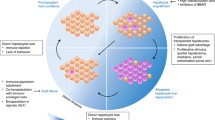
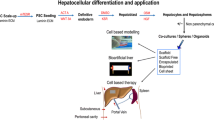
Abstract
Purpose of Review
Significant recent scientific developments have occurred in the field of liver repopulation and regeneration. While techniques to facilitate liver repopulation with donor hepatocytes and different cell sources have been studied extensively in the laboratory, in recent years, clinical hepatocyte transplantation (HT) and liver repopulation trials have demonstrated new disease indications and also immunological challenges that will require the incorporation of a fresh look and new experimental approaches.
Recent Findings
Growth advantage and regenerative stimulus are necessary to allow donor hepatocytes to proliferate. Current research efforts focus on mechanisms of donor hepatocyte expansion in response to liver injury/preconditioning. Moreover, the latest clinical evidence shows that important obstacles to HT include optimizing engraftment and limited duration of effectiveness, with hepatocytes being lost to immunological rejection. We will discuss alternatives for cellular rejection monitoring, as well as new modalities to follow cellular graft function and near-to-clinical cell sources.
Summary
HT partially corrects genetic disorders for a limited period of time and has been associated with reversal of acute liver failure. The main identified obstacles that remain to make HT a curative approach include improving engraftment rates, and methods for monitoring cellular graft function and rejection. This review aims to discuss current state of the art in clinical HT and provide insights into innovative approaches taken to overcome these obstacles.
Similar content being viewed by others
Abbreviations
- ALF:
-
Acute liver failure
- CLI:
-
Cherenkov illumination imaging
- ESC:
-
Embryonic stem cells
- hAECs:
-
Human amnion epithelial cells
- HT:
-
Hepatocyte transplantation
- iPSCs:
-
Induced pluripotent stem cells
- MPIO:
-
Micron-sized iron oxide particles
- MSC:
-
Mesenchymal stem cells
- OLT:
-
Orthotopic liver transplantation
- PAI:
-
Photoacoustic imaging
- SERI:
-
Surface-enhanced Raman imaging
References
Papers of particular interest, published recently, have been highlighted as: ••Of major importance
Dhawan A, Puppi J, Hughes RD, Mitry RR. Human hepatocyte transplantation: current experience and future challenges. Nat Rev Gastroenterol Hepatol. 2010;7:288–98.
Allen KJ, Mifsud NA, Williamson R, Bertolino P, Hardikar W. Cell-mediated rejection results in allograft loss after liver cell transplantation. Liver Transpl. 2008;14:688–94.
Darwish AA, Sokal E, Stephenne X, Najimi M, de Goyet Jde V, Reding R. Permanent access to the portal system for cellular transplantation using an implantable port device. Liver Transpl. 2004;10:1213–5.
Dhawan A, Mitry RR, Hughes RD. Hepatocyte transplantation for liver-based metabolic disorders. J Inherit Metab Dis. 2006;29:431–5.
Dhawan A, Mitry RR, Hughes RD, Lehec S, Terry C, Bansal S, et al. Hepatocyte transplantation for inherited factor VII deficiency. Transplantation. 2004;78:1812–4.
Fox IJ, Chowdhury JR, Kaufman SS, Goertzen TC, Chowdhury NR, Warkentin PI, et al. Treatment of the Crigler-Najjar syndrome type I with hepatocyte transplantation. N Engl J Med. 1998;338:1422–6.
Grossman M, Rader DJ, Muller DW, Kolansky DM, Kozarsky K, Clark BJ 3rd, et al. A pilot study of ex vivo gene therapy for homozygous familial hypercholesterolaemia. Nat Med. 1995;1:1148–54.
Horslen SP, McCowan TC, Goertzen TC, Warkentin PI, Cai HB, Strom SC, et al. Isolated hepatocyte transplantation in an infant with a severe urea cycle disorder. Pediatrics. 2003;111:1262–7.
Jorns C, Nowak G, Nemeth A, Zemack H, Mork LM, Johansson H, et al. De novo donor-specific HLA antibody formation in two patients with Crigler-Najjar syndrome type I following human hepatocyte transplantation with partial hepatectomy preconditioning. Am J Transplant. 2016;16:1021–30.
Lysy PA, Najimi M, Stephenne X, Bourgois A, Smets F, Sokal EM. Liver cell transplantation for Crigler-Najjar syndrome type I: update and perspectives. World J Gastroenterol. 2008;14:3464–70.
Meyburg J, Das AM, Hoerster F, Lindner M, Kriegbaum H, Engelmann G, et al. One liver for four children: first clinical series of liver cell transplantation for severe neonatal urea cycle defects. Transplantation. 2009;87:636–41.
Muraca M, Gerunda G, Neri D, Vilei MT, Granato A, Feltracco P, et al. Hepatocyte transplantation as a treatment for glycogen storage disease type 1a. Lancet. 2002;359:317–8.
Puppi J, Tan N, Mitry RR, Hughes RD, Lehec S, Mieli-Vergani G, et al. Hepatocyte transplantation followed by auxiliary liver transplantation—a novel treatment for ornithine transcarbamylase deficiency. Am J Transplant. 2008;8:452–7.
Sokal EM, Smets F, Bourgois A, Van Maldergem L, Buts JP, Reding R, et al. Hepatocyte transplantation in a 4-year-old girl with peroxisomal biogenesis disease: technique, safety, and metabolic follow-up. Transplantation. 2003;76:735–8.
Stephenne X, Najimi M, Sibille C, Nassogne MC, Smets F, Sokal EM. Sustained engraftment and tissue enzyme activity after liver cell transplantation for argininosuccinate lyase deficiency. Gastroenterology. 2006;130:1317–23.
Stephenne X, Najimi M, Smets F, Reding R, de Veille de Goyet J, Sokal EM. Cryopreserved liver cell transplantation controls ornithine transcarbamylase deficient patient while awaiting liver transplantation. Am J Transplant. 2005;5:2058–61.
Bilir BM, Guinette D, Karrer F, Kumpe DA, Krysl J, Stephens J, et al. Hepatocyte transplantation in acute liver failure. Liver Transpl. 2000;6:32–40.
Fisher RA, Bu D, Thompson M, Tisnado J, Prasad U, Sterling R, et al. Defining hepatocellular chimerism in a liver failure patient bridged with hepatocyte infusion. Transplantation. 2000;69:303–7.
Khan AA, Habeeb A, Parveen N, Naseem B, Babu RP, Capoor AK, et al. Peritoneal transplantation of human fetal hepatocytes for the treatment of acute fatty liver of pregnancy: a case report. Trop Gastroenterol. 2004;25:141–3.
Schneider A, Attaran M, Meier PN, Strassburg C, Manns MP, Ott M, et al. Hepatocyte transplantation in an acute liver failure due to mushroom poisoning. Transplantation. 2006;82:1115–6.
Strom SC, Chowdhury JR, Fox IJ. Hepatocyte transplantation for the treatment of human disease. Semin Liver Dis. 1999;19:39–48.
Habibullah CM, Syed IH, Qamar A, Taher-Uz Z. Human fetal hepatocyte transplantation in patients with fulminant hepatic failure. Transplantation. 1994;58:951–2.
Horslen SP, Fox IJ. Hepatocyte transplantation. Transplantation. 2004;77:1481–6.
Mito M, Kusano M, Kawaura Y. Hepatocyte transplantation in man. Transplant Proc. 1992;24:3052–3.
•• Soltys KA, Setoyama K, Tafaleng EN, Soto Gutierrez A, Fong J, Fukumitsu K, Nishikawa T, et al. Host conditioning and rejection monitoring in hepatocyte transplantation in humans. J Hepatol. 2017;66(5):987–1000. Hepatocyte transplantation can potentially be used to treat genetic liver disorders but its application in clinical practice has been impeded by inefficient hepatocyte engraftment and the inability to monitor rejection of transplanted liver cells. In this study, we first show in non-human primates that pretreatment of the host liver with radiation improves the engraftment of transplanted liver cells. We then used this knowledge in a series of clinical hepatocyte transplants in patients with genetic liver disorders to show that radiation pretreatment and rejection risk monitoring are safe and, if optimized, could improve engraftment and long-term survival of transplanted hepatocytes in patients.
Gramignoli R, Vosough M, Kannisto K, Srinivasan RC, Strom SC. Clinical hepatocyte transplantation: practical limits and possible solutions. Eur Surg Res. 2015;54:162–77.
Khan Z, Strom SC. Hepatocyte transplantation in special populations: clinical use in children. Methods Mol Biol. 2017;1506:3–16.
Huppert SS, Campbell KM. Emerging advancements in liver regeneration and organogenesis as tools for liver replacement. Curr Opin Organ Transplant. 2016;21:581–7.
Jorns C, Ellis EC, Nowak G, Fischler B, Nemeth A, Strom SC, et al. Hepatocyte transplantation for inherited metabolic diseases of the liver. J Intern Med. 2012;272:201–23.
Hansel MC, Gramignoli R, Skvorak KJ, Dorko K, Marongiu F, Blake W, et al. The history and use of human hepatocytes for the treatment of liver diseases: the first 100 patients. Curr Protoc Toxicol. 2014;62(14 12):11–23.
Hughes RD, Mitry RR, Dhawan A. Current status of hepatocyte transplantation. Transplantation. 2012;93:342–7.
Soltys KA, Soto-Gutierrez A, Nagaya M, Baskin KM, Deutsch M, Ito R, et al. Barriers to the successful treatment of liver disease by hepatocyte transplantation. J Hepatol. 2010;53:769–74.
Bartlett DC, Newsome PNA. Modified protocol for the isolation of primary human hepatocytes with improved viability and function from normal and diseased human liver. Methods Mol Biol. 2017;1506:61–73.
Lo B, Parham L. Ethical issues in stem cell research. Endocr Rev. 2009;30:204–13.
Strom S, Fisher R. Hepatocyte transplantation: new possibilities for therapy. Gastroenterology. 2003;124:568–71.
Tsiaoussis J, Newsome PN, Nelson LJ, Hayes PC, Plevris JN. Which hepatocyte will it be? Hepatocyte choice for bioartificial liver support systems. Liver Transpl. 2001;7:2–10.
Horner R, Kluge M, Gassner J, Nosser M, Major RD, Reutzel-Selke A, et al. Hepatocyte isolation after laparoscopic liver resection. Tissue Eng Part C Methods. 2016;22:839–46.
Li AP. Human hepatocytes: isolation, cryopreservation and applications in drug development. Chem Biol Interact. 2007;168:16–29.
Vondran FW, Katenz E, Schwartlander R, Morgul MH, Raschzok N, Gong X, et al. Isolation of primary human hepatocytes after partial hepatectomy: criteria for identification of the most promising liver specimen. Artif Organs. 2008;32:205–13.
Kleine M, Riemer M, Krech T, DeTemple D, Jager MD, Lehner F, et al. Explanted diseased livers—a possible source of metabolic competent primary human hepatocytes. PLoS One. 2014;9:e101386.
Alexandre E, Cahn M, Abadie-Viollon C, Meyer N, Heyd B, Mantion G, et al. Influence of pre-, intra- and post-operative parameters of donor liver on the outcome of isolated human hepatocytes. Cell Tissue Bank. 2002;3:223–33.
Alexandrova K, Griesel C, Barthold M, Heuft HG, Ott M, Winkler M, et al. Large-scale isolation of human hepatocytes for therapeutic application. Cell Transplant. 2005;14:845–53.
Gramignoli R, Tahan V, Dorko K, Skvorak KJ, Hansel MC, Zhao W, et al. New potential cell source for hepatocyte transplantation: discarded livers from metabolic disease liver transplants. Stem Cell Res. 2013;11:563–73.
Hewes JC, Riddy D, Morris RW, Woodrooffe AJ, Davidson BR, Fuller B. A prospective study of isolated human hepatocyte function following liver resection for colorectal liver metastases: the effects of prior exposure to chemotherapy. J Hepatol. 2006;45:263–70.
Kawahara T, Toso C, Douglas DN, Nourbakhsh M, Lewis JT, Tyrrell DL, et al. Factors affecting hepatocyte isolation, engraftment, and replication in an in vivo model. Liver Transpl. 2010;16:974–82.
Lloyd TD, Orr S, Patel R, Crees G, Chavda S, Vadyar H, et al. Effect of patient, operative and isolation factors on subsequent yield and viability of human hepatocytes for research use. Cell Tissue Bank. 2004;5:81–7.
Richert L, Alexandre E, Lloyd T, Orr S, Viollon-Abadie C, Patel R, et al. Tissue collection, transport and isolation procedures required to optimize human hepatocyte isolation from waste liver surgical resections. A multilaboratory study. Liver Int. 2004;24:371–8.
Serralta A, Donato MT, Orbis F, Castell JV, Mir J, Gomez-Lechon MJ. Functionality of cultured human hepatocytes from elective samples, cadaveric grafts and hepatectomies. Toxicol in Vitro. 2003;17:769–74.
•• Lee SM, Schelcher C, Laubender RP, Frose N, Thasler RM, Schiergens TS, et al. An algorithm that predicts the viability and the yield of human hepatocytes isolated from remnant liver pieces obtained from liver resections. PLoS One. 2014;9:e107567. Isolated human primary hepatocytes are an essential in vitro model for basic and clinical research. For successful application as a model, isolated hepatocytes need to have a good viability and be available in sufficient yield. This study identifies donor characteristics, intra-operative factors, tissue processing, and cell isolation parameters that affect the viability and yield of human hepatocytes. By developing an accessible algorithm, projected viability can be determined even before isolation of hepatocytes, so that donors that result in high viability and yield can be identified.
Bartlett DC, Hodson J, Bhogal RH, Youster J, Newsome PN. Combined use of N-acetylcysteine and liberase improves the viability and metabolic function of human hepatocytes isolated from human liver. Cytotherapy. 2014;16:800–9.
Bhogal RH, Hodson J, Bartlett DC, Weston CJ, Curbishley SM, Haughton E, et al. Isolation of primary human hepatocytes from normal and diseased liver tissue: a one hundred liver experience. PLoS One. 2011;6:e18222.
Izamis ML, Calhoun C, Uygun BE, Guzzardi MA, Price G, Luitje M, et al. Simple machine perfusion significantly enhances hepatocyte yields of ischemic and fresh rat livers. Cell Med. 2013;4:109–23.
Izamis ML, Perk S, Calhoun C, Uygun K, Yarmush ML, Berthiaume F. Machine perfusion enhances hepatocyte isolation yields from ischemic livers. Cryobiology. 2015;71:244–55.
Bruinsma BG, Sridharan GV, Weeder PD, Avruch JH, Saeidi N, Ozer S, et al. Metabolic profiling during ex vivo machine perfusion of the human liver. Sci Rep. 2016;6:22415.
Nativ NI, Yarmush G, So A, Barminko J, Maguire TJ, Schloss R, et al. Elevated sensitivity of macrosteatotic hepatocytes to hypoxia/reoxygenation stress is reversed by a novel defatting protocol. Liver Transpl. 2014;20:1000–11.
Yarmush G, Santos L, Yarmush J, Koundinyan S, Saleem M, Nativ NI, Schloss RS, et al. Metabolic flux distribution during defatting of steatotic human hepatoma (HepG2) cells. Metabolites 2016;6(1):1.
Duret C, Moreno D, Balasiddaiah A, Roux S, Briolotti P, Raulet E, et al. Cold preservation of human adult hepatocytes for liver cell therapy. Cell Transplant. 2015;24:2541–55.
Puts CF, Berendsen TA, Bruinsma BG, Ozer S, Luitje M, Usta OB, et al. Polyethylene glycol protects primary hepatocytes during supercooling preservation. Cryobiology. 2015;71:125–9.
Jorns C, Gramignoli R, Saliem M, Zemack H, Mork LM, Isaksson B, et al. Strategies for short-term storage of hepatocytes for repeated clinical infusions. Cell Transplant. 2014;23:1009–18.
Bonora-Centelles A, Donato MT, Lahoz A, Pareja E, Mir J, Castell JV, et al. Functional characterization of hepatocytes for cell transplantation: customized cell preparation for each receptor. Cell Transplant. 2010;19:21–8.
Gramignoli R, Tahan V, Dorko K, Venkataramanan R, Fox IJ, Ellis EC, et al. Rapid and sensitive assessment of human hepatocyte functions. Cell Transplant. 2014;23:1545–56.
Weber A, Groyer-Picard MT, Franco D, Dagher I. Hepatocyte transplantation in animal models. Liver Transpl. 2009;15:7–14.
Sigal SH, Rajvanshi P, Gorla GR, Sokhi RP, Saxena R, Gebhard DR Jr, et al. Partial hepatectomy-induced polyploidy attenuates hepatocyte replication and activates cell aging events. Am J Phys. 1999;276:G1260–72.
Abdalla EK. Portal vein embolization (prior to major hepatectomy) effects on regeneration, resectability, and outcome. J Surg Oncol. 2010;102:960–7.
Furrer K, Tian Y, Pfammatter T, Jochum W, El-Badry AM, Graf R, et al. Selective portal vein embolization and ligation trigger different regenerative responses in the rat liver. Hepatology. 2008;47:1615–23.
Guha C, Parashar B, Deb NJ, Sharma A, Gorla GR, Alfieri A, et al. Liver irradiation: a potential preparative regimen for hepatocyte transplantation. Int J Radiat Oncol Biol Phys. 2001;49:451–7.
Guha C, Sharma A, Gupta S, Alfieri A, Gorla GR, Gagandeep S, et al. Amelioration of radiation-induced liver damage in partially hepatectomized rats by hepatocyte transplantation. Cancer Res. 1999;59:5871–4.
Koenig S, Yuan Q, Krause P, Christiansen H, Rave-Fraenk M, Kafert-Kasting S, et al. Regional transient portal ischemia and irradiation as preparative regimen for hepatocyte transplantation. Cell Transplant. 2011;20:303–11.
Yamanouchi K, Zhou H, Roy-Chowdhury N, Macaluso F, Liu L, Yamamoto T, et al. Hepatic irradiation augments engraftment of donor cells following hepatocyte transplantation. Hepatology. 2009;49:258–67.
Dagher I, Boudechiche L, Branger J, Coulomb-Lhermine A, Parouchev A, Sentilhes L, et al. Efficient hepatocyte engraftment in a nonhuman primate model after partial portal vein embolization. Transplantation. 2006;82:1067–73.
Pourcher G, El-Kehdy H, Kanso F, Groyer-Picard MT, Gaillard M, Trassard O, et al. Volumetric portal embolization: a new concept to improve liver regeneration and hepatocyte engraftment. Transplantation. 2016;100:344–54.
Kabarriti R ZW, Yaffe H, Liu L, Asp P, Tome WA, et al. Delaying transplantation by 24 hours after preparative hepatic irradiation enhances engraftment and proliferation of transplanted hepatocytes in mouse liver. In. 90:S174–S175. ed. Int J Radiat Oncol; 2014.
Nygaard S, Barzel A, Haft A, Major A, Finegold M, Kay MA, et al. A universal system to select gene-modified hepatocytes in vivo. Sci Transl Med. 2016;8:342ra379.
Gustafson E, Asif S, Kozarcanin H, Elgue G, Meurling S, Ekdahl KN, Nilsson B. Control of IBMIR induced by fresh and cryopreserved hepatocytes by low molecular weight dextran sulfate versus heparin. Cell Transplant. 2017;26(1):71–81.
Lee CA, Dhawan A, Smith RA, Mitry RR, Fitzpatrick E. Instant blood-mediated inflammatory reaction in hepatocyte transplantation: current status and future perspectives. Cell Transplant. 2016;25:1227–36.
Han B, Lu Y, Meng B, Qu B. Cellular loss after allogenic hepatocyte transplantation. Transplantation. 2009;87:1–5.
Hayashi C, Ito M, Ito R, Murakumo A, Yamamoto N, Hiramatsu N, et al. Effects of edaravone, a radical scavenger, on hepatocyte transplantation. J Hepatobiliary Pancreat Sci. 2014;21:919–24.
Asif S, Ekdahl KN, Fromell K, Gustafson E, Barbu A, Le Blanc K, et al. Heparinization of cell surfaces with short peptide-conjugated PEG-lipid regulates thromboinflammation in transplantation of human MSCs and hepatocytes. Acta Biomater. 2016;35:194–205.
Meier RP, Montanari E, Morel P, Pimenta J, Schuurman HJ, Wandrey C, et al. Microencapsulation of hepatocytes and mesenchymal stem cells for therapeutic applications. Methods Mol Biol. 2017;1506:259–71.
Mitry RR, Jitraruch S, Iansante V, Dhawan A. Alginate encapsulation of human hepatocytes and assessment of microbeads. Methods Mol Biol. 2017;1506:273–81.
Sgroi A, Mai G, Morel P, Baertschiger RM, Gonelle-Gispert C, Serre-Beinier V, et al. Transplantation of encapsulated hepatocytes during acute liver failure improves survival without stimulating native liver regeneration. Cell Transplant. 2011;20:1791–803.
Teramura Y, Oommen OP, Olerud J, Hilborn J, Nilsson B. Microencapsulation of cells, including islets, within stable ultra-thin membranes of maleimide-conjugated PEG-lipid with multifunctional crosslinkers. Biomaterials. 2013;34:2683–93.
Jitraruch S, Dhawan A, Hughes RD, Filippi C, Lehec SC, Glover L, Mitry RR. Cryopreservation of hepatocyte microbeads for clinical transplantation. Cell Transplant. 2017;26(8):1341–1354.
Jitraruch S, Dhawan A, Hughes RD, Filippi C, Soong D, Philippeos C, et al. Alginate microencapsulated hepatocytes optimised for transplantation in acute liver failure. PLoS One. 2014;9:e113609.
Ohashi K, Yokoyama T, Yamato M, Kuge H, Kanehiro H, Tsutsumi M, et al. Engineering functional two- and three-dimensional liver systems in vivo using hepatic tissue sheets. Nat Med. 2007;13:880–5.
Uygun BE, Soto-Gutierrez A, Yagi H, Izamis ML, Guzzardi MA, Shulman C, et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010;16:814–20.
Yokoyama T, Ohashi K, Kuge H, Kanehiro H, Iwata H, Yamato M, et al. In vivo engineering of metabolically active hepatic tissues in a neovascularized subcutaneous cavity. Am J Transplant. 2006;6:50–9.
Zhang S, Zhang B, Chen X, Chen L, Wang Z, Wang Y. Three-dimensional culture in a microgravity bioreactor improves the engraftment efficiency of hepatic tissue constructs in mice. J Mater Sci Mater Med. 2014;25:2699–709.
Fox IJ, Chowdhury JR. Hepatocyte transplantation. Am J Transplant. 2004;4(Suppl 6):7–13.
Ambrosino G, Varotto S, Strom SC, Guariso G, Franchin E, Miotto D, et al. Isolated hepatocyte transplantation for Crigler-Najjar syndrome type 1. Cell Transplant. 2005;14:151–7.
Oldhafer F, Bock M, Falk CS, Vondran FW. Immunological aspects of liver cell transplantation. World J Transplant. 2016;6:42–53.
Castellaneta A, Thomson AW, Nayyar N, de Vera M, Mazariegos GV. Monitoring the operationally tolerant liver allograft recipient. Curr Opin Organ Transplant. 2010;15:28–34.
Ashokkumar C, Bentlejewski C, Sun Q, Higgs BW, Snyder S, Mazariegos GV, et al. Allospecific CD154+ B cells associate with intestine allograft rejection in children. Transplantation. 2010;90:1226–31.
Ashokkumar C, Shapiro R, Tan H, Ningappa M, Elinoff B, Fedorek S, et al. Allospecific CD154+ T-cytotoxic memory cells identify recipients experiencing acute cellular rejection after renal transplantation. Transplantation. 2011;92:433–8.
Ashokkumar C, Talukdar A, Sun Q, Higgs BW, Janosky J, Wilson P, et al. Allospecific CD154+ T cells associate with rejection risk after pediatric liver transplantation. Am J Transplant. 2009;9:179–91.
Hogen R, DiNorcia J, Dhanireddy K. Antibody-mediated rejection: what is the clinical relevance? Curr Opin Organ Transplant. 2017;22:97–104.
Zhang M, Wang H, Tan S, Navarro-Alvarez N, Zheng Y, Yang YG. Donor CD47 controls T cell alloresponses and is required for tolerance induction following hepatocyte allotransplantation. Sci Rep. 2016;6:26839.
Ide K, Wang H, Tahara H, Liu J, Wang X, Asahara T, et al. Role for CD47-SIRPalpha signaling in xenograft rejection by macrophages. Proc Natl Acad Sci U S A. 2007;104:5062–6.
Navarro-Alvarez N, Yang YG. Lack of CD47 on donor hepatocytes promotes innate immune cell activation and graft loss: a potential barrier to hepatocyte xenotransplantation. Cell Transplant. 2014;23:345–54.
Wang H, Madariaga ML, Wang S, Van Rooijen N, Oldenborg PA, Yang YG. Lack of CD47 on nonhematopoietic cells induces split macrophage tolerance to CD47null cells. Proc Natl Acad Sci U S A. 2007;104:13744–9.
Wang H, Wu X, Wang Y, Oldenborg PA, Yang YG. CD47 is required for suppression of allograft rejection by donor-specific transfusion. J Immunol. 2010;184:3401–7.
Heyn C, Ronald JA, Mackenzie LT, MacDonald IC, Chambers AF, Rutt BK, et al. In vivo magnetic resonance imaging of single cells in mouse brain with optical validation. Magn Reson Med. 2006;55:23–9.
Shapiro EM, Sharer K, Skrtic S, Koretsky AP. In vivo detection of single cells by MRI. Magn Reson Med. 2006;55:242–9.
Slotkin JR, Cahill KS, Tharin SA, Shapiro EM. Cellular magnetic resonance imaging: nanometer and micrometer size particles for noninvasive cell localization. Neurotherapeutics. 2007;4:428–33.
YL W, Ye Q, Foley LM, Hitchens TK, Sato K, Williams JB, et al. In situ labeling of immune cells with iron oxide particles: an approach to detect organ rejection by cellular MRI. Proc Natl Acad Sci U S A. 2006;103:1852–7.
Roach DR, Garrett WM, Welch G, Caperna TJ, Talbot NC, Shapiro EM. Magnetic cell labeling of primary and stem cell-derived pig hepatocytes for MRI-based cell tracking of hepatocyte transplantation. PLoS One. 2015;10:e0123282.
Rodriguez-Porcel M. In vivo imaging and monitoring of transplanted stem cells: clinical applications. Curr Cardiol Rep. 2010;12:51–8.
von der Haar K, Lavrentieva A, Stahl F, Scheper T, Blume C. Lost signature: progress and failures in in vivo tracking of implanted stem cells. Appl Microbiol Biotechnol. 2015;99:9907–22.
Wang P, Petrella F, Nicosia L, Bellomi M, Rizzo S. Molecular imaging of stem cell transplantation for liver diseases: monitoring, clinical translation, and theranostics. Stem Cells Int. 2016;2016:4058656.
Hickey RD, Mao SA, Amiot B, Suksanpaisan L, Miller A, Nace R, et al. Noninvasive 3-dimensional imaging of liver regeneration in a mouse model of hereditary tyrosinemia type 1 using the sodium iodide symporter gene. Liver Transpl. 2015;21:442–53.
Rezvani M, Grimm AA, Willenbring H. Assessing the therapeutic potential of lab-made hepatocytes. Hepatology. 2016;64:287–94.
Baxter M, Withey S, Harrison S, Segeritz CP, Zhang F, Atkinson-Dell R, et al. Phenotypic and functional analyses show stem cell-derived hepatocyte-like cells better mimic fetal rather than adult hepatocytes. J Hepatol. 2015;62:581–9.
Cameron K, Tan R, Schmidt-Heck W, Campos G, Lyall MJ, Wang Y, et al. Recombinant laminins drive the differentiation and self-organization of hESC-derived hepatocytes. Stem Cell Rep. 2015;5:1250–62.
Duan Y, Catana A, Meng Y, Yamamoto N, He S, Gupta S, et al. Differentiation and enrichment of hepatocyte-like cells from human embryonic stem cells in vitro and in vivo. Stem Cells. 2007;25:3058–68.
Hay DC, Fletcher J, Payne C, Terrace JD, Gallagher RC, Snoeys J, et al. Highly efficient differentiation of hESCs to functional hepatic endoderm requires ActivinA and Wnt3a signaling. Proc Natl Acad Sci U S A. 2008;105:12301–6.
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72.
Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–20.
Tolosa L, Pareja E, Gomez-Lechon MJ. Clinical application of pluripotent stem cells: an alternative cell-based therapy for treating liver diseases? Transplantation. 2016;100:2548–57.
Bissig-Choisat B, Wang L, Legras X, Saha PK, Chen L, Bell P, et al. Development and rescue of human familial hypercholesterolaemia in a xenograft mouse model. Nat Commun. 2015;6:7339.
Imagawa K, Takayama K, Isoyama S, Tanikawa K, Shinkai M, Harada K, et al. Generation of a bile salt export pump deficiency model using patient-specific induced pluripotent stem cell-derived hepatocyte-like cells. Sci Rep. 2017;7:41806.
Strom SC, Gramignoli R. Human amnion epithelial cells expressing HLA-G as novel cell-based treatment for liver disease. Hum Immunol. 2016;77:734–9.
Akle C, McColl I, Dean M, Adinolfi M, Brown S, Fensom AH, et al. Transplantation of amniotic epithelial membranes in patients with mucopolysaccharidoses. Exp Clin Immunogenet. 1985;2:43–8.
Bembi B, Comelli M, Scaggiante B, Pineschi A, Rapelli S, Gornati R, et al. Treatment of sphingomyelinase deficiency by repeated implantations of amniotic epithelial cells. Am J Med Genet. 1992;44:527–33.
Scaggiante B, Pineschi A, Sustersich M, Andolina M, Agosti E, Romeo D. Successful therapy of Niemann-Pick disease by implantation of human amniotic membrane. Transplantation. 1987;44:59–61.
Miki T. A rational strategy for the use of amniotic epithelial stem cell therapy for liver diseases. Stem Cells Transl Med. 2016;5:405–9.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Financial Disclosure
This work was supported by grants from NIH, RO1 AI122369 to I.J.F and DK099257 to A.S.-G., and the Pittsburgh Liver Research Center Seed Grant to J.E.S. and A.S.-G.
Additional information
This article is part of the Topical Collection on Cellular Transplants
Rights and permissions
About this article
Cite this article
Squires, J.E., Soltys, K.A., McKiernan, P. et al. Clinical Hepatocyte Transplantation: What Is Next?. Curr Transpl Rep 4, 280–289 (2017). https://doi.org/10.1007/s40472-017-0165-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40472-017-0165-6