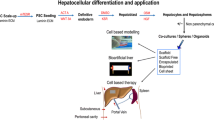
Abstract
Purpose of Review
Liver stem cells have been proposed as alternatives or additions for whole liver transplantations to accommodate the donor liver shortage. Various sources of liver stem cells have been described in experimental animal studies. Here we aim to compare the various studies.
Recent Findings
Irrespective of the experimental design, the percentage of long-lasting survival and functional recovery of transplanted cells is generally very low. An exception to this are the proliferating hepatocytes transplanted into Fah(-/-) Rag2−/−IL2rg−/− mice; here 4-month post-transplantation around 65% repopulation was observed, and 11/14 mice survived in contrast to zero survival in sham-treated animals.
Summary
Taking the different cellular sources for the organoids, the different maturation status of the transplanted cells, and the variable animal models into account, a paper-to-paper comparison is compromised. This lack of objective comparison restricts the translation of these model studies into clinical practice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The liver, in weight one of the body’s largest organs, has a crucial role in a plethora of biological functions. Among these are functions associated with lipid metabolism (energy storage), ammonium removal, bile acid synthesis, and the production of clotting factors and (de)toxification of compounds delivered to the liver via the intestinal tract. Not to mention liver-specific viruses, but the liver is continuously exposed to toxins and drugs and especially in the well-fed Western World with high levels of lipids [1]. Although the liver has an enormous regenerative capacity, the insults can be too severe for the liver regeneration potential. Reviews on the mechanism of liver regeneration are numerous, including discussions on the various flavors of liver stem cells involved in the daily tear-and-wear on the recovery from more severe incidental insults (e.g. [2,3,4,5,6,7].). Recently, innumerous reviews on organoids as translational models and for developmental studies have been published (e.g., [8,9,10,11,12,13,14].).
This review focusses on the potential of liver organoids to be used as alternatives for liver transplantation. Organoids for disease modeling and or improved toxicology models are not addressed here. The readers will realize that the term organoid and the cell types constituting an organoid are currently far from clear-cut. Therefore, emphasis in this review is on the various types of liver organoid transplantations described today and current hurdles hampering the clinical application of transplantable liver organoids as alternatives for whole liver transplantations.
The Start
Due to the lack of suitable organs for liver transplantation, around 25% of the patients on the waiting list for whole liver transplantation die before they receive a matching donor liver [1, 15]. The percentage of successful transplantations is high, but lifelong immunosuppression and the costs associated herewith are enormous [16, 17]. Moreover, it is anticipated that the number of people requiring liver transplantation is likely to increase in the next decades in view of the long-term detrimental effects of especially HBV and the increase in people suffering from the deleterious effects of obesity on the liver function, especially if the nonalcoholic fatty liver disease (NAFLD) progresses into nonalcoholic steatohepatitis (NASH) [18]. Undoubtedly, alternatives for whole liver transplantation are therefore urgently needed.
The recent advances in liver organoid culture open novel alternative pathways. Organoids are most often defined as an in vitro 3D-multicellular cluster derived from stem/progenitor cells, capable of self-renewal and self-organization, that recapitulates the function of the tissue from which it was derived [19, 20]. These functional, not necessarily architectural, mini-organs have been described initially for the intestine [21], not surprisingly in view of the enormous turnover of the intestine. In 2013 a landmark paper first described mouse liver organoids derived from single Leucine-rich repeat-containing G protein coupled receptor 5+ (Lgr5, G-receptor coupled receptor 49) liver stem cells [22••]. Lgr5, and its homologs Lgr4 and Lgr6, are receptors for R-spondin, which upon binding of R-spondin inactivate the E3 ligases Znrf3 and Rnf43. This, in turn, results in an increased concentration of the Wnt receptors Frizzled and Lrp5/6 on the cell surface and a high activation of the canonical Wnt/beta-catenin signaling pathway. Single Lgr5+ cells, isolated by means of FACS, can initiate organoid formation in vitro, which can be expanded in culture under growth conditions with active Wnt signaling and can be driven into a hepatocyte-like phenotype under differentiation conditions. Moreover, it turned out that functional hepatocytes could be generated upon transplantation of clonally expanded organoids in fumarylacetoacetate hydrolase Fah(-/-) mice, a model for severe liver damage. In the following years, liver organoids from humans and other non-primate mammals were described [23,24,25,26]. The cells from these 3D culture systems were applied in liver transplantations, disease modeling, and liver developmental studies. Under expansion conditions, these cells were proliferating in culture for over 8 months while remaining genetically stable as assayed by means of whole genome sequencing and/or karyotyping [23, 24, 26]. This suggests that the organoids are not likely to form tumors in vivo, and therefore the genetic stability fulfilled a requirement for transplantation.
Of note, organoids derived from Lgr5+ stem cells are pure epithelial structures. In contrast, the liver consists of various cell types in order to perform its multiple functions: hepatocytes (biotransformation, coagulation factors) account for about 60–70% of the cells, whereas other cell types are less abundant, e.g., stellate cells (vitamin A storage, 5–8%), Kupffer cells (liver macrophages, 10–15%), cholangiocytes (bile duct cells, 3–5%), and liver sinusoidal endothelial cells (blood vessel lining, 15–20%). This cellular variability poses pressure on the use of the word “organoid” for stem/progenitor-derived 3D liver cultures, since those “organoids” do not self-organize into a structural entity constituting the various (non-epithelial) liver cells types. However, for the sake of simplicity, the term “organoids” in the following applies to 3D stem/progenitor-derived in vitro cultures mimicking to a certain degree some aspects of the livers function.
Transplantation with Liver Organoids
The first description of transplanted liver organoids, expanded mouse Lgr5+ cells from liver ductal origin into Fah(-/-) Rag(-/-) IL2rg(-/-) mice, revealed that as little as in 3 out of 5 mice, some Alb+/Fah+/CYP+ cell clusters were detected 2–3 months post-transplantation. These cells accounted for around 0.1–1% of the total liver volume [22••]. A few years later, transplantation of human liver organoids into retrorsine/CCl4-treated Balbc/nude mice [23] and rat liver organoids into Fah(-/-) IL2rg(-/-) rats [25] has been described. Although the successful recovery from induced liver damage was obtained in 2 out of 6 mice and 7 out of 11 rats in those studies, again only a few % reconstitution of the liver volume was achieved. One positive exception was observed in 1 rat, where almost 20% of the liver volume was repopulated by donor cells [25].
In addition to lgr5+-derived organoids, the cellular basis of liver organoids can also arise from induced pluripotent stem cells (iPSs) that are differentiated toward hepatocyte- or cholangiocyte-like cells. In this respect, the findings by Takebe and co-workers are of great interest [27••, 28]. In contrast to the above-mentioned Lgr5+ adult stem cells, the iPS-derived hepatocyte-like organoids self-organized into 3D-multicellular structures on the basis of a mixture of iPS cells (or fetal derived hepatocyte-like cells) in combination with HUVECs and MSCs. Upon transplantation of these buds into immunodeficient NOD/SCID mice, functional hepatocyte cords were formed lining with the sinusoidal endothelium. Most strikingly here was the connection with the blood system of the recipient mice.
The cellular source of transplantable liver cells is even more widespread, and then Lgr5+ and iPSCs derived donor cells only. For instance, extrahepatic cholangiocytes can be the origin of the cells in a transplanted organoid [29]. Here a damaged bile duct epithelium is reconstituted under the kidney capsule by transplantation of organoids from extrahepatic cholangiocytes in NSG-mice [29]. The choice here for this cell type makes sense in view of the damaged target organ to be addressed with the transplantation.
Transplantations with Hepatocytes from Non-adult-Hepatocyte Sources
Taking into consideration the damaged cell types of specific liver diseases, it is conceivable that hepatocytes are at the basis of transplantation to recover damaged hepatocytes. Therefore, iHEPS (directly reprogrammed hepatocytes) were differentiated toward hepatocytes including CYP activity [30]. In combination with a PCL scaffold, the differentiated iHEPS normalized ALT and bilirubin levels in Fah(-/-) Rag2(-/-) IL2rg(-/-) mice, whereas AST levels remained elevated [30]. This is indicative of a partial recovery from the liver damage by the transplanted cells.
Yet another cellular source was utilized by Hu et al. [31], who compared transplantation efficiency of fetal hepatocyte-derived organoids with pediatric primary hepatocyte organoids [31]. Three-month post-transplantation in Fah(-/-) Rag2(-/-) NOD IL2rg(-/-) mice nodules of Alb+/MRP2+/Cyp2E1+ cells were still detected. Furthermore, the proliferation marker Ki67 indicated that these cells were actively proliferating. The albumin secretion was slightly better in the pediatric compared to the fetal-derived organoids.
Massive cell proliferation was achieved upon TNF alpha stimulation of mouse bile duct-derived hepatocytes [32]. In the Fah (-/-) mice classical model for liver cell transplantation, 3–4 months post-transplantation revealed only a few percentages of repopulated cells that were Ki67+ positive. A very high repopulation was observed in Fah(-/-) Rag2(-/-) IL2rg(-/-) mice transplanted with bi-potent proliferating hepatocytes [33]. Not only the authors showed a way to reach enormous cell numbers but the transplantation results were impressive. Eleven out of 14 mice survived (in contrast to zero in the sham-operated mice), and on average 65% of repopulation of the recipient liver was achieved.
One of the very few studies in rats was a transplantation of iPS-derived hepatic stem cells into Gunn rats, the classical model for the Crigler-Najjar syndrome [34]. Upon maturation of the transplanted stem cells in the recipient Gunn rats, hyperbilirubinemia was severely reduced (about one third), and human uridine diphosphate glucuronosyltransferase 1 family polypeptide A1 (UGT1A1) expressing cells were detected in less than 1% of the cells.
The Misty Road
Taking the results of all these studies, it is obvious that a comparison of the various studies is difficult. In view of the different cellular sources for the organoids, different maturation status of the transplanted cells, variable animal models, and biological parameters described, a paper-to-paper comparison is hampered. A summary of the various organoid transplantation studies is presented in Table 1. Recently transplantation studies were performed with autologous and gene-corrected canine livers organoids in COMMD1-deficient dogs [35]. Due to a large deletion encompassing exon-2 of the COMMD1-gene [36], these dogs develop copper toxicosis, at higher speed and more pronounced as compared to for instance Wilson disease [37];[38]). Preliminary data point in the same direction as for the rodent models, with the engraftment of donor cells being ineffective on the long run. In the model of inherited copper toxicosis, the follow-up period was up to 2 years post-transplantation.
One obvious drawback of these initial studies was the cellular composition of these organoids. They consisted mainly of partially differentiated hepatic epithelial cells (at best covering a limited array of hepatocyte functions, e.g., albumin production and a few biotransformation enzymes). The cellular heterogeneity and architecture of the liver were not taken into account, as was the possible heterogeneity in hepatocyte-like cells within one organoid (or between organoids of same donor, not to mention variations between donors). On top of this, various research groups developing liver organoids used different sources to acquire organoids consisting of hepatocyte (or cholangiocyte) like cells. This makes the road toward successful organoid transplantations quite challenging.
The Next Hurdle Toward the Finish
Mathematically speaking, the most cost-effective means to overcome organ shortage is related to lifestyle changes (prevention of liver diseases) and the stimulation to acquire more donor organs [39]. Although cost-effective in theory and in view of the highly effective current HCV-treatment regimens improvements are expected, donor shortage will remain an obstacle in the years to come.
Therefore, alternatives for whole liver transplantations will be needed as urgently as before. So what hampers clinical application of organoids as alternatives for whole liver transplantations? The culture of organoids most often requires undefined Matrigel, a compound extracted from the Engelbreth-Holm-Swarm (EHS) mouse sarcoma. Apart from ethical concerns related to the usage of mice for the production of Matrigel, the variable functionality and the largely unknown and possibly variable constitution of Matrigel clearly block FDA approval. Therefore, it is of the utmost importance to investigate a more robust, defined, and animal-free alternatives for Matrigel. This is a highly competitive field of biotechnology nowadays. In order to make all the claims on “highly potential” alternatives for Matrigel for liver organoid culture comparable with each other, some guidelines regarding liver functionality are required, if not urgently needed. These guidelines should include, among other parameters, a minimal list of defined characteristics of a functional hepatocyte, a functional cholangiocyte, or other liver cell types to be included in the organoid system. As long as such guidelines are not implemented, the field of Matrigel alternatives remains open for “wild claims.” Even more difficult is the issue of cellular heterogeneity within one organoid and between organoids of the same donor. This heterogeneity was clearly described in human iPS-derived organoids by means of single cell RNA-Seq, which revealed several cell clusters related to the various cell types harbored in the organoids such as hepatocyte-, stellate-, and Kupffer-like cells [40••]. Even within the large hepatocyte-like cell clusters, an enormous variation in RNA expression was observed. This multicell-type organoid provided a novel approach to study liver fibrosis, especially with the incorporation of atomic force microscopy to evaluate the stiffness of the organoids as a measure for fibrogenesis. For other types of organoids, the heterogeneity of the cells within an organoid is not yet fully appreciated and opens new lines of research. For instance, after disrupting an organoid, does it reconstitute in the same 3D cellular makeup as before or is reorganization of an organoid a random process? In addition to the variations between organoid types, there is also a lack of means to compare the various transplantation studies with each other. ISSCR guidelines exist for stem cell research and clinical translation (https://www.isscr.org/membership/policy/2016-guidelines/guidelines-for-stem-cell-research-and-clinical-translation). This, however, has not cultivated a paper-to-paper comparison and improved the clear repetition of an experiment. For RT-qPCR, this problem was addressed by the MIQE-precise guidelines, nowadays obligatory for numerous scientific journals, providing a checklist with minimal requirements needed for proper comparison and repetition [41]. Based on the success of the MIQE-precise guidelines, an initiative to make something similar for organoid transplantation studies is provided in Table 2. The required information is presented in the studies discussed above although a paper-to-paper comparison is difficult in view of the various measurements. A checklist would simplify the search for experimental details and facilitate the comparison and reproducibility of the described studies. This is beneficial for reviewers, readers, and those who want to repeat/continue on the published papers.
The authors remain positive about the future of transplantable liver organoids. Different approaches are already ongoing in that respect, varying from developments with decellularized liver scaffolds [42•, 43,44,45, 46•], organoid cultures combined with bioprinting, upscaling and improved differentiation protocols [31,32,33, 47]; (Schneeberger et al., accepted), more and better predictive animal models (e.g., [35]), and so forth. Those combined efforts all seem to pave the way for transplantable liver organoids. But yet unknown hurdles are to be expected (“the known unknowns”), including ethical issues [48], as always in the translation of models into clinical practice. These prospects keep us motivated as never before.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Younossi ZM, Koening AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease- meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84.
Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004;5:836–47.
Riehle KJ, Dan YY, Campbell JS, Fausto N. New concepts in liver regeneration. J Gastroenterol Hepatol. 2011;35:923–9.
Fausto N, Campbell JS, Riehle KJ. Liver regeneration. J Hepatol. 2012;57:692–4.
Forbes SJ, Rosenthal N. Preparing the ground for tissues regeneration: from mechanisms to therapy. Nat Med. 2014;20:857–69.
Michalopoulos GK. Liver regeneration and normal liver tissue maintenance. Hepatology. 2017;65:1384–92.
Van Haele M, Snoeck J, Roskams T. Human liver regeneration: an etiology dependent process. Int J Mol Sci 2019:20 in press doi: https://doi.org/10.3390/ijms20092332.
Ader M, Tanaka EM. Modeling human development in 3D culture. Curr Opin Cell Biol. 2014;31:23–8.
Nantasanti S, de Bruin A, Rothuizen J, Penning LC, Schotanus BA. Concise review: organoids are a powerful tool for the study of liver disease and personalized treatment design in humans and animals. Stem Cells Transl Med. 2016;5:325–30.
Ober EA, Lemaigre FP. Development of the liver: insights into organ and tissue morphogenesis. J Hepatol. 2018;68:1049–62.
Akbari S, Sevinç GG, Ersoy N, Basak O, Kaplan K, Sevinç K, et al. Robust, long-term culture of endoderm-derived hepatic organoids for disease modeling. Stem Cell Rep. 2019;8:627–41.
Haugobook SJ, Ferrer M, Ottinger EA. In vitro and in vivo translational models for rare livers diseases. Biochim Biophys Ata, Mol Bas Dis. 2019;1865:1003–18.
Prior N, Inacio P, Huch M 2019. Liver organoids: from basic research to therapeutic applications. Gut: in press doi :https://doi.org/10.1136/gutjnl-2019-319256.
Fiorotto R, Amenduni M, Mariotti V, Fabris L, Spirli C, Strazzabosco M. Liver diseases in the dish: iPSC and organoids as a new approach to modeling liver diseases. Biochim Biophys Acta Mol basis Dis. 2019;1865(5):920–8. https://doi.org/10.1016/j.bbadis.2018.08.038Epub 2018 Sep 5.
Zhang J, Zhao X, Liang ML, Demirci U, Wang S. A decade of progress in liver regenerative medicine. Biomaterials. 2018a;157:161–76.
Jorns C, Ellis EC, Nowak G, Fischler B, Nemeth A, Strom SC, et al. Hepatocyte transplantation for inherited metabolic diseases of the liver. J Intern Med. 2012;272:201–23.
Forbes SJ, Gupta S, Dhawan A. A cell therapy for liver disease: from liver transplantation to cell factory. J Hepatol. 2015;62:S157–69.
Younossi ZM, Stepanova M, Younossi Y, Golabi P, Mishra A, Rafiq N, Henry L 2019. Epidemiology of chronic livers diseases in the USA in the past three decades. Gut in press. doi:https://doi.org/10.1136/gutjnl-2019-318813.
Lancaster MA, Knoblich JA. Organogenesis in a dish: modeling development and disease using organoid technology. Science. 2014;345:1247125.
Huch M, Koo BK. Modeling mouse and human development using organoid cultures. Development. 2015;142:3113–25.
Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–5.
•• Huch M, Dorrell C, Boj SF, van Es JH, Li VS, van de Wetering M, et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. 2013;494:247–50 This is the first description of liver organoids derived from single Lgr5+ cells.
Huch M, Gehart H, van Boxtel R, Hamer K, Blokzijl F, Verstegen MM, et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell. 2015;160:299–312.
Nantasanti S, Spee B, Kruitwagen HS, Chen C, Geijsen N, Oosterhoff LA, et al. Disease modeling and gene therapy of copper storage disease in canine hepatic organoids. Stem Cell Rep. 2015;5:895–907.
Kuijk EW, Rasmussen S, Blokzijl F, Huch M, Gehart H, Toonen P, et al. Generation and characterization of rat liver stem cell lines and their engraftment in a rat model of liver failure. Sci Rep. 2016;6:22154.
Kruitwagen HS, Oosterhoff LA, Vernooij IGWH, Schrall IM, van Wolferen ME, Bannink F, et al. Long-term adult feline liver organoid cultures for disease modeling of hepatic steatosis. Stem Cell Rep. 2017;8:822–30.
•• Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T, et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499:481–4 This paper describes a self-organizing liver bud based on iPSC, mesenchymal stromal cells and endothelial cells.
Takebe T, Koike N, Sekine K, Fujiwara R, Amiya T, Zheng YW, et al. Engineering of human hepatic tissue with functional vascular networks. Organogenesis. 2014 Apr-Jun;10(2):260–7.
Sampaziotis F, Justin AW, Tysoe OC, Sawiak S, Godfrey EM, Upponi SS, et al. Reconstruction of the mouse extrahepatic biliary tree using primary human extrahepatic cholangiocyte organoids. Nat Med. 2017;23:954–63.
Rashidi H, Luu NT, Alwahsh SM, Ginai M, Alhaque S, Dong H, et al. 3D human liver tissue from pluripotent stem cells displays stable phenotype in vitro and supports compromised liver function in vivo. Arch Toxicol. 2018;92:3117–29.
Hu H, Gehart H, Artegiani B, Löpez-Iglesias C, Dekkers F, Basak O, et al. Long-term expansion of functional mouse and human hepatocytes as 3D organoids. Cell. 2018;175:1591–606.
Peng WC, Logan CY, Fish M, Anbarchian T, Aguisanda F, Álvarez-Varela A, et al. Inflammatory cytokine TNFα promotes the long-term expansion of primary hepatocytes in 3D culture. Cell. 2018;175:1607–19.
Zhang K, Zhang L, Liu W, Ma X, Cen J, Sun Z, et al. In vitro expansion of primary human hepatocytes with efficient liver repopulation capacity. Cell Stem Cell. 2018;23:806–19.
Fourrier A, Delbos F, Menoret S, Collet C, Thi Thuy LT, Myara A, et al. Regenerative cell therapy for the treatment of hyperbilirubinemic Gunn rats with fresh and frozen human induced pluripotent stem cells-derived hepatic stem cells. Xenotransplantation. 2019;25:e12544. https://doi.org/10.1111/xen.12544.
Kruitwagen HS, Fieten H, Penning LC. Towards bioengineered liver stem cell transplantation studies in a preclinical dog model for inherited copper toxicosis. Bioengineering (Basel). 2019;6:E88.
van de Sluis B, Rothuizen J, Pearson PL, van Oost BA, Wijmenga C. Identification of a new copper metabolism gene by positional cloning in a purebred dog population. Hum Mol Genet. 2002;11:165–73.
Favier RP, Spee B, Schotanus BA, van den Ingh TS, Fieten H, Brinkhof B, et al. COMMD1-deficient dogs accumulate copper in hepatocytes and provide a good models for chronic hepatitis and fibrosis. PloS-One. 2012;7:e42158.
Favier RP, Spee B, Fieten H, van den Ingh TS, Schotanus BA, Brinkhof B, et al. Aberrant expression of copper associated genes after copper accumulation in COMMD1-deficine dogs. J Trace Elem Med Biol. 2015;29:347–53.
El-Agroudy NN, Kurzbach A, Rodionov RN, O’Sullivan J, Roden M, Birkenfeld AL, et al. Are lifestyle therapies effective for NAFLD treatment? Trends Endocrinol Metab. 2019;30:701–9.
•• Ouchi R, Togo S, Kimura M, Shinozawa T, Koido M, Koike H, et al. Modeling Steatohepatitis in Humans with Pluripotent Stem Cell-Derived Organoids. Cell Metab. 2019;30:374–84 This paper shown, based on single cell RNA-sequencing, the highly variable population of cells that constitute liver organoids based on iPS cells.
Bustin SA, Beaulieu JF, Huggett J, Jaggi R, Kibenge FS, Olsvik PA, et al. MIQE précis: Practical implementation of minimum standard guidelines for fluorescence-based quantitative real-time PCR experiments. BMC Mol Biol. 2010;11:74.
• Baptista PM, Siddiqui MM, Lozier G, Rodriguez SR, Atala A, Soker S. The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology. 2011;53:604–17 This is the first description of the generation of a vascularized human liver after decellularization.
Lang R, Stern MM, Smith L, Liu Y, Bharadwaj S, Liu G, et al. Three-dimensional culture of hepatocytes on porcine liver tissue-derived extracellular matrix. Biomaterials. 2011;32:7042–52.
Chen C, Pla-Palacín I, Baptista PM, Shang P, Oosterhoff LA, van Wolferen ME, et al. Hepatocyte-like cells generated by direct reprogramming from murine somatic cells can repopulate decellularized livers. Biotechnol Bioeng. 2018;115:2807–16.
Verstegen MMA, Willemse J, van den Hoek S, Kremers GJ, Luider TM, van Huizen NA, et al. Decellularization of whole human liver grafts using controlled perfusion for transplantable organ bioscaffolds. Stem Cells Dev. 2017;26:1304–15.
• Vyas D, Baptista PM, Brovold M, Moran E, Gaston B, Booth C, et al. Self-assembled liver organoids recapitulate hepatobiliary organogenesis in vitro. Hepatology. 2018;67:750–61 A model was presented to study liver development and diseases based on self-assembling liver progenitor cells in a acellularized scaffold.
Takebe T, Sekine K, Kimura M, Yoshizawa E, Ayano S, Koido M, et al. Massive and reproducible production of liver buds entirely from human pluripotent stem cells. Cell Rep. 2017;21:2661–70.
Bredenoord AL, Clevers H, Knoblich JA. Human tissues in a dish: the research and ethical implications of organoid technology. Science. 2017 Jan;20:355(6322).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Pedro Baptista and Louis Penning declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Tissue Engineering and Regeneration
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Baptista, P.M., Penning, L.C. Transplantable Liver Organoids, Too Many Cell Types to Choose: a Need for Scientific Self-Organization. Curr Transpl Rep 7, 18–23 (2020). https://doi.org/10.1007/s40472-020-00266-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40472-020-00266-2