Abstract
Background
Tenosynovial giant cell tumor (TGCT) is a rare, locally aggressive tumor of the joints, bursa, and tendon sheath that can cause considerable pain and substantial morbidity. Although surgery is the primary treatment for patients with TGCT, surgical resection is associated with high rates of recurrence, particularly for patients with diffuse TGCT. Pexidartinib, a colony-stimulating factor 1 receptor inhibitor, is approved by the US Food and Drug Administration for the treatment of adult patients with symptomatic TGCT associated with severe morbidity or functional limitations and not amenable to improvement with surgery.
Case Description
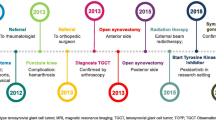
A 32-year-old man presented with intra-articular diffuse TGCT with pain and received noncurative treatment for 5 years (2014–2019). In 2019, the patient was found to have extensive disease accompanied by pain and limited range of motion. The patient’s case was presented to a sarcoma multidisciplinary tumor board, who determined that surgery would cause significant morbidity and macroscopic residual tumor. As a result of the extent of disease, young age, and otherwise good health, treatment with pexidartinib was started through a compassionate use program at 800 mg/day. After dose reductions to pexidartinib at 400 mg/day and then 200 mg/day as a result of creatine phosphokinase elevations, the patient achieved a complete response after 2 years of treatment; pain was reduced and mobility was restored. The patient reported no side effects related to pexidartinib treatment. Treatment was stopped in 2022 for future family planning. After pexidartinib therapy was interrupted, the patient’s wife had a successful pregnancy and delivery; however, the disease showed a slow but constant clinical deterioration, with a reduction in the range of movement of the affected knee and an apparent increase in widespread TGCT nodules.
Conclusion
Our case is unique because it provides support for pexidartinib use as upfront therapy for TGCT, instead of surgery, in selected cases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Surgery is the primary treatment for patients with tenosynovial giant cell tumor (TGCT); however, surgical resection is associated with high rates of disease recurrence, particularly for patients with diffuse TGCT. |
Pexidartinib, a colony-stimulating factor 1 receptor inhibitor, is approved by the US Food and Drug Administration for the treatment of adult patients with symptomatic TGCT associated with severe morbidity or functional limitations and not amenable to improvement with surgery. |
Here, we report the case of a patient with TGCT who achieved a complete response after 2 years of pexidartinib treatment, including reduced pain, restored mobility, and no side effects to treatment. |
Our case is unique because it provides support for pexidartinib use as upfront therapy for TGCT, instead of surgery, in selected cases. |
Introduction
Tenosynovial giant cell tumor (TGCT) is a rare, locally aggressive tumor of the joints, bursa, and tendon sheaths with a highly variable clinical presentation that includes pain, swelling, and limited range of motion and can result in substantial morbidity [1,2,3]. TGCT is caused by overexpression of colony-stimulating factor 1 (CSF1), which is often due to the chromosomal translocation t(1;2) that fuses CSF1 to COL6A3 [2,3,4]. Surgery is the mainstay of treatment for TGCT; however, not all tumors are amenable to surgery because of the risk of severe morbidity, and the recurrence rate is approximately 55% for patients with diffuse TGCT (d-TGCT) [1,2,3, 5]. Systemic therapy is a nonsurgical treatment option for such patients.
Pexidartinib is a small molecule tyrosine kinase inhibitor with selective activity against the CSF1 receptor [6]. The phase 3 ENLIVEN study enrolled 120 patients (pexidartinib, n = 61; placebo, n = 59) who were treated with pexidartinib (1000 mg/day loading dose p.o. for 2 weeks, followed by 800 mg/day). At week 25, 24 (39%) patients in the pexidartinib group achieved a response per Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 compared with no responses in the placebo group [7]. The proportion of patients with a response increased to 53% by RECIST v1.1 after approximately 1 year [7]. In comparison with placebo, pexidartinib increased relative range of motion and significantly improved physical function [7]. The most common treatment-emergent adverse events in patients receiving pexidartinib were hair color changes, fatigue, increased hepatic enzymes, and dysgeusia [7]. On the basis of these data, pexidartinib was approved in the USA for the treatment of adult patients with symptomatic TGCT associated with severe morbidity or functional limitations and not amenable to improvement with surgery [8]. Pexidartinib is only available through a Risk Evaluation and Mitigation Strategy program because of the risk of serious and potentially fatal hepatotoxicity [8]. We describe a patient who received pexidartinib as upfront therapy, in place of surgery, for a severe case of TGCT of the knee.
Case Report
A 32-year-old man with a history of hypertension presented in 2014 with pain and swelling in his right knee. A biopsy and magnetic resonance imaging (MRI) were conducted and revealed intra-articular d-TGCT. The patient did not undergo surgery at this time because of limited symptoms and after discussion with an orthopedic surgeon at a different center. Instead, a wait-and-see approach was used from the 2014 biopsy until the 2016 biopsy. In 2016, the patient underwent arthroscopic biopsy with partial removal of tumor tissue and injection of intra-articular corticosteroids; this treatment was not intended to be curative. Two years later in 2018, the patient was treated with methotrexate, hydroxychloroquine, and etanercept for 3 months; these treatments were suggested by a rheumatologist.
The patient presented to our clinic in October 2019, at which time a full workup was performed, including MRI. Sagittal images of the patient’s knee in October 2019 (Fig. 1) show the extent of disease, with a large tumor and substantial swelling around the knee. At this time, the patient was generally in good health, with a numeric rating scale (NRS) pain score of 5 and was using nonsteroidal anti-inflammatory drugs as needed to manage pain. He had functional limitations and a range of motion of 10–100°. As a result of the extent of disease, the patient’s case was discussed with a sarcoma multidisciplinary tumor board (MDTB) to determine the treatment approach. The MDTB discussed multiple options, including open surgery using an anterior or posterior incision, arthroscopic surgery, medical therapy, and a wait-and-see approach. The patient had no prior surgery, except for arthroscopic biopsy of the knee, and significant disease. The consensus of the MDTB was that surgery was expected to cause significant morbidity in this patient, with the intraoperative risk of neurovascular damage and possible postsurgical joint stiffness, in addition to almost certainty that the patient would not obtain complete relief from the disease. Given the patient’s symptomatic disease and the tumor not being amenable to improvement with surgery, treatment with pexidartinib was considered.
The MDTB agreed to prescribe pexidartinib, and the patient started pexidartinib at 800 mg daily (400 mg, twice daily) through a compassionate use program. After 4 months of treatment, the patient’s creatine phosphokinase (CPK) levels increased and the dose of pexidartinib was reduced to 400 mg daily; after 2 months, CPK levels continued to increase and the dose was further reduced to 200 mg daily. During this time, the Risk Evaluation and Mitigation Strategy program was followed and the CPK elevations were discussed with the drug manufacturer (Daiichi Sankyo, Inc.). Because there was no increase in the patient’s bilirubin or creatinine levels, pexidartinib treatment continued at the reduced dose.
After approximately 2 years of pexidartinib therapy (September 2021), a complete response was observed (Fig. 2); the patient reported an NRS pain score of 1–2 with decreased pain frequency and intensity and a complete recovery of range of motion in the affected knee. The patient also reported no side effects related to pexidartinib treatment. After the profound response to pexidartinib, additional treatments were considered, including surgery, continuation of pexidartinib, and suspension of pexidartinib with possible rechallenge if symptoms returned. The MDTB recommended continued treatment with pexidartinib at a dose of 200 mg/day, and MRI scans were repeated every 3 months.
In March 2022, the patient decided to stop pexidartinib for future family planning; pexidartinib therapy was interrupted and the patient’s wife had a successful pregnancy and delivery. In June 2022, 3 months after interrupting pexidartinib therapy, minimal clinical progression was observed with an NRS pain score of 0 and decreasing range of motion; these findings were validated by MRI showing relapse of TGCT. The patient showed a slow but constant clinical deterioration, with a reduction in the range of movement of the affected knee and an apparent increase in widespread TGCT nodules.
In January 2023, the patient’s pain had increased to an NRS pain score of 3 and he had limitations in daily activities (e.g., climbing steps of a staircase without pain, walking for long distances without stopping) and generally showed a worsening quality of life and MRI showed RECIST v1.1 progression of the disease (Fig. 3). At this point, because the pexidartinib compassionate use program was closed, the MDTB’s recommendation was to resume systemic treatment with an investigational CSF1 receptor inhibitor, which is currently ongoing within a clinical trial at the time of this report. The patient provided written informed consent for the publication of this case report and accompanying images.
Discussion
Treatment goals for patients with TGCT include long-term tumor eradication or control of tumor burden and improvement of musculoskeletal symptoms [3]. The successful use of pexidartinib in this patient provides support for the use of this treatment modality as upfront therapy for TGCT, with consideration of symptoms, functional limitations, surgical prognosis, and treatment side effects.
Although the standard-of-care treatment for TGCT is surgery, this is not always possible, as observed in the present report. Furthermore, tumors often recur, and repeated surgeries may be required [9]. The ENLIVEN study showed that 39% of patients had stable disease with pexidartinib treatment, with an additional 39% achieving partial or complete response to therapy [7]. In a pooled analysis of patients treated with pexidartinib for a median of 19 months (range 1–76+ months), the response rate per RECIST v1.1 was 60% [10].
The patient described in this report experienced a complete tumor response after 2 years of pexidartinib treatment, which was accompanied by reduced pain and improved range of motion. The patient had CPK elevations that led to dose reduction. These elevations were discussed at length with the study sponsor, and it was determined that discontinuation was not necessary. During the time the patient started pexidartinib, CPK was routinely monitored. The treatment was well tolerated over 4 years of treatment, including CPK normalization after dose reduction to 200 mg. Importantly, the reduced dose was also effective with improved response over time.
Pexidartinib is associated with a serious risk of hepatotoxicity; in an analysis of patients treated with pexidartinib across four clinical studies, 95% of patients with TGCT experienced liver test abnormalities, 5 (4%) patients experienced serious mixed or cholestatic injury with all events occurring within the first 8 weeks of treatment [11]. The patient described here did not have liver enzyme elevations. The pexidartinib prescribing information and the Risk Evaluation and Mitigation Strategy program provide guidance and support regarding the monitoring of liver enzymes and the management of any elevations during the first few months of pexidartinib treatment [8].
In this case study, all possible treatment options were discussed with the patient, and the MDTB determined that surgery would have negatively impacted the patient. If the patient had not been experiencing symptoms, active surveillance with a “watch and wait” approach and routine imaging could have been taken (Fig. 4). However, given the young age of the patient and the extent and severity of disease, treatment with pexidartinib was chosen as the first option in an attempt to avoid the morbidity of the joint, considering the high risk of relapse/progression after surgery. This treatment approach aligns with the current labeling recommendations for pexidartinib in patients with TGCT associated with severe morbidity or functional limitations and not amenable to surgery because the patient in this case report was not eligible for surgery [8]. Pexidartinib is currently the only approved systemic treatment for patients with TGCT; however, it is not available in all countries. In countries without approved systemic therapy, consensus guidelines recommend participation in a clinical trial or off-label CSF1 receptor inhibitors [12]. Standard treatment for symptomatic TGCT is surgery when it can be accomplished without significant morbidity. However, for patients with difficult to manage, symptomatic disease or with moderate/severe functional impairment for which surgery would be associated with significant morbidity, medical management with systemic treatments is considered. CSF1 receptor inhibitors are considered the standard systemic treatment for adult patients with TGCT. Currently, pexidartinib is the only approved CSF1 receptor inhibitor, in the USA, South Korea, and Taiwan, for adult patients with symptomatic TGCT associated with severe morbidity or functional limitations and not amenable to improvement with surgery [8, 13, 14]. Other CSF1 receptor inhibitors are available for patients either as investigational agents in ongoing clinical trials for TGCT or by off-label treatment, when available [12].
Several other case reports of neoadjuvant use of pexidartinib have been reported in the literature. Bernthal et al. reported on three separate patients who were treated with pexidartinib prior to surgery, after surgery, or at both times to improve outcomes in patients with TGCT [15]. In one of these patients, neoadjuvant pexidartinib over 16 months reduced the tumor size and allowed for successful total hip arthroplasty. The second patient had d-TGCT of the foot and recurrence after surgery; pexidartinib treatment resulted in disease stabilization and symptom control. The third patient with d-TGCT of the knee was able to successfully eliminate disease with surgery plus pre- and postoperative pexidartinib [15]. In a separate report, neoadjuvant pexidartinib was used in a 32-year-old patient for recurrence of d-TGCT in the shoulder, neck, and chest that could not be cured with resection or amputation; pexidartinib therapy reduced tumor masses by approximately 80% and the patient underwent limb-salvage surgery, after which the patient resumed adjuvant pexidartinib [16]. Finally, pexidartinib was initiated in a 47-year-old woman with extensive d-TGCT of the right knee who was not a surgical candidate; pexidartinib treatment reduced tumor size, which allowed the patient to return to work and reduce the use of pain medications [17]. Taken together, these cases suggest that use of neoadjuvant targeted therapy that reduces tumor burden may allow for successful resection and reconstruction to restore joint functionality, redefining inoperable cases of TGCT [16].
Given the dramatic response in this patient with severe TGCT not amenable to surgery, future studies are warranted to investigate the benefits of CSF1 receptor inhibitors, such as pexidartinib, (1) to reduce TGCT burden of disease prior to surgery (neoadjuvant setting) or (2) as the sole treatment option to reduce tumor size, reduce symptoms, and improve function of the involved joint or joints (upfront therapy), followed by treatment dose reduction or delays (maintenance treatment) or treatment interruption (drug holidays) and rechallenge (Fig. 4).
Conclusion
This case report detailing the upfront use of pexidartinib adds to the literature on upfront use of CSF1 receptor inhibitors for the treatment of d-TGCT and highlights the importance of multidisciplinary care and an individualized treatment approach based on the patient’s tumor location and type, symptoms, functional impact, extent of response to treatments, and needs specific to young adults, including reproductive health and timing.
Data Availability
Clinical and imaging data are available upon request.
References
Palmerini E, Longhi A, Donati DM, Staals EL. Pexidartinib for the treatment of adult patients with symptomatic tenosynovial giant cell tumor: safety and efficacy. Expert Rev Anticancer Ther. 2020;20:441–5.
Healey JH, Bernthal NM, van de Sande M. Management of tenosynovial giant cell tumor: a neoplastic and inflammatory disease. J Am Acad Orthop Surg Glob Res Rev. 2020;4(e20):00028.
Vaynrub A, Healey JH, Tap W, Vaynrub M. Pexidartinib in the management of advanced tenosynovial giant cell tumor: focus on patient selection and special considerations. Onco Targets Ther. 2022;15:53–66.
West RB, Rubin BP, Miller MA, et al. A landscape effect in tenosynovial giant-cell tumor from activation of CSF1 expression by a translocation in a minority of tumor cells. Proc Natl Acad Sci USA. 2006;103:690–5.
Fang H, He R, Chiu A, et al. Genetic factors in acute myeloid leukemia with myelodysplasia-related changes. Am J Clin Pathol. 2020;153:656–63.
Tap WD, Wainberg ZA, Anthony SP, et al. Structure-guided blockade of CSF1R kinase in tenosynovial giant-cell tumor. N Engl J Med. 2015;373:428–37.
Tap WD, Gelderblom H, Palmerini E, et al. Pexidartinib versus placebo for advanced tenosynovial giant cell tumour (ENLIVEN): a randomised phase 3 trial. Lancet. 2019;394:478–87.
TURALIO® (pexidartinib) capsules, for oral use [package insert]. Basking Ridge, NJ: Daiichi Sankyo, Inc.; November 2023.
Verspoor FG, van der Geest IC, Vegt E, Veth RP, van der Graaf WT, Schreuder HW. Pigmented villonodular synovitis: current concepts about diagnosis and management. Future Oncol. 2013;9:1515–31.
Gelderblom H, Wagner AJ, Tap WD, et al. Long-term outcomes of pexidartinib in tenosynovial giant cell tumors. Cancer. 2021;127:884–93.
Lewis JH, Gelderblom H, van de Sande M, et al. Pexidartinib long-term hepatic safety profile in patients with tenosynovial giant cell tumors. Oncologist. 2021;26:e863–73.
Stacchiotti S, Dürr HR, Schaefer IM, et al. Best clinical management of tenosynovial giant cell tumour (TGCT): a consensus paper from the community of experts. Cancer Treat Rev. 2023;112: 102491.
Pexidartinib. Korean Ministry of Food and Drug Safety (MFDS). 2021. https://www.mfds.go.kr/eng/brd/m_19/view.do?seq=70437. Accessed 24 July 2024.
Pexidartinib. Taiwan Food and Drug Administration (FDA). 2022. https://www.fda.gov.tw/eng/searchin.aspx?q=pexidartinib. Accessed 24 July 2024.
Bernthal NM, Randall RL, Zeitlinger LN, Geiger EJ, Healey JH. Complementary effects of surgery and pexidartinib in the management of patients with complex diffuse-tenosynovial giant cell tumor. Case Rep Orthop. 2022;2022:7768764.
Geiger EJ, Jensen AR, Singh AS, Nelson SD, Bernthal NM. Use of neoadjuvant pexidartinib with limb salvage surgery for diffuse tenosynovial giant cell tumor: a case report. J Orthop Sci. 2024;29:458–62.
Giustini N, Bernthal NM, Bukata SV, Singh AS. Tenosynovial giant cell tumor: case report of a patient effectively treated with pexidartinib (PLX3397) and review of the literature. Clin Sarcoma Res. 2018;8:14.
Acknowledgements
The authors thank MariaPia Cumani for her graphic work and figure support.
Medical Writing/Editorial Assistance
Medical writing and editorial assistance were provided by Miranda Tradewell, PhD, of Lumanity Scientific Inc., and were financially supported by Daiichi Sankyo, Inc.
Funding
Medical writing and editorial assistance for the development of this case report were financially supported by Daiichi Sankyo, Inc. Daiichi Sankyo, Inc. is also covering publication costs including the journal’s Rapid Service Fee.
Author information
Authors and Affiliations
Contributions
Emanuela Palmerini conceived of the presented idea and was responsible for methodology, investigation, obtaining resources, and supervision. Emanuela Palmerini and Gianmarco Tuzzato curated and validated the data and contributed to visualization of the images. Giuliano Peta was responsible for evaluating the images and selecting the images for the case report. All authors contributed to the writing (original draft; review and editing) of the case report and reviewed and approved the final case report.
Corresponding author
Ethics declarations
Conflict of Interest
Emanuela Palmerini has served on advisory boards for Daiichi Sankyo, Inc., Deciphera Pharmaceuticals, EUSA Pharma, and SynOx Therapeutics. Giuliano Peta and Gianmarco Tuzzato report no financial competing interests.
Ethical Approval
The patient provided written informed consent for the publication of this case report and accompanying images.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Palmerini, E., Peta, G. & Tuzzato, G. Pexidartinib Upfront in a Case of Tenosynovial Giant Cell Tumor: Proof of Concept for a Treatment Paradigm Shift. Oncol Ther (2024). https://doi.org/10.1007/s40487-024-00298-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40487-024-00298-z