Abstract
Introduction
The TRANSFORM study demonstrated that an immunosuppression based on a combination of calcineurin inhibitors and de-novo mTOR inhibitors (mTORi) is safe and effective in kidney transplant recipients. However, data that validate this approach in clinical practice are currently missing.
Materials and methods
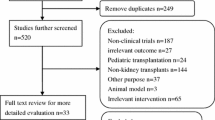
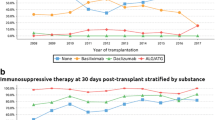
Analysis of 401 kidney transplant recipients transplanted from June 2013 to December 2016. All patients received tacrolimus with prednisone in combination with either mycophenolate (n = 186) or mTORi (either everolimus or sirolimus, n = 215). A propensity score to receive mTORi was calculated based on the inverse probability of treatment weighting (IPTW) from the following parameters: age and sex of donor and recipient, BMI, previous transplants, diabetes, cPRA, dialysis before transplantation, dialysis vintage, type of donor, ABO-incompatibility, HLA-mismatches, induction and ischemia time. Median follow-up was 2.6 [1.9; 3.7] years.
Results
Cox-regression analysis suggests good results for mTORi versus MPA in terms of 1-year biopsy-proven acute rejection (BPAR, P = 0.063), 1-year graft loss (P = 0.025) and patient survival (P < 0.001). Results observed for BPAR and graft failure were largely attributed to those patients that would have been excluded by the TRANSFORM because of some exclusion criteria (52.9% of the population, P = 0.003 for 1-year BPAR and P = 0.040 for graft loss). In patients who met selection criteria for TRANSFORM, no effect of treatment for BPAR or graft failure was observed, while the beneficial effect on overall survival persisted.
Conclusions
In a real-life setting, a protocol based on de-novo mTORi with tacrolimus and prednisone could be employed as a standard immunosuppressive regimen and was associated with good outcomes.



Similar content being viewed by others
References
Yanik EL, Siddiqui K, Engels EA (2015) Sirolimus effects on cancer incidence after kidney transplantation: a meta-analysis. Cancer Med 4(9):1448–1459
Alberú J, Pascoe MD, Campistol JM et al (2011) Lower malignancy rates in renal allograft recipients converted to sirolimus-based, calcineurin inhibitor-free immunotherapy: 24-month results from the CONVERT Trial. Transplantation 92(3):303–310
Tedesco- Silva H, Felipe C, Ferreira A et al (2015) Reduced incidence of cytomegalovirus infection in kidney transplant recipients receiving everolimus and reduced tacrolimus doses. Am J Transpl 15(10):2655–2664
Cervera C, Cofan F, Hernandez C et al (2016) Effect of mammalian target of rapamycin inhibitors on cytomegalovirus infection in kidney transplant recipients receiving polyclonal antilymphocyte globulins: a propensity score-matching analysis. Transpl Int 29(11):1216–1225
Ekberg H, Bernasconi C, Tedesco-Silva H et al (2009) Calcineurin inhibitor minimization in the symphony study: observational results 3 years after transplantation. Am J Transpl 9(8):1876–1885
Flechner SM, Glyda M, Cockfield S et al (2011) The ORION Study: comparison of two sirolimus-based regimens versus tacrolimus and mycophenolate mofetil in renal allograft recipients. Am J Transpl 11(8):1633–1644
Sharif A, Shabir S, Chand S, Cockwell P, Ball S, Borrows R (2011) Meta-analysis of calcineurin-inhibitor-sparing regimens in kidney transplantation. J Am Soc Nephrol 22(11):2107–2118
Pascual J, Diekmann F, Fernández-Rivera C et al (2017) Recomendaciones para el uso de everolimus en trasplante renal de novo: falsas creencias, mitos y realidades. Nefrología 37(3):253–266
Ventura-Aguiar P, Campistol JM, Diekmann F (2016) Safety of mTOR inhibitors in adult solid organ transplantation. Expert Opin Drug Saf 15(3):303–319
Cibrik D, Silva HT, Vathsala A et al (2013) Randomized trial of everolimus-facilitated calcineurin inhibitor minimization over 24 months in renal transplantation. Transplantation 95(7):933–942
Mühlbacher F, Neumayer H-H, del Castillo D et al (2014) The efficacy and safety of cyclosporine reduction in de novo renal allograft patients receiving sirolimus and corticosteroids: results from an open-label comparative study. Transpl Int 27(2):176–186
Pascual J, Berger SP, Witzke O et al (2018) Everolimus with reduced calcineurin inhibitor exposure in renal transplantation. J Am Soc Nephrol 29:1979–1991
Rosenbaum PR, Rubin DB (1983) The central role of the propensity score in observational studies for causal effects. Biometrika 70:41–55
D’Agostino RB Jr (1998) Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 17:2265–2281
Austin PC (2009) Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput 38(6):1228–1234
Säemann MD, Haidinger M, Hecking M, Hörl WH, Weichhart T (2009) The multifunctional role of mTOR in Innate immunity: implications for transplant immunity. Am J Transpl 9(12):2655–2661
Weichhart T, Costantino G, Poglitsch M (2008) The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity 29(4):565–577
Bechstein WO, Paczek L, Wramner L et al (2013) European Rapamune Tacrolimus Study Group. A comparative, randomized trial of concentration-controlled sirolimus combined with reduced-dose tacrolimus or standard-dose tacrolimus in renal allograft recipients. Transp Proc 45(6):2133–2140
Ma MKM, Yung S, Chan TM (2018) mTOR inhibition and kidney diseases. Transplantation 102(2S Suppl 1):S32–S40
Xie X, Jiang Y, Lai X, Xiang S, Shou Z, Chen J (2015) mTOR inhibitor versus mycophenolic acid as the primary immunosuppression regime combined with calcineurin inhibitor for kidney transplant recipients: a meta-analysis. BMC Nephrol 16(1):91
Gatault P, Bertrand D, Büchler M et al (2016) Eight-year results of the Spiesser study, a randomized trial comparing de novo sirolimus and cyclosporine in renal transplantation. Transpl Int 29(1):41–50
Budde K, Lehner F, Sommerer C et al (2012) conversion from cyclosporine to everolimus at 4.5 months post transplant: 3-year results from the randomized ZEUS Study. Am J Transpl 12(6):1528–1540
de Fijter JW, Holdaas H, Øyen O et al (2017) Early conversion from calcineurin inhibitor- to everolimus-based therapy following kidney transplantation: results of the randomized ELEVATE trial. Am J Transpl 17(7):1853–1867
Lebranchu Y, Thierry A, Toupance O et al (2009) Efficacy on renal function of early conversion from cyclosporine to sirolimus 3 months after renal transplantation: concept study. Am J Transpl 9(5):1115–1123
Thierry A, Thervet E, Vuiblet V (2011) Long-term impact of subclinical inflammation diagnosed by protocol biopsy one year after renal transplantation. Am J Transpl 11(10):2153–2161
Holdaas H, Rostaing L, Serón D (2011) Conversion of long-term kidney transplant recipients from calcineurin inhibitor therapy to everolimus: a randomized, multicenter, 24-month study. Transplantation 92(4):410–418
Schena FP, Pascoe MD, Alberu J et al (2009) Conversion from calcineurin inhibitors to sirolimus maintenance therapy in renal allograft recipients: 24-month efficacy and safety results from the CONVERT Trial. Transplantation 87(2):233–242
Torres F, Ríos J, Saez-Peñataro J, Pontes C (2017) Is propensity score analysis a valid surrogate of randomization for the avoidance of allocation bias? Semin Liver Dis 37(3):275–286
Gouëffic Y, Potter-Perigo S, Chan CK, Johnson PY, Braun K, Evanko SP, Wight TN (2007) Sirolimus blocks the accumulation of hyaluronan (HA) by arterial smooth muscle cells and reduces monocyte adhesion to the ECM. Atherosclerosis 195(1):23–30
Paoletti E, Citterio F, Corsini A, Potena L, Rigotti P, Sandrini S, Bussalino E, Stallone G, ENTROPIA Project (2019) Everolimus in kidney transplant recipients at high cardiovascular risk: a narrative review. J Nephrol. https://doi.org/10.1007/s40620-019-00609-y(Epub ahead of print)
Andrassy J, Hoffmann VS, Rentsch M (2012) Is cytomegalovirus prophylaxis dispensable in patients receiving an mtor inhibitor-based immunosuppression? A systematic review and meta-analysis. Transpl J 94(12):1208–1217
Ferrer IR, Wagener ME, Robertson JM et al (2010) Cutting edge: rapamycin augments pathogen-specific but not graft-reactive CD8 + T cell responses. J Immunol 185(4):2004–2008
Hirsch HH, Vincenti F, Friman S et al (2013) Polyomavirus BK replication in de novo kidney transplant patients receiving tacrolimus or cyclosporine: a prospective, randomized, multicenter study. Am J Transpl 13(1):136–145
Suwelack B, Malyar V, Koch M, Sester M, Sommerer C (2012) The influence of immunosuppressive agents on BK virus risk following kidney transplantation, and implications for choice of regimen. Transpl Rev 26(3):201–211
Nashan B, Gaston R, Emery V et al (2012) Review of cytomegalovirus infection findings with mammalian target of rapamycin inhibitor-based immunosuppressive therapy in de novo renal transplant recipients. Transpl J 93(11):1075–1085
Vitko S, Margreiter R, Weimar W et al (2005) Three-year efficacy and safety results from a study of everolimus versus mycophenolate mofetil in de novo renal transplant patients. Am J Transpl 5(10):2521–2530
Murakami N, Riella LV, Funakoshi T (2014) Risk of metabolic complications in kidney transplantation after conversion to mTOR Inhibitor: a systematic review and meta-analysis. Am J Transpl 14(10):2317–2327
Knoll GA, Kokolo MB, Mallick R et al (2014) Effect of sirolimus on malignancy and survival after kidney transplantation: systematic review and meta-analysis of individual patient data. BMJ 349:g6679
Chapman JR, Rangan GK (2010) Why do patients develop proteinuria with sirolimus? Do we have the answer? Am J Kidney Dis 55(2):213–216
Diekmann F, Andrés A, Oppenheimer F (2012) mTOR inhibitor—associated proteinuria in kidney transplant recipients. Transpl Rev 26(1):27–29
Lui SL, Chan KW, Tsang R, Yung S, Lai KN, Chan TM (2006) Effect of rapamycin on renal ischemia-reperfusion injury in mice. Transpl Int 19(10):834–839
Cucchiari D, Molina-Andujar A, Montagud-Marrahi E et al (2019) Use of de-novo mTOR inhibitors in hypersensitzed kidney trasplant recipients: experience from clinical practice. Transplantation. https://doi.org/10.1097/tp.0000000000003021(Epub ahead of print)
Piotti G, Cremaschi E, Maggiore U (2017) Once-daily prolonged-release tacrolimus formulations for kidney transplantation: what the nephrologist needs to know. J Nephrol 30(1):53–61
Bestard O, Cravedi P (2017) Monitoring alloimmune response in kidney transplantation. J Nephrol 30(2):187–200
Funding
This study has been partially funded by Redes Tematicas De Investigacion Cooperativa En Salud; REDINREN (RD16/0009/0023) co-funded by ISCIII-Subdirección General de Evaluación and Fondo Europeo de Desarrollo Regional (FEDER) “Una manera de hacer Europa”, and Secretaria d’Universitats i Recerca and CERCA Programme del Departament d’Economia i Coneixement de la Generalitat de Catalunya (2017-SGR-1331).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
DC has received speaker fees from Novartis and travel support from Astellas and Novartis. PVA has received travel support from Astellas, Novartis, Chiesi, Sandoz and speaker fees from Alexion and Mallinkrodt. GJP has received travel support from Sandoz. NE has received travel support from Novartis, Astellas and Chiesi. IR received speaker fees from Novartis and travel support from Sandoz, Pfizer y Astellas. FT has received DSMB fees from ImClone, Daiichi-Sankyo Pharma Development, ArQule and Rovi and speaker fees from Bayer. JMC has had consultancy agreements with Pfizer, Wyeth, Novartis and Roche; has received research funding from Pfizer and Novartis; and has been a scientific advisor or board member for Novartis and Pfizer and has received lecture fees from Alexion. JR has received DSMB fees from SOLTI and speaker fees in biostatistical education from SOLTI, Boehringer Ingelheim, Grunenthal, AMGEN and Novartis without relation of the present work. FD has received research grants and speaker fees from Astellas, Neovii, Novartis, Pfizer, Teva, Transplant Biomedicals. FO has received speaker fees from Novartis, Sanofi and Neovii and travel support from Novartis. AMJ, ENM, EDSA, FC, MS, JVT and MJR reported no conflict of interest. The results presented in this paper have not been published previously in whole or part, except in abstract format
Ethical statement
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cucchiari, D., Ríos, J., Molina-Andujar, A. et al. Combination of calcineurin and mTOR inhibitors in kidney transplantation: a propensity score analysis based on current clinical practice. J Nephrol 33, 601–610 (2020). https://doi.org/10.1007/s40620-019-00675-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-019-00675-2