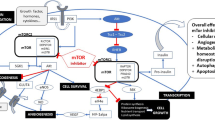
Abstract
The use of mTOR inhibitors evokes debate in transplantation. Numerous clinical trials have explored the role of the mTOR inhibitors sirolimus and everolimus in kidney transplantation, but the results have been mixed; they have not shown a clear benefit over the current regimens. However, many of these trials were impacted by the difficulties and uncertainties in managing side effects and the dosing of the mTOR inhibitors. Being aware of the effective treatments available for these side effects, understanding the pathophysiology of the mTOR pathway allows patients to remain on therapy. Successful side effect management means that the longer term, advantageous benefits of mTOR inhibition can be gained by the kidney recipient and means that it is time to use de novo mTOR inhibitors in kidney recipients.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Calne RY, White DJ, Thiru S, et al. Cyclosporin A in patients receiving renal allografts from cadaver donors. Lancet. 1978;2:1323–7.
Nankivel BJ, Burrows RJ, Fung CL, et al. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349:2326.
Shihab F, Christians U, Smith L, et al. Focus on mTOR inhibitors and tacrolimus in renal transplantation: pharmacokinetics, exposure-response relationships, and clinical outcomes. Transpl Immunol. 2014;31:22–32.
Thomson AW, Woo J. Immunosuppressive properties of FK-506 and rapamycin. Lancet. 1989;19:443–4.
Vezina C, Kudelski A, Sehgal SN. Rapamycin (AY-22,989) a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J Antibiot. 1975;28:721–6.
Morris RE, Meiser BM. Identification of a new pharmacologic action for an old compound. Med Sci Res. 1989;I17:609–10.
Meiser BM, Wang J, Morris RE. Rapamycin: a new and highly active immunosuppressive macrolide with an efficacy superior to cyclosporine. In: Progress in Immunology. 1989. p. 1195–8.
Calne RY, Collier DS, Lim S, et al. Rapamycin for immunosuppression in organ allografting. Lancet. 1989;2:227.
Gummert JF, Ikonen T, Morris RE. New immunosuppressive drugs: a review. J Am Soc Nephrol. 1999;10:1366–80.
Whiting PH, Woo J, Adam BJ, et al. Toxicity of rapamycin – a comparative and combination study with cyclosporine at immunotherapeutic dosages in the rat. Transplantation. 1991;52:203–8.
Dumont FJ, Su Q. Mechanism of action of the immunosuppressant rapamycin. Life Sci. 1996;58:373–95.
Corthay A. A three-cell model for activation of naïve T helper cells. Scan J Immuol. 2006;64:93–6.
Mahalati K, Kahan BD, et al. Clinical pharmacokinetics of sirolimus. Clin Pharmacokinet. 2001;40:573–85.
Benjamin D, Columbi M, Moroni C, et al. Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat Rev Drug Discov. 2011;10:868–80.
Kahan BD. Efficacy of sirolimus compared with azathioprine for reduction of acute renal allograft rejection: a randomized multicenter study. The Rapamune US Study Group. Lancet. 2000;356:194–202.
Macdonald AS, Rapamune Global Study Group. A worldwide, phase III, randomized, controlled safety and efficacy study of a sirolimus/cyclosporine regimen for prevention of acute rejection in recipients of primary mismatched renal allografts. Transplantation. 2001;71:271–80.
Johnson RW, Kreis H, Oberbauer R, et al. Sirolimus allows early cyclosporine withdrawal in renal transplantation resulting in improved renal function and lower blood pressure. Transplantation. 2001;72:777–86.
Oberbauer R, Kreis H, Johnson RW, et al. Long-term improvement in renal function with sirolimus after early cyclosporine withdrawal in renal transplant recipients: 2-year results of the Rapamune Maintenance Regimen Study. Transplantation. 2003;76:364–70.
Oberbauer R, Segoloni G, Campistol JM, et al. Early cyclosporine withdrawal from a sirolimus-based regimen results in better renal allograft survival and renal function at 48 months after transplantation. Transpl Int. 2005;18:22–8.
Larson TS, Dean PG, Stegall MD, et al. Complete avoidance of calcineurin inhibitors in renal transplantation: a randomized trial comparing sirolimus and tacrolimus. Am J Transplant. 2006;6:514–22.
Flechner SM, Glyda M, Cockfield S, et al. The ORION study: comparison of two sirolimus-based regimens versus tacrolimus and mycophenolate mofetil in renal allograft recipients. Am J Transplant. 2011;11:1633–44.
Kahan B, Wong RL, Carter C, et al. A phase I study of a 4-week course of SDZ-RAD (RAD) quiescent cyclosporine-prednisone-treated renal transplant recipients. Transplantation. 1999;68:1100–6.
Vitko S, Margreiter R, Weimar W, et al. Three-year efficacy and safety results from a study of everolimus versus mycophenolate mofetil in de novo renal transplant patients. Am J Transplant. 2005;5:2521–30.
Lorber MI, Mugaonkar S, Butt KM, et al. Everolimus versus mycophenolate mofetil in the prevention of rejection in de novo renal transplant recipients: a 3-year randomized, multicenter, phase III study. Transplantation. 2005;80:244–52.
Vitko S, Margreiter R, Weimar W, et al. Everolimus (Certican) 12-month safety and efficacy versus mycophenolate mofetil in de novo renal transplant recipients. Transplantation. 2004;1532–40.
Vitko S, Tedesco H, Eris J, et al. Everolimus with optimized cyclosporine dosing in renal transplant recipients: 6-month safety and efficacy results of two randomized studies. Am J Transplant. 2004;4:626–35.
Cibrik D, Silva HT, Vathsala A, et al. Randomized trial of everolimus-facilitated calcineurin inhibitor minimization over 24 months in renal transplantation. Transplantation. 2013;95:933–42.
Su L, Tam N, Deng R, et al. Everolimus-based calcineurin-inhibitor sparing regimens for kidney transplant recipients: a systematic review and meta-analysis. Int Urol Nephrol. 2014;46:2035–44. This large meta-analysis of seven studies and 2,067 kidney recipients demonstrates improved renal function associated with reduced CMV infection, as well as increased rejection and adverse events.
Akselband Y, Harding MW, Nelson PA. Rapamycin inhibits spontaneous and fibroblast growth factor beta-stimulated proliferation of endothelial cells and fibroblasts. Transplant Proc. 1991;23:2833–6.
Ventura-Aguiar P, Campistol JM, Diekman F. Safety of mTOR inhibitors in adult solid organ transplantation. Exp Op Drug Safety. 2016;15:303–19. This review describes both the early side effects of mTOR inhibitors such as lymphoceles formation and wound healing issues, as well as the longer term issues such as infections and proteinuria, offering management solutions.
Shegogue D, Trohanowksa M. Mammalian target of rapamycin positively regulates collagen type 1 production via a phosphatidylinositol 3-kinase-independent pathway. J Bio Chem. 2004;279:23166–75.
Sehgal SN. Rapamune (RAPA, rapamycin, sirolimus): mechanism of action of immunosuppressive effects results from blockade of signal transduction and inhibition of cell cycle progression. Clin Biochem. 1998;31:335–40.
Bongelo RGB, Fuhro R, Wang Z, et al. Rapamycin ameliorates proteinuria-associated tubulointerstitial inflammation and fibrosis in experimental membranous nephropathy. J Am Soc Nephrol. 2005;16:2063–72.
Knight RJ, Villa M, Laskey R, et al. Risk factors for impaired wound healing in sirolimus-treated renal transplant recipients”. Clin Transplant. 2007;21:460–5.
McKenna GJ, Klintmalm GB.: The role of mTOR Inhibitors in Solid Organ Transplantation. In: Molecules to Medicine with mTOR, 1st Edition. Academic Press 2016, 293-311
Nashan B, Critterio F. Wound healing complications and the use of mammalian target of rapamycin inhibitors in kidney transplantation: a critical review of the literature. Transplantation. 2012;94:547–61.
Pengel LH, Liu LG, Morris PJ. Do wound complications or lymphoceles occur more often in solid organ transplant recipients on mTOR inhibitors ? A systematic review of randomized controlled trials. Transpl Int. 2011;24:1216–30.
Cooper M, Wiseman AC, Zibari G, et al. Wound events in kidney transplant patients receiving de novo everolimus: a pooled analysis of three randomized controlled trials. Clin Transplant. 2013;27:E625–35. Data from three prospective, randomized controlled trials were pooled to examine the dose-dependent impact of everolimus on wound healing, and lymphoceles in comparison to controls.
Dantal J, Berthoux F, Moal MC, et al. Efficacy and safety of de novo or early everolimus with low cyclosporine in deceased-donor kidney transplant recipients at specified risk of delayed graft function: 12-month results of a randomized multicenter trial. Tranpl Int. 2010;23:1084–93.
Albano L, Berthoux F, Moal MC, et al. Incidence of delayed graft function and wound healing complications after deceased-donor kidney transplantation is not affection by de novo everolimus. Transplantation. 2009;88:69–76.
Tiong HY, Flechner SM, Zhou L, et al. A systematic approach to minimizing wound problems for de novo sirolimus-treated kidney transplant recipients. Transplantation. 2009;87:296–302.
Campistol JM, Cockwell P, Diekmann F, et al. Practical recommendations for the early use of m-TOR inhibitors (sirolimus) in renal transplantation. Transpl Int. 2009;22:681–7.
Kaplan B, Qazi Y, Wellen JR, et al. Strategies for the management of adverse events associated with mTOR inhibitors. Transplant Rev. 2014;28:126–33. This review gives an comprehensive overview of the side effects of mTOR inhibitor therapies, and offers useful tips for managing wound-healing, metabolic, and renal side effects.
Campistol JM, de Fijter JW, Flechner SM, et al. mTOR inhibitor-associated dermatologic and mucosal problems. Clin Transplant. 2010;24:149–56.
McKenna GJ, Trotter JF. Sirolimus conversion for renal dysfunction in liver transplant recipients: the devil really is in the details. Am J Transplant. 2012;12:521–2.
Halimi JM, Laouad I, Buchler M, et al. Early low-grade proteinuria: causes, short-term evoluation and long-term consequences in renal transplantation. Am J Transplant. 2005;5:2281–8.
Fernandez-Fresnedo G, Escallada R, Rodrigo E, et al. The risk of cardiovascular disease associated with proteinuria in renal transplant patients. Transplantation. 2002;73:1345–8.
Stallone G, Infance B, Grandaliano G, et al. Management of side effects of sirolimus therapy. Transplantation. 2009;87(Supp 8):S23–6.
Diekmann F, Guttierrez-Dalmau A, Lopez S, et al. Influence of sirolimus on proteinuria in de novo kidney transplantation with expanded criteria donors: comparison of two CNI-free protocols. Nephrol Dial Transplant. 2007;22:2316–21.
Muller-Krebs S, Weber L, Tsobaneli J, et al. Cellular effects of everolimus and sirolimus on podocytes. PLoS One. 2013;8, e80340.
Letavernier E, Bruneval P, Vandermeersch S, et al. Sirolimus interacts with pathways essential for podocyte integrity. Nephrol Dial Transplant. 2009;24:630–8.
Abbata M, Zoja C, Remuzzi G. How does proteinuria cause progressive renal damage ? J Am Soc Neprhol. 2006;17:2974–84.
Wiseman AC, McCague K, Kim Y, et al. The effect of everolimus versus mycophenolate upon proteinuria following kidney transplant and relationship to graft outcome. Am J Transplant. 2013;13:442–9. This large study highlights the dose-dependent risk of proteinuria with everolimus use in kidney transplant recipients, and correlates it to GFR and graft survival.
Blanco S, Vaquero M, Gomez-Guerrero C, et al. Potential role of angiotensin-converting enzyme inhibitors and statins on early podocyte damage in a model of type 2 diabetes mellitus, obesity, and mild hypertension. Am J Hypertens. 2005;18:557–65.
Zoja C, Corna D, Gagliardini E, et al. Adding a statin to a combination of ACE inhibitor and ARB normalizes proteinuria in experiemental diabetes, which translates into full renoprotection. Am J Physiol Renal Physiol. 2010;299:F1203–11.
Webster AC, Lee VW, Chapman JR, et al. Target of rapamycin inhibitors (TOR-I; sirolimus and everolimus) for primary immunosuppression in kidney transplant recipients. Cochrane Database Syst Rev. 2006;2:CD004290.
Schrem H, Barg-Hock H, Strassbourg CP, et al. Aftercare for patients with transplant organs. Dtsch Arztebl Int. 2009;106:148–56.
Krynitz B, Edgren G, Lindelof B, et al. Risk of skin cancer and other malignancies in kidney, liver, heart and lung transplant recipients 1970 to 2008 – a Swedish population-based study. Int J Cancer. 2013;132:1429–38.
Alter M, Satzger I, Schrem H, et al. Non-melanoma skin cancer is reduced after switch of immunosuppression to mTOR inhibitors in organ transplant recipients. J Dtsch Dermatol Ges. 2014;12:480–8.
Salgo R, Gossman J, Schofer H, et al. Switch to a sirolimus-based immunosuppression in long-term renal transplant recipients: reduced rate of (pre-) malignancies and nonmelanoma skin cancer in a prospective randomized, assessor-blinded controlled clinical trial. Am J Transplant. 2010;10:1385–93.
Campbell SB, Walker R, Tai SS, et al. Randomized controlled trial of sirolimus for renal transplant recipients at high risk for nonmelanoma skin cancer. Am J Transplant. 2012;12:1146–56.
Hoogendijk-van den Akker JM, Harden PN, Holtsma AJ, et al. Two-year randomized controlled prospective trial converting treatment of stable renal transplant recipients with cutaneous invasive squamous cell carcinomas to sirolimus. J Clin Oncol. 2013;31:1317–27.
Euvard S, Morelon E, Rostaing L, et al. Sirolimus and secondary skin-cancer prevention in kidney transplantation. N Engl J Med. 2012;369:329–39. This study examined the impact of conversion to sirolimus immunosuppression on the development of cutaneous squamous cell carcinoma, in a multicenter randomized controlled trial.
Alberu J, Pascoe MD, Campistol JM, et al. Lower malignancy rates in renal allograft recipients converted to siorlimus-based calcineurin inhibitor-free immunotherapy: 24 month results from the CONVERT trial. Transplantation. 2011;92:302–10.
Ramos E, Drachenberg CB, Papadimitriou JC, et al. Clinical course of polyoma virus nephropathy in 67 renal transplant patients. J Am Soc Nephrol. 2002;13:2145–51.
Jacobi J, Prignitz A, Buttner M, et al. BK viremia and polyomavirus nephropathy in 352 kidney transplants; risk factors and potential role of mTOR inhibition. BMC Nephrol. 2013;14:207. This study demonstrated that a switch of immunosuppression to an mTOR inhibitor in patients with BK viremia was associated with rapid BK viral clearance and stable graft function.
Liacini A, Seamone ME, Muruve DA, et al. Anti-BK virus mechanism of sirolimus and leflunomide alone and in combination: toward a new therapy for BK virus inactivation. Transplantation. 2010;90:1450–7.
Egli A, Kohli S, Dickenmann M, et al. Inhibition of polyomavirus BK-specific T-cell responses by immunosuppressive drugs. Transplantation. 2009;88:1161–8.
Hirsch HH, Yakhontova K, Lu M, et al. BK polyomavirus replication in renal tubular epithelial cells is inhibited by sirolimus, but activated by tacrolimus through a pathway involving FKBP-12. Am J Transplant. 2016;16:821–32. This study outlines that sirolimus exerts an opposite effect to tacrolimus on BK virus by inhibiting BK viral replication. This occurs via a mechanism that is dependent on the cellular protein FKBP-12.
Polanco N, Gonzalez ME, Folgueira MD, et al. Everolimus-based immunosuppression therapy for BK nephropathy. Transplant Proc. 2015;47:57–61.
Tohme FA, Kalil RS, Thomas CP. Conversion to a sirolimus-based regimen is associated with lower incidence of BK viremia in low-risk kidney transplant recipients. Transpl Infect Dis. 2015;17:66–72.
Pontrelli P, Rossini M, Infante B, et al. Rapamycin inhibits PAI-1 expression and reduces interstitial fibrosis and glomerulosclerosis in chronic allograft nephropathy. Transplantation. 2008;85:125–34.
Alpay N, Ozkok A, Caliskan Y, et al. Influence of conversion calcineurin inhibitors to everolimus on fibrosis, inflammation, tubular damage and vascular function in renal transplant patients. Clin Exp Nephrol. 2014;18:961–7. Serum pro-inflammatory and pro-fibrogenic cytokines were measured in stable renal transplant recipients following conversion to everolimus, showing a reduction in these cytokines along with improved renal function.
Ruiz JC, Campistol JM, Grinyo JM, et al. Early cyclosporine A withdrawal in kidney transplant recipients receiving sirolimus prevents progression of chronic pathologic allograft lesions. Transplantation. 2004;78:1312–8.
Stallone G, Di Paolo S, Schena A, et al. Early withdrawal of cyclosporine improves 1 year kidney graft structure and function in sirolimus treated patients. Transplantation. 2003;75:998–1003.
Flechner SM, Kurian SM, Solez K, et al. De novo kidney transplantation without use of calcineurin inhibitors preserves renal structure and function at two years. Am J Transpl. 2004;4:1776–85.
Kasiska BL, Guijaro C, Massy ZA, et al. Cardiovascular disease after renal transplantation. J Am Soc Nephrol. 1996;7:158–65.
Morales JM. Cardiovascular risk profile in patients treated with sirolimus after renal transplantation. Kidney Int Suppl. 2005;93:S69–73.
Blum CB. Effects of Sirolimus on lipids in renal allograft recipients: an analysis using the Framingham risk model. Am J Transplant. 2002;2:551–9.
McKenna GJ, Trotter JF, Klintmalm E, et al. Sirolimus and cardiovascular disease risk in liver transplantation. Transplantation. 2013;95:215–21. This study demonstrates the impact of long term sirolimus use on reducing cardiovascular disease risk in transplant recipients.
Castro C, Campistol JM, Sancho D, et al. Rapamycin attenuates atherosclerosis induced by dietary cholesterol in apolipoprotein-deficient mice through a p27Kip1-independent pathway. Atherosclerosis. 2004;172:31–8.
Chen WQ, Zhong L, Zhang K, et al. Oral rapamycin attenuates inflammation and enhances stability of atherosclerotic plaques in rabbits independents of serum lipid levels. Br J Pharmacol. 2009;156:941–51.
Zhao L, Ding T, Cyrus T, et al. Low-dose oral sirolimus reduces atherogenesis, vascular inflammation and modulates plaque composition in mice lacking the LDL receptor. Br J Pharmacol. 2009;156:774–85.
Ma KL, Varghese Z, Ku U, et al. Sirolimus inhibits endogenous cholesterol synthesis induced by inflammatory stress in human vascular smooth muscles cells. Am J Physiol Heart Circ Physiol. 2010;298:H16146–H1651.
Ma KL, Ruan XZ, Powis SH, et al. Anti-atherosclerotic effects of sirolimus on human vascular smooth muscle cells. Am J Physiol Hear Circ Physiol. 2007;292:H2721–8.
Elloso MM, Azrolan N, Sehgal SN, et al. Protective effect of the immunosuppressant sirolimus against aortic atherosclerosis in apo E-deficient mice. Am J Transplant. 2003;3:562–9.
Poon M, Marx SO, Gallo R, et al. Rapamycin inhibits vascular smooth muscle cell migration. J Clin Invest. 2005;98:2277–83.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Greg J. McKenna declares that he has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Kidney Transplantation
Rights and permissions
About this article
Cite this article
McKenna, G.J. Is It Time to Use De Novo mTOR Inhibitors Posttransplant?. Curr Transpl Rep 3, 244–253 (2016). https://doi.org/10.1007/s40472-016-0111-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40472-016-0111-z