Abstract
Drought stress is one of the most severe abiotic stresses affecting adversely plant growth, crop production, and various metabolic processes. Using seaweed extract in mitigating water stress adverse effects is highly important for plant production. The present study discussed the physiological role of seaweed extract (Sargassum denticulatum) in improving wheat tolerance to water stress.
Water stress (40% of field capacity) caused significant decreases in wheat plant growth parameters (shoot height, fresh, and dry weights of the shoot) as well as with significant decreases in chlorophyll content and starch. Total soluble sugars, free amino acids, proline, and phenolic compounds contents increased in stressed wheat plants irrigated every three weeks compared with control plants. The foliar application of seaweed extract 2% enhanced all growth and yield parameters and more accumulation of the organic solutes in leaves of water-stressed plants. These increases correlated with significant increases in total phenolic contents as compared with control plants. The trnL intron and psbA-trnH intergenic regions of cpDNA were amplified from extracted total genomic DNA. The results indicated that the variation among psbA-trnH intergenic region was more than trnL intron region to distinct the variation of wheat treatments as responsible to water deficit.
Foliar spray of seaweeds extract was effective in improving wheat performance by enhancing compatible osmolytes, antioxidant compounds and enhancing variation among non-coding chloroplast DNA (cpDNA) regions trnL intron and psbA-tnH as a response to water deficit.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
One of the most important crops in the world is wheat. So, it is necessary to increase wheat production to satisfy the needs of the rapid population growth (Fred et al. 2006). Nowadays, an increase in wheat crop production occupies the main objective of agriculture policy in the Egyptian government, by improving wheat varieties and improving agricultural practices. Marine algae are one of the most important marine resources in the world and are generally used as human food, animal feed and considering raw material for several industries. More than fifteen million metric tons of seaweed products is used annually as nutrient supplements and biostimulants in agricultural and horticultural crops production (FAO 2006). It is used as biofertilizers in agriculture and horticulture (Al-Shakankery et al. 2014). These products improve seeds germination, increase plant tolerance to environmental stresses, and enhance crop yield (Kumari et al. 2011). Beneficial effects from the use of seaweed extracts as natural regulators have induced increased crop yield and plant vigor to resist adverse environmental effects (Featonby-Smith and Van Staden 1983). Drought is considered a crucial factor that affects growth and crop production in arid and semiarid regions. Some plants have a set of physiological adaptations that allow tolerating water stress conditions. From these adaptations, drought induced an accumulation of total phenolic compound, total free amino acids, and a marked increase in the proline content (Abdelgawad et al 2015). Zhang and Erivn (2008) used seaweed extract to raise plant tolerance to environmental stresses. They reported that the application of seaweed extract improves seedling growth of wheat plants during growth period. Abd El-Baky et al. (2014) focused on water relations, photosynthesis, and the accumulation of specific metabolites. Despite reducing photosynthetic activity, accumulation of organic acids, osmolytes, as well as changes in carbohydrate metabolism are typical physiological and biochemical responses to stress. Synthesis of osmoprotectants, osmolytes, or compatible solutes is one of the mechanisms that plants have evolved for adaptation to water deficit, which acts as osmotic balancing agents, accumulated in plant cells in response to stress (Sadak et al. 2010). Chloroplasts are one of the most important organelles in green plants, seaweed, etc., which act as environmental sensors. Chloroplasts play an important role in many plants cell functions, including photosynthesis, carbon fixation, and stress response (Zdravka et al. 2017). Furthermore (Sun and Guo 2016) mentioned that the chloroplast represents a major organelle that can be very important when studying the role of desiccation stress. Besides the very important role of the chloroplast in cellular communication, retrograde signaling plays an important role in the adaptive responses of plants to stress. Non-coding chloroplast DNA (cpDNA) regions tend to evolve more rapidly than do coding regions, by the accumulation of insertions/deletions at a rate at least equal to that for nucleotide substitutions (Wolfe et al. 1987; Zurawski and Clegg 1987; Clegg and Zurawski 1992). The trnL-F intergenic spacer and psbA-trnH intergenic regions of cpDNA are non-coding characters, and this region is more variable than the coding regions. Some studies on the non-coding regions of cpDNA showed higher variations and more often mutation than that of coding regions (Taberlet et al. 1991; Jansen et al. 2008; Skuza et al. 2020). On the other hand, the trnH-psbA intergenic spacer region is one of the most variable regions of the plastid genome in terms of having the highest percentages of variable sites (Shaw et al. 2007) and can offer high levels of species discrimination (Kress et al. 2005; Shaw et al. 2007).
The hypothesis of this study is that Sargassum denticulatum extract may promote plant growth under drought stress and may affect chloroplast genome.
Therefore, the current investigation first intended to study the possible effects of local seaweed extracts with low doses on wheat growth under water stress. The second aim is to study seaweed's efficiency of chloroplast response to a severe and prolonged water deficit.
2 Materials and methods
This work was carried out through two experiments; the first experiment was a preliminary (laboratory) experiment that aimed to study the effect of different concentrations of Sargassum denticulatum extractions on the growth of wheat plants seedling and to choose the best concentration of seaweeds for the treatment of plants in the second experiment. The second was a greenhouse experiment to study the differential responses of wheat plants, Giza 168 cultivar (Triticum aestivum L.) for water stress and foliar treatment with seaweed extract (Sargassum denticulatum). The work was conducted in Faculty of Women for Arts, Science and Education, Botany Department Ain shams University, Cairo, Egypt, during two successive winter seasons 2017/2018 and 2018/2019. Wheat seeds, Giza 168 cultivar were obtained from the Agricultural Research Centre, Ministry of Agriculture, and Land Reclamation, Egypt. The marine seaweeds Sargassum denticulatum were collected from Ras Sedr (Lat. 29° 37′ N; Long 32° 41′ E).
2.1 Preparation of seaweed algal extracts – Samples of seaweeds were air-dried (26–30 °C) with indirect light for 10 days followed by oven-drying for 48 h at 60 °C. Dried seaweeds were crushed and powdered with a mixer grinder, and then, the powdered seaweed samples were stored in the airtight container for the future use. To 500 g of powdered seaweeds, 5000 ml of sterile distilled water was added, and contents were stood at room temperature for 60 min {ratio 1:10 (w/v)}. Then the extracts were filtered through a filter paper and stored at 4 °C for further experimental studies. The filtered was used as 10% (w/v) aqueous seaweed extract. Three different concentrations, i.e., 0% control, 1%, 2%, and 3% for S. denticulatum, was prepared using distilled water (Anisimov et al. 2013).
2.2 Algal analysis – Pigments (chlorophyll a, chlorophyll b, and carotenoids) were estimated by spectrophotometer according to Timothy et al. (2013). The elements, calcium, potassium, magnesium, sodium, manganese, copper, zinc, iron, phosphate, and nitrogen, were estimated by the Inductively Coupled Plasma-Emission Spectrometry (ICP-ES) with Ultra Sonic Nebulizer (USN) (Eaton and Franson 2005). Amino acids were detected according to George (2019). Total protein content was determined as Bradford (1976). Total carbohydrates content was estimated by phenol–sulfuric acid method (Dubois et al. 1956), and total soluble lipid content was determined by a method described by Van Handel (1985). Phenols contents were determined according to Singleton et al. (1999). Growth hormones, abscisic acid, cytokinin, gibberellic acid, salicylic acid, and brassinosteroids, were detected according to Arteca et al. (1980).
2.3 Preliminary experiment – A homogenous lot of seeds of the wheat plants were selected for uniformity of size, shape, and viability. Before germinating, the seeds were surface sterilized by soaking for 3 min in 2.5% sodium hypochlorite solution and washed several times with distilled water. The sterilized seeds were presoaked in distilled water (control) and different concentrations of algal extract (1, 2, and 3% for S. denticulatum) for 12 h. Subsequently, the seeds were allowed to drain for one hour. The seeds were transferred to sterile Petri dishes containing two sheets of Whitman number 1 filter paper moistened with water. Each Petri dish contained 20 seeds, and each treatment was replicated 3 times. The seeds were allowed to germinate at 25 °C in the darkness. The germination percentage was recorded after 7 days. At the end of the experimental period (14 days), seedlings, shoot, and root length as well as fresh and dry matter were recorded.
2.4 Greenhouse experiment – Wheat seeds that were previously sterilized were grown in pots (diameter 35 cm and depth 40 cm) containing 7 kg soil. Characteristics of the soil were as follows: texture, sandy loam; pH, 7.7; ECe, 0.23 dS m-1, and organic matter, 0.41%. Before planting calcium superphosphate (15.5% P2O5) and potassium sulfate (48% K2O) at the rate of 3.0 and 1.50 g/pot were added, respectively. The fertilization with ammonium sulfate (20.5% N) at the rate of 6.86 g/pot was added in two equal doses, and the first one was added after 2 weeks from sowing and the second 2 weeks later. Ten seeds per pot and five replicates were used for each treatment. Five plants after seedling were let to grow in each pot. Preliminary experiments showed that a 2% concentration of seaweed extract (S. denticulatum) exhibited the best performance for wheat plants seedling. Therefore, it was chosen to treat plants in the pot experiment. Treatment sets were as follows:
2.5 First set: control and irrigation intervals – Irrigation every week, soil moisture content depleted from 100 to 70% of field capacity (FC). Irrigation every two weeks, soil moisture content depleted from 100 to 55% of FC. Irrigation every three weeks, soil moisture content depleted from 100 to 40% of FC (soil FC was determined on dry weight basis of irrigated pots after keeping saturated soil for 24 h under free drainage).
2.6 Second set: seaweeds treatments – The second set was subjected to the same water regime as in the first set, and after two weeks from sowing, wheat seedlings were sprayed with seaweed extract 2% (S. denticulatum) and then sprayed again after 45 days from sowing. The first group was sprayed with distilled water only at the same times as the second group was sprayed with seaweed extract.
At 70 days after sowing, samples from the two sets were taken to study growth parameters in terms of morphological parameters (plant height, fresh, and dry weight), photosynthetic pigments (chlorophyll a, chlorophyll b, and carotenoids) of leaves, carbohydrate (total soluble sugars and starch), and some osmoprotectants as proline, total free amino acid, and phenolic compounds contents.
2.7 Yield determination – At maturity, plant samples were collected randomly to record yield and its components such as plant height, spike length, number of spikelet’s/spikes, the weight of grain/plant, and 1000 seeds weight.
2.8 Phytochemical analysis – Photosynthetic pigments content, total chlorophyll a and b, and carotenoid contents in fresh leaves were determined using the method of Lichtenthaler and Buschmann (2001). Total soluble sugars (TSS) were extracted and determined by Yemm and Willis (1956). Starch was measured as reducing sugars by Nelson’s method (Nelson 1944) and expressed in maltose equivalents. Proline content was assayed according to the method described by Bates et al. (1973). Free amino acid content (FAA) was extracted according to the method described by Vartanain et al. (1992) and determined by spectrocolorimeter according to Yemm et al. (1955). Phenolic content was assayed by Diaz and Martin (1972).
2.9 DNA extraction and amplification – Total genomic DNA was extracted from fresh leaves using the DNeasy plant mini kit (Qiagen). The universal primer pairs for trnL-F (C + D Primers) and trnH-psbA regions were amplified as recommended by Taberlet et al. (1991) and Hamilton (1999), (Table 1 and Fig. 3). PCRs were carried out in 25 µl reaction volumes and included 2 µl of genomic DNA, 3 µl of a 10 × reaction buffer, 3 µl of 2 mM MgCl2, 3 µl of 1 mM dNTP solution, 3 pmol of each primer, and 0.25 U RedTaqTM polymerase (Sigma-Aldrich, St. Louis, USA). The final volume was adjusted with deionized distilled water. The cycling parameters included an initial denaturation step at 96 °C for 5 min followed by 30 cycles of 96 °C for 30 s, 50 °C for 30 s, and 72 °C for 40 s. A final extension step at 72 °C for 5 min completed the reactions. Negative controls were kept for all the primers which included all the components of the reaction mixture except template DNA, and it was replaced with nuclease-free water. Amplified products were observed on 1.5% agarose gel (1XTBE) with ethidium bromide staining.
2.10 Statistical analysis – The data were statistically analyzed using a one-way analysis of variance (ANOVA 1) as described by Snedecor and Cockran (1969). The means were compared by LSD using SPSS (version 16).
3 Results
3.1 Algal analysis – The photosynthetic pigments like chlorophyll ‘a,’ chlorophyll ‘b,’ and carotenoids content were estimated in S. denticulatum presented in Table 2.
The macro- and micronutrients as mg/g of the brown marine alga S. denticulatum revealed the presence of nitrogen (168.3 mg/g), phosphorus (26.12 mg/g), potassium (73.54 mg/g), magnesium (60.67 mg/g), calcium (78.93 mg/g), copper (0.012 mg/g), manganese (0.441 mg/g), zinc (0.737 mg/g), and iron (6.91 mg/g). Among the elements estimated, nitrogen (168.3 mg/g) as macronutrients and iron (6.91 mg/g) as micronutrients were found to be abundant in the alga. Similarly, in the case of phytohormones analysis (µg/g), cytokinin (84.51) was found to be more when compared to abscisic acid (57.93), gibberellic acid (38.25), salicylic acid (6.35), and brassinosteroids (2.19). The percentage of phenolic compound recorded in S. denticulatum was higher (15.2) than protein, carbohydrate, and lipid (11.87, 7.65, and 3.19), respectively. The chemical properties of the extract for seaweed S. denticulatum have been analyzed, and their values are given in Table 3.
Amino acids analysis of S. denticulatum (Table 4) led to the detection of 17 amino acids with reasonably resolved separations. The seaweeds contained all the essential amino acids at different proportions. The total amino acid content was (3.76 µg/g) and the highest essential amino acid was glutamic (0.56 µg/g), and the most limiting amino acid was histidine followed by cysteine.
3.2 Preliminary experiment – The effect of different concentrations of S. denticulatum extractions on germination percentage and growth parameter of wheat seedling is shown in Table 5. The highest rate of germination (97.2%) was found on application of the S. denticulatum liquid fertilizer at 2% concentration, followed by 93.3 and 92.5%, the result obtained on using 1 and 3%, respectively, as compared with the control seeds (92.3%). The highest shoot length (21 cm) and root length (19.7 cm) were observed at 2% concentration of alga. The maximum total fresh weight (0.40 g/plant) and total dry weight (0.022 g/plant) were recorded on seeds treated with 2% concentration of alga liquid fertilizer as compared to the control values (0.32 g/plant) for total fresh weight and total dry weight (0.012 g/plant). In our experiments, the use of 2% seaweed liquid extracts of S. denticulatum significantly promoted the rate of growth on seedling of wheat. Concentrations of (1 and 3%) were found to show lower effect on all the parameters studied.
4 Pot experiment
4.1 Changes in yield parameters – Data illustrated in Table 6 show that plant length, spike length, spike weight, number of spikelets/spike number of seeds/spike, seeds yield/plant (cm), weight of seeds/spike (g), and weight of 1000 seeds (g) of plant attained the highest values for control plants and were declined progressively by increasing water intervals. A significant increase was detected in all plants treated with 2% S. denticulatum extractions compared with control. Seaweeds sprayed plants gave significantly higher yield and yield components than non-sprayed plants.
5 Phytochemical analysis
5.1 Changes in pigments, TSS, and starch contents – Changes in total soluble sugars and starch contents of wheat leaves treated with seaweeds and grown under normal and water deficit are presented in Table 7. The data show a decrease in pigments content (Chl. a, Chl. B, and carotenoids) in wheat leaves under water stress. However, 2% of S. denticulatum extract increased the pigment content when compared to control plants under the same conditions. Data clearly show that wheat plant sprayed with 2% S. denticulatum extractions significantly increased the starch contents of leaves as compared to plants under the same water regime and without spraying with the extract.
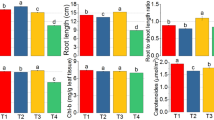
5.2 Changes in proline, FAA, and phenolic contents – Data presented in Figs. 1 and 2 showed the effect of foliar application by 2% of S. denticulatum extracts on proline, free amino acids, and phenolic contents in wheat plants. The exposure of wheat plants to water deficit led to significant increases in proline, free amino acids, and phenolic content of wheat leaves. In the meantime, foliar with 2% S. denticulatum extraction led to marked decreased in FAA and proline content compared to plants grown under the same conditions. Seaweeds foliar treatment caused significant increases in phenolic content as compared with untreated plants.
5.3 Amplification of cpDNA coding for trnL intron and psbA-trnH intergenic region – The PCR amplification of the trnL intron was successfully obtained for all foliar treatments with seaweeds extract with or without water deficit and control plants irrigated every 3 weeks. Meanwhile, amplification was not successful for the control plants irrigated every 1 and 2 weeks. The length of the amplified trnL gene was 530 bp (Fig. 3).
Agarose gel showing amplified trnL intron (C-D) primer and trnH-psb intergenic region for wheat plants irrigated every 1. one week, 2. two weeks, 3. three weeks, 4. foliar plants with seaweeds extract irrigated every one week, 5. foliar plants with seaweeds extract irrigated every two weeks, 6. foliar plants with seaweeds extract irrigated every three weeks, Lane M: 100 bp. Ladder
PCR amplification of psbA-trnH intergenic region was successfully obtained for all treatments of wheat plants except for control plants treated with a long-term dehydration period of three weeks. The samples had a PCR product of about 321 bp. Thus, it can be assumed that the chloroplast psbA-trnH intergenic region gene missing from the chloroplast genome may be involved with water-deficit responses, necessitating further investigation.
6 Discussion
The goal of this study was to see how effective Sargassum denticulatum is at increasing wheat drought tolerance. According to Zodape (2001) and Tarakhovskaya et al. (2007) seaweed products contain growth regulators (auxins, cytokinin, and gibberellins), amino acids, and mineral elements that positively promote plant development and division. In response to the treatment with varied concentrations of the seaweed extracts under research, statistically significant changes in shoot length, root length, and seedling, fresh and dry weights were detected. Seed soaking in 2% Sargassum denticulatum showed a favorable response in growth characteristics in this regard. The inclusion of several growth-promoting chemicals could explain the higher seedling growth. Seedling growth was higher with Sargassum denticulatum liquid fertilizer. The existence of nutritional contents in seaweeds may also contribute to their ability to promote growth (Kalaivanan and Venkatesalu 2012). Pre-soaking wheat seeds in various doses of algal extract improved all seedling growth criteria. The use of 2% alga resulted in the greatest increases in all growth criteria. Drought, the most critical environmental stress, stifles plant growth and development, reduces plant yield, and impairs agricultural plant performance more than any other factor. Due to the fall in turgor pressure, growth is one of the most drought-sensitive processes (Kusaka et al. 2005). Water deficit in soybean plants, according to Anjum et al. (2011), lowered plant height, lowest node height, leaf area, stem diameter, number of nodes, and branches by decreasing the soil's water potential.
The effect of 2% extract of S. denticulatum gave increase in germination rate and shoot length of Triticum aestivum L. as compared to control. The increase in germination percentage might be due to the presence of growth promoting substances such as gibberellins, cytokinins, micronutrients (Fe, Cu, CO, Zn, Mn, Mo, and Ni), vitamins, and amino acids. Our results coincided with those of earlier studies on Cajanus cajan (Mohan et al. 1994), Vigna sinensis (Sivasankari et al. 2006), and Zea mays (Al-Shakankery et al. 2014). The beneficial effects of Sargassum extract application could be due to improved root proliferation and establishment, allowing plants to mine more nutrients in a balanced proportion from further away and deeper soil layers.
Photosynthesis is required for plant growth and reproduction, and chlorophyll is the green pigment responsible for light absorption and photosynthesis (Nelson and Cox 2004; Marschner 2011). According to Mourey-Bringuier (1986), algal extracts improve pigment concentration by means of phytohormones included in these extracts, mainly cytokinin, which protects chlorophyll from degradation under water stress and increases nitrogen assimilation. Seaweed liquid fertilizer boosted total chlorophyll and carotenoids content in Cyamopsis tetragonoloba at a lower dosage (20%) with or without chemical fertilizer (Arumugam et al. 2009), possibly due to the presence of plant growth agents in the seaweed extract used (Mostafa and Zheekh 1999). Our results are also consistent with those reported by Salma et al. (2014), which show that plant tolerance to abiotic stress and particularly water stress is significantly improved by applying algal extracts. Similar observations were reported for the effect of extracts of Chaetomorpha antennina and Rosenvingea intricate on the chlorophyll contents of Abelmoschus esculentus and Raphanus sativus plants (Thirumaran et al. 2006 and 2007). The increases in pigments may be due to the presence of high amount of magnesium in the liquid fertilizers of S. denticulatum. Marine algae contain cytokinins, gibberellins, auxins, auxin-like, and other growth promoting compounds (Yokoya et al. 2010).
Plants' acquisition of drought resistance is intimately linked to the buildup of soluble carbohydrates (Hoekstra et al. 2001). The increase in sugar concentration could be due to starch degradation (Fischer and Höll 1991). Ketabchi and Shahrtash (2011) indicated that soluble sugar concentrations increased at the same time as starch quantities declined. In this study, foliar treatments with algal extract increased starch accumulation in all water intervals, which could be related to seaweed extract's promoter effect on chlorophyll levels.
Proline is important for osmotic adjustment, stability, and protection of enzymes, proteins, and membranes from drought-induced osmotic stress (Ashraf and Foolad 2007). With drought stress, foliar applications with seaweed extract lowered proline and free amino acid levels in wheat plants. Our findings are consistent with those of previous soybean research (Rathore et al. 2009). In Vigna sinensis L., similar findings were reported (Sivasankari et al. 2006).
Many plants have shown an increase in phenol content in various tissues when subjected to osmotic stress (Mansori et al. 2019). These increases could be related to total phenols' role as a key regulator of plant metabolic processes and, as a result, overall plant development (Abdallah et al. 2015). Furthermore, phenols serve as a substrate for numerous antioxidant enzymes, reducing the effects of drought stress (Bakry et al. 2012). Another mechanism behind phenolic compounds' antioxidant benefits is their ability to reduce membrane mobility (Sadak 2016 b). Furthermore, phenolic compounds have an antioxidant effect as free radical scavengers due to their reactivity as electron or hydrogen donors, which helps to stabilize and delocalize unpaired electrons, as well as their role as transition metal ions chelators (Huang et al. 2005).
The increase in phenolic content in treated plants can explain the favorable effect of seaweed extract on wheat drought resistance. Abiotic and biotic stressors, on the other hand, can cause phenolic buildup in plants (Yamasaki et al. 1995). Active oxygen species are produced during drought stress and can cause oxidative damage (Ortega-Villasante et al. 2016). Enzymatic and non-enzymatic antioxidative systems, such as phenolic compounds, which have perfect structural chemistry for free radical scavenging action, mitigate the harmful effects of reactive oxygen species (Ahmed et al. 2010). The polyphenol-derived radical is able to stabilize and delocalize the unpaired electron, as well as the high reactivity of phenolic compounds as hydrogen or electron donors (chain-breaking function). The osmotic adjustment in plants under drought stress is caused by the accumulation of compatible osmolytes at high concentrations.
The non-coding characters trnL-F intergenic spacer and psbA-trnH intergenic area of cpDNA are more changeable than the coding sections. Non-coding portions of cpDNA have been found to have more variants and mutations than coding sections in several studies (Skuza et al. 2020). According to our PCR experiments, the trnL intron was shown to be present in long-term drought-stressed plants for three weeks, as well as all foliar treatments with seaweed extract with or without drought stress and lacking in control plants irrigated for one and two weeks.
The precise loss of an intron has been reported from other chloroplast genes (e.g., Downie and Palmer 1992; Wallace and Cota 1996; Jansen et al. 2008). Such a process has been already reported for the loss of both group I and group II introns in plants, fungi, and animals (e.g., Campagna and Downie 1998; Hu 2006; Jeffares et al. 2006). Other mechanisms of intron loss could be simple genomic deletion and in-frame intron deletion (Niu et al. 2005). The chloroplast psbA-trnH intergenic region gene is losing from the chloroplast genome may be as a response to severe drought stress (3 weeks). These findings suggest that sequencing analysis should be used in more research. The failure of psbA-trnH intergenic region amplification in seedling plants could be due to nucleotide variations as single-nucleotide substitutions that prevent PCR amplification (Bru et al. 2008). This indicates that the foliar treatments with seaweed extract may have reduced the harmful effect of severe drought stress. The results showed that the investigated regions of the chloroplast genome are variable in wheat plant treatments with or without seaweed extract under drought conditions. Thus, it can be assumed the chloroplast psbA-trnH intergenic region gene missing from the chloroplast genome as a response to severe drought stress (Sun and Guo 2016). The psbA gene encodes the D1 reaction center protein of photosystem II. The D1 protein is the primary target of the damage, and it is sacrificed in order to avoid complete inactivation and disassembly of PSII (Nixon et al. 2010).
The role of chloroplast psbA UTRs in the regulation of gene expression has been investigated intensively for more than twenty years (Zurawski et al. 1982); preservation of its structure influences psbA longevity, processing, and maturation.
Finally, we may conclude that foliar spraying seaweed extract (S. denticulatum) with antioxidant components (phenolics), suitable osmolytes, and some osmoprotectant molecules such TSS, proline, and free amino acids improved wheat performance. As a response to water scarcity, seaweed extract increases diversity in the non-coding chloroplast DNA (cpDNA) regions trnL intron and psbA-trnH. Marine algae are one of the forms of biological fertilization that does not contaminate the environment, increases crop yield, and aids in water conservation, which is now the most important aspect of Egyptian agriculture.
References
Abd El-Baky HH, Hussein MM, El-Baroty GS (2014) Induces of antioxidant compounds and salt tolerance in wheat plant, irrigated with seawater as response to application of microalgae spray. Am J Agric Biol Sci. https://doi.org/10.3844/ajabssp.2014.127.137
Abdallah MM, El-Bassiouny HM, Bakry BA, Sadak MS (2015) Effect of Arbuscular mycorrhiza and glutamic acid on growth, yield, some chemical composition and nutritional quality of wheat plant grown in newly reclaimed sandy soil. Res J Pharm Biol Chem Sci 6:1038–1054
Abdelgawad ZA, Thoria RM, Afiah SA, Al-Agwany HH (2015) Drought and salt stress on growth, osmolytes protein and isozymes in Vicia faba L. Genotypes Egypt J Agron 37:93–119
Ahmad P, Jaleel CA, Salem MA, Nabi G, Sharma S (2010) Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit Rev Biotechnol 30:161–75. https://doi.org/10.3109/07388550903524243
Al-Shakankery FM, Hamouda RA, Ammar MM (2014) The promotive effect of different concentrations of marine algae as biofertilizers on growth and yield of maize (Zea mays L.) plants. J Chem Biol Phys Sci 4:43201–43211
Anisimov MM, Chaikina EL, Klykov AG, Rasskazov VA (2013) Effect of seaweeds extracts on the growth of seedling roots of buckwheat (Fagopyrum esculentum Moench) is depended on the season of algae collection. Agric Sci Dev 2:67–75
Anjum SA, Wang LC, Farooq M, Hussain M, Xue LL, Zou C (2011) Brassinolide application improves the drought tolerance in maize through modulation of enzymatic antioxidants and leaf gas exchange: brassinolide improves drought tolerance in maize. J Agron Crop Sc 197:177–185. https://doi.org/10.1111/j.1439-037X.2010.00459.x
Arteca RN, Poovaiah BW, Smith OE (1980) Use of high performance liquid chromatography for the determination of endogenous hormone levels in Solanum tuberosum L. subjected to carbon dioxide enrichment of the root zone. Plant Physiol 65:1216–1219. https://doi.org/10.1104/pp.65.6.1216
Arumugam R, Anantharaman P (2009) Effect of seaweed liquid fertilizer on growth and pigment concentration of Abelmoschus esculentus (l) medikus. J Agron 2:57–66
Ashraf MF, Foolad MR (2007) Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot 59:206–216. https://doi.org/10.1016/j.envexpbot.2005.12.006
Bakry BA, El-Hariri DM, Sadak MS, El-Bassiouny HM (2012) Drought stress mitigation by foliar application of salicylic acid in two linseed varieties grown under newly reclaimed sandy soil. J Appl Sci Res 8:3503–3514
Bates LS, Waldan RP, Teare LD (1973) Rapid determination of free proline under water stress studies. Plant Soil 39:205–207. https://doi.org/10.1007/BF00018060
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Bru D, Martin-Laurent F, Philippot L (2008) Quantification of the detrimental effect of a single primer-template mismatch by real-time PCR using the 16S rRNA gene as an example. Appl Environ Microbiol 74:1660–1663. https://doi.org/10.1128/AEM.02403-07
Campagna ML, Downie SR (1998) The intron in chloroplast gene rpl16 is missing from the flowering plant families Geraniaceae, Goodeniaceae, and Plumbaginaceae. Trans Illinois State Acad Sci 91:1–11
Clegg MT, Zurawski G (1992) Chloroplast DNA and the study of plant phylogeny: present status and future prospects. In: Soltis PS, Soltis DE, Doyle JJ (eds) Molecular systematics of Plants. Chapman and Hall, New York, pp 1–13
Diaz D H, Martin G C (1972) Peach seed dormancy in relation to endogenous inhibitors and applied growth substances. Am Soc Hort Sci J
Downie SR, Palmer JD (1992) Use of chloroplast DNA rearrangements in reconstructing plant phylogeny. In: Soltis PS, Soltis DE, Doyle JJ (eds) Molecular systematics of plants. Springer, Boston, pp 14–35. https://doi.org/10.1007/978-1-4615-3276-7_2
Dubois M, Gilles KA, Hamilton JK, Rebers PT, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356. https://doi.org/10.1021/ac60111a017
Eaton AD, Franson MA (2005) American public health association (APHA) standard methods for the examination of water & wastewater. American Public Health Association, Washington
FAO (2006). Yearbook of fishery statistics, vol 98(1–2). Food and Agricultural Organisation of the United Nations, Rome
Featonby-Smith BC, Van Staden J (1983) The effect of seaweed concentrate and fertilizer on the growth of Beta vulgaris. Z Pflanzenphysiol 112:155–162. https://doi.org/10.1016/s0044-328x(83)80030-0
Fischer C, Höll W (1991) Food reserves of scots pine (Pinus sylvestris L.): I. Seasonal changes in the carbohydrate and fat reserves of pine needles. Trees 5:187–195. https://doi.org/10.1007/BF00227524
Fred G, Kurt S, Sherif I (2006) Egypt grain and feed annual. USAA Foreign Agric Service Gain Rep N2:6007
George WL (2019) Official method of analysis of AOAC international. Volume 1, chapter 4, p. 9–13. 21st Edition
Hamilton MB (1999) Four primer pairs for the amplification of chloroplast intergenic regions with intraspecific variation. Mol Ecol 8:521–523
Hoekstra FA, Golovina E, Buitink J (2001) Mechanisms of plant desiccation tolerance. Trends Plant Sci 6:431–438. https://doi.org/10.1016/s1360-1385(01)02052-0
Hu K (2006) Intron exclusion and the mystery of intron loss. FEBS Lett 580:6361–6365. https://doi.org/10.1016/j.febslet.2006.10.048
Huang C, He W, Guo J, Chang X, Su P, Zhang L (2005) Increased sensitivity to salt stress in an ascorbate-deficient Arabidopsis mutant. J Exp Bot 56:3041–3049. https://doi.org/10.1093/jxb/eri301
Jansen RK, Wojciechowski MF, Sanniyasi E, Lee SB, Daniell H (2008) Complete plastid genome sequence of the chickpea (Cicer arietinum) and the phylogenetic distribution of rps12 and clpP intron losses among legumes (Leguminosae). Mol Phylogenet Evol 48:1204–1217. https://doi.org/10.1016/j.ympev.2008.06.013
Jeffares DC, Mourier T, Penny D (2006) The biology of intron gain and loss. Trends Genet 22:16–22. https://doi.org/10.1016/j.tig.2005.10.006
Kalaivanan C, Venkatesalu V (2012) Utilization of seaweed Sargassum myriocystum extracts as a stimulant of seedlings of Vigna mungo (L.) Hepper. Span J Agric Res 2:466–470. https://doi.org/10.5424/sjar/2012102-507-10
Ketabchi S, Shahrtash M (2011) Effects of methyl jasmonate and cytokinin on biochemical responses of maize seedlings infected by Fusarium moniliforme. Asian J Exp Biol Sci 2:299–305
Kress WJ, Wurdack KJ, Zimmer EA, Weight LA, Janzen DH (2005) Use of DNA barcodes to identify flowering plants. Proc Natl Acad Sci USA 102:8369–8374
Kumari R, Kaur I, Bhatnagar AK (2011) Effect of aqueous extract of Sargassum johnstonii Setchell & Gardner on growth, yield and quality of Lycopersicon esculentum Mill. J Appl Phycol 23:623–633. https://doi.org/10.1007/s10811-011-9651-x
Kusaka M, Lalusin AG, Fujimura T (2005) The maintenance of growth and turgor in pearl millet (Pennisetum glaucum [L.] Leeke) cultivars with different root structures and osmo-regulation under drought stress. Plant Sci 168:1–14. https://doi.org/10.1016/j.plantsci.2004.06.021
Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids: measurement and characterization by UV-Vis spectroscopy. Curr Protocol Food Anal Chem 1:F4–F3. https://doi.org/10.1002/0471142913.faf0403s01
Mansori M, Farouk IA, Hsissou D, El Kaoua M (2019) Seaweed extract treatment enhances vegetative growth and antioxidant parameters in water stressed Salvia officinalis L. Mater Environ Sci 10:756–766
Marschner H (2011) Marschner’s mineral nutrition of higher plants. Academic press
Mohan VR, Venkataraman Kumar V, Murugeswari R, Muthuswami S (1994) Effect of crude and commercial seaweed extracts on seed germination and seedling growth in Cajanus cajan L. Phykos 33:47–51
Mostafa ME, Zheekh L (1999) Effect of seaweed extracts on seed germination, seedling growth and some metabolic process of Vicia faba L. Cytobios 100:23–25
Mourey Bringuier A N (1986) Recherches sur les modalites d'emploi et les modes d'action des micropulverisats d'ascophyllum nodosum chez glycine max placee en situation de stress (Doctoral dissertation, Rennes 1).
Nelson N (1944) A photometric adaptation of the Somogyi method for the determination of glucose. J Biol Chem 153:375–380. https://doi.org/10.1016/s0021-9258(18)71980-7
Nelson D L, Cox M M (2004) Principles of bioenergetics. Lehninger, Principles of Biochemistry (4th ed., pp. 489–520). New York, USA: WH Freeman and company.
Niu DK, Hou WR, Li SW (2005) mRNA-mediated intron losses: evidence from extraordinarily large exons. Mol Biol Evol 22:1475–1481. https://doi.org/10.1093/molbev/msi138
Nixon PJF, Michoux J, Yu M, Boehm JK (2010) Recent advances in understanding the assembly and repair of photosystem II. Ann Bot 106:1–16
Ortega-Villasante C, Burén S, Barón-Sola Á, Martínez F, Hernández LE (2016) In vivo ROS and redox potential fluorescent detection in plants: Present approaches and future perspectives. Methods 109:92–104. https://doi.org/10.1016/j.ymeth.2016.07.009
Rathore SS, Chaudhary DR, Boricha GN, Ghosh A, Bhatt BP, Zodape ST, Patolia JS (2009) Effect of seaweed extract on the growth, yield and nutrient uptake of soybean (Glycine max) under rainfed conditions. South Afr J Bot. https://doi.org/10.1016/j.sajb.2008.10.009
Sadak MS (2016) Mitigation of drought stress on fenugreek plant by foliar application of trehalose. Int J Chem Technol Res 9:147–155
Sadak MS, Abdelhamid MT, El-Saady AK (2010) Physiological responses of faba bean plant to ascorbic acid grown under salinity stress. Egypt J Agron 32:89–106
Salma L, Aymen EM, Maher S, Hassen A, Chérif H, Halima C, Mimoun E (2014) Effect of seaweed extract of Sargassum vulgare on germination behavior of two bean cultivars (Phaseolus vulgaris L) under salt stress. IOSR J Agric Vet Sci 7:116–120. https://doi.org/10.9790/2380-0721116120
Shaw J, Lickey EB, Schilling EE, Small RL (2007) Comparison of wholechloroplast genome sequences to choose noncoding regions for phylogenetic studies in angiosperms. Tortoise Hare III Am J Bot 94:275–288
Singleton VL, Orthofer R, Lamuela-Raventós RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol 299:152–178. https://doi.org/10.1016/s0076-6879(99)99017-1
Sivasankari S, Venkatesalu V, Anantharaj M, Chandrasekaran M (2006) Effect of seaweed extracts on the growth and biochemical constituents of Vigna sinensis. Bioresour Technol 97:1745–1751. https://doi.org/10.1016/j.biortech.2005.06.016
Skuza L, Filip E, Szućko I, Bocianowski J (2020) SPInDel Analysis of the Non-Coding Regions of cpDNA as a More Useful Tool for the Identification of Rye (Poaceae: Secale) Species. Int J Mol Sci 21:9421
Bello I, Snedecor GW, Cochran WG (1970) Statistical methods. Econometrica 38:372. https://doi.org/10.2307/1913021
Sun AZ, Guo FQ (2016) Chloroplast retrograde regulation of heat stress responses in plants. Front Plant Sci 7:398. https://doi.org/10.3389/fpls.2016.00398
Taberlet P, Gielly L, Pautou G, Bouvet J (1991) Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Mol Biol 17:1105–1109. https://doi.org/10.1007/bf00037152
Tarakhovskaya ER, Maslov YI, Shishova MF (2007) Phytohormones in algae. Russ J Plant Physiol 54:163–170. https://doi.org/10.1134/s1021443707020021
Thirumaran G, Karmakar P, Anantharaman P (2006) Effect of seaweed extracts used as fertilizer for Abelmoschus esculentus. J Ecobiol 19:373
Thirumaran G, Anantharaman P, Kannan L (2007) Effect of seaweed extracts used as a liquid fertilizer in the radish (Raphanus sativus). J Ecobiol 20:49
Timothy R P, Yoshiaki M, Carol M L (2013) A manual of chemical and biological methods for sea water Analysis
Van Handel E (1985) Rapid determination of total lipids in mosquitoes. J Am Mosq Control Assoc 1:302–304
Vartanian N, Hervochon P, Marcotte L, Larher F (1992) Proline accumulation during drought rhizogenesis in Brassica napus var. Oleifera J Plant Physiol 140:623–628. https://doi.org/10.1016/s0176-1617(11)80799-6
Wallace RS, Cota JH (1996) An intron loss in the chloroplast gene rpo C1 supports a monophyletic origin for the subfamily cactoideae of the Cactaceae. Curr Genet 29:275–281
Wolfe KH, Li WH, Sharp PM (1987) Rates of nucleotide substitution vary greatly among plant mitochondrial, chloroplast, and nuclear DNAs. Proc Natl Acad Sci USA 84:9054–9058. https://doi.org/10.1007/bf02221558
Yamasaki H, Heshiki R, Ikehara N (1995) Leaf-goldenning induced by high light inFicus microcarpa L. f., a tropical fig. J Plant Res 108:171–180. https://doi.org/10.1007/BF02344341
Yemm EW, Cocking EC, Ricketts RE (1955) The determination of amino acids with ninhydrin. Analyst 80:209–214
Yemm EW, Willis AJ (1956) The respiration of barley plants: IX New phytologist. Metabol Roots During Assim Nitrogen. https://doi.org/10.1111/j.1469-8137.1956.tb05283.x
Yokoya NS, Stirk WA, Van Staden J, Novák O, Turečková V, Pěnčí k A, Strnad M, (2010) Endogenous cytokinins, auxins, and abscisic acid in red algae from Barazil. J Phycol 46:1198–1205. https://doi.org/10.1111/j.1529-8817.2010.00898.x
Ivanova Z, Sablok G, Daskalova E, Zahmanova G, Apostolova E, Yahubyan G, Baev V (2017) Frontiers in plant science front. Plant Sci. https://doi.org/10.3389/fpls.2017.00204
Zhang X, Ervin EH (2008) Impact of seaweed extract-based cytokinins and zeatin riboside on creeping bentgrass heat tolerance. Crop Sci 48:364–370. https://doi.org/10.2135/cropsci2007.05.0262
Zodape ST (2001) Seaweeds as a biofertilizer. J Sci Ind Res 60:378–382
Zurawski G, Clegg MT (1987) Evolution of higherplant chloroplast DNA-encoded genes: Implications for structure-function and phylogenetic studies. Annual Rev PI Physiol 38:398–418
Zurawski G, Bohnert HJ, Whitfeld PR, Bottomley W (1982) Nucleotide sequence of the gene for the Mr 32,000 thylakoid membrane protein from Spinacia oleracea and Nicotiana debneyi predicts a totally conserved primary translation product of Mr 38,950. Proc Natl Acad Sci 79:7699–7703. https://doi.org/10.1073/pnas.79.24.7699
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ali, A.H., Said, E.M. & Abdelgawad, Z.A. The role of seaweed extract on improvement drought tolerance of wheat revealed by osmoprotectants and DNA (cpDNA) markers. Braz. J. Bot 45, 857–867 (2022). https://doi.org/10.1007/s40415-022-00820-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40415-022-00820-5