methyltransferase pathway. Nutrients. 2013;5(9):3481–95.
Corbin KD, Zeisel SH. Choline metabolism provides novel insights into nonalcoholic fatty liver disease and its progression. Curr Opin Gastroenterol. 2012;28(2):159–65.
Fonseca V, Keebler M, Dicker-Brown A, Desouza C, Poirier LA, Murthy SN, et al. The effect of troglitazone on plasma homocysteine, hepatic and red blood cell S-adenosyl methionine, and S-adenosyl homocysteine and enzymes in homocysteine metabolism in Zucker rats. Metab Clin Exp. 2002;51(6):783–6.
Cordero P, Gomez-Uriz AM, Campion J, Milagro FI, Martinez JA. Dietary supplementation with methyl donors reduces fatty liver and modifies the fatty acid synthase DNA methylation profile in rats fed an obesogenic diet. Genes Nutr. 2013;8(1):105–13.
Dahlhoff C, Worsch S, Sailer M, Hummel BA, Fiamoncini J, Uebel K, et al. Methyl-donor supplementation in obese mice prevents the progression of NAFLD, activates AMPK and decreases acyl-carnitine levels. Mol Metab. 2014;3(5):565–80.
Kumar S, Pamulapati H, Tikoo K. Fatty acid induced metabolic memory involves alterations in renal histone H3K36me2 and H3K27me3. Mol Cell Endocrinol. 2016;422:233–42.
Tikoo K, Sharma E, Amara VR, Pamulapati H, Dhawale VS. Metformin improves metabolic memory in high fat diet (HFD)-induced renal dysfunction. J Biol Chem. 2016;291(42):21848–56.
Lieber CS, Packer L. S-Adenosylmethionine: molecular, biological, and clinical aspects—an introduction. Am J Clin Nutr. 2002;76(5):1148S–50S.
Honda Y, Kessoku T, Sumida Y, Kobayashi T, Kato T, Ogawa Y, et al. Efficacy of glutathione for the treatment of nonalcoholic fatty liver disease: an open-label, single-arm, multicenter, pilot study. BMC Gastroenterol. 2017;17(1):96.
Elshorbagy AK, Nijpels G, Valdivia-Garcia M, Stehouwer CD, Ocke M, Refsum H, et al. S-Adenosylmethionine is associated with fat mass and truncal adiposity in older adults. J Nutr. 2013;143(12):1982–8.
Elshorbagy AK, Jerneren F, Samocha-Bonet D, Refsum H, Heilbronn LK. Serum S-adenosylmethionine, but not methionine, increases in response to overfeeding in humans. Nutr Diabetes. 2016;6:e192.
Zheng M, Zhang M, Yang J, Zhao S, Qin S, Chen H, et al. Relationship between blood levels of methyl donor and folate and mild cognitive impairment in Chinese patients with type 2 diabetes: a case-control study. J Clin Biochem Nutr. 2014;54(2):122–8.
van Driel LM, Eijkemans MJ, de Jonge R, de Vries JH, van Meurs JB, Steegers EA, et al. Body mass index is an important determinant of methylation biomarkers in women of reproductive ages. J Nutr. 2009;139(12):2315–21.
Schalinske KL, Smazal AL. Homocysteine imbalance: a pathological metabolic marker. Adv Nutr. 2012;3(6):755–62.
Tawfik A, Mohamed R, Elsherbiny NM, DeAngelis MM, Bartoli M, Al-Shabrawey M. Homocysteine: a potential biomarker for diabetic retinopathy. J Clin Med. 2019;8(1):E121.
Iwasaki H. Impaired PRMT1 activity in the liver and pancreas of type 2 diabetic Goto-Kakizaki rats. Life Sci. 2009;85(3–4):161–6.
Lee JH, Park GH, Lee YK, Park JH. Changes in the arginine methylation of organ proteins during the development of diabetes mellitus. Diabetes Res Clin Pract. 2011;94(1):111–8.
Wortham M, He L, Gyamfi M, Copple BL, Wan YJ. The transition from fatty liver to NASH associates with SAMe depletion in db/db mice fed a methionine choline-deficient diet. Dig Dis Sci. 2008;53(10):2761–74.
Yun KU, Ryu CS, Oh JM, Kim CH, Lee KS, Lee CH, et al. Plasma homocysteine level and hepatic sulfur amino acid metabolism in mice fed a high-fat diet. Eur J Nutr. 2013;52(1):127–34.
Brinkmann SJ, Worner EA, van Leeuwen PA. Strict glucose control and artificial regulation of the NO-ADMA-DDAH system in order to prevent endothelial dysfunction. J Physiol. 2016;594(11):2775–6.
Lee W, Lee HJ, Jang HB, Kim HJ, Ban HJ, Kim KY, et al. Asymmetric dimethylarginine (ADMA) is identified as a potential biomarker of insulin resistance in skeletal muscle. Sci Rep. 2018;8(1):2133.
Di Franco M, Lucchino B, Conti F, Valesini G, Spinelli FR. Asymmetric dimethyl arginine as a biomarker of atherosclerosis in rheumatoid arthritis. Mediat Inflamm. 2018;2018:3897295.
Zhou S, Zhu Q, Li X, Chen C, Liu J, Ye Y, et al. Asymmetric dimethylarginine and all-cause mortality: a systematic review and meta-analysis. Sci Rep. 2017;7:44692.
Ge K. Epigenetic regulation of adipogenesis by histone methylation. Biochim Biophys Acta. 2012;1819(7):727–32.
Lee J, Saha PK, Yang QH, Lee S, Park JY, Suh Y, et al. Targeted inactivation of MLL3 histone H3-Lys-4 methyltransferase activity in the mouse reveals vital roles for MLL3 in adipogenesis. Proc Natl Acad Sci U S A. 2008;105(49):19229–34.
Cho YW, Hong S, Jin Q, Wang L, Lee JE, Gavrilova O, et al. Histone methylation regulator PTIP is required for PPARgamma and C/EBPalpha expression and adipogenesis. Cell Metab. 2009;10(1):27–39.
Li J, Huang J, Li JS, Chen H, Huang K, Zheng L. Accumulation of endoplasmic reticulum stress and lipogenesis in the liver through generational effects of high fat diets. J Hepatol. 2012;56(4):900–7.
Terashima M, Barbour S, Ren J, Yu W, Han Y, Muegge K. Effect of high fat diet on paternal sperm histone distribution and male offspring liver gene expression. Epigenetics. 2015;10(9):861–71.
Skinner MK. What is an epigenetic transgenerational phenotype? F3 or F2. Reprod Toxicol. 2008;25(1):2–6.
Donkin I, Barres R. Sperm epigenetics and influence of environmental factors. Mol Metab. 2018;14:1–11.
Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21(3):381–95.
Shinkai Y, Tachibana M. H3K9 methyltransferase G9a and the related molecule GLP. Genes Dev. 2011;25(8):781–8.
Wang L, Xu S, Lee JE, Baldridge A, Grullon S, Peng W, et al. Histone H3K9 methyltransferase G9a represses PPARgamma expression and adipogenesis. EMBO J. 2013;32(1):45–59.
Wang Q, Wang X, Lai D, Deng J, Hou Z, Liang H, et al. BIX-01294 promotes the differentiation of adipose mesenchymal stem cells into adipocytes and neural cells in Arbas Cashmere goats. Res Vet Sci. 2018;119:9–18.
Jang MK, Kim JH, Jung MH. Histone H3K9 demethylase JMJD2B activates adipogenesis by regulating H3K9 methylation on PPARgamma and C/EBPalpha during adipogenesis. PLoS One. 2017;12(1):e0168185.
Lu H, Lei X, Zhang Q. Liver-specific knockout of histone methyltransferase G9a impairs liver maturation and dysregulates inflammatory, cytoprotective, and drug-processing genes. Xenobiotica. 2018;23:1–13.
Bricambert J, Alves-Guerra MC, Esteves P, Prip-Buus C, Bertrand-Michel J, Guillou H, et al. The histone demethylase Phf2 acts as a molecular checkpoint to prevent NAFLD progression during obesity. Nat Commun. 2018;9(1):2092.
Liu X, Strable MS, Ntambi JM. Stearoyl CoA desaturase 1: role in cellular inflammation and stress. Adv Nutr. 2011;2(1):15–22.
Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53:401–26.
Funding
This work was supported by funding from HMRF (03143966) and the Hong Kong Polytechnic University (Internal grant 1.55XX.99ZK and SFHMRF1819) (to C.M.W).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors received no financial support in the writing of this manuscript. The opinions expressed in this publication are those of the authors and do not necessarily reflect those of the company who employs them.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Epigenetics
Rights and permissions
About this article
Cite this article
Xu, L., Yeung, M.H.Y., Yau, M.Y.C. et al. Role of Histone Acetylation and Methylation in Obesity. Curr Pharmacol Rep 5, 196–203 (2019). https://doi.org/10.1007/s40495-019-00176-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40495-019-00176-7