Abstract
The recent availability of 124I, due in part to the spread of PET scanners, has opened up new possibilities for performing pre-therapeutic dosimetric studies in patients with differentiated thyroid cancer. 124I PET/CT has remarkable clinical potential: for disease staging and the ablation of thyroid remnants, but also for studying patients at high risk or with suspected local relapses and/or metastases; furthermore, it has a low stunning risk. Many clinical studies have shown the superiority of 124I PET/CT versus 131I conventional imaging, which is attributable to the possibility of combining morphological and highly specific functional imaging data, avoiding most of the known pitfalls of 131I scanning. 124I PET/CT can be used to perform dosimetry, avoiding the side effects of 131I, to tailor treatments, instead of using fixed therapeutic activities, and to evaluate mean absorbed doses both to target lesions (thereby allowing adequate therapy planning and staging) and to non-target organs such as the salivary glands. In addition, the concomitant use of 18F-FDG PET/CT allows the detection of non-iodine-avid lesions, discriminating these from simultaneously occurring iodine-positive lesions. This review analyzes clinical studies on 124I PET/CT in patients with differentiated thyroid cancer, and suggests possible future applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Expression of the sodium iodide symporter (NIS), the key cellular feature for specific iodine uptake, is an essential prerequisite for the treatment of differentiated thyroid cancer (DTC) [1]. Indeed, for more than 50 years, radioiodine therapy with 131I (RAIT) has been the first line of treatment for patients with DTC after surgery.
The primary objective of RAIT is to achieve ablation of remnants, although it is also used in the treatment of local relapses or metastases, and to improve accuracy in diagnosis and long-term follow-up. However, the use of RAIT is somewhat controversial. The European guidelines [2] categorized DTC as: (1) very low-risk, (2) low-risk, and (3) high-risk. Patients in the first group, being considered to have an excellent prognosis after surgery, do not derive benefit from of RAIT; in other words, in these patients, its high cost and radiobiological risk would not be offset by any clinical improvement in the status of the disease. In high-risk patients, on the other hand, the use of radioiodine is recommended, since the advantages outweigh the risks. For those classified as low-risk, the use of radioiodine may decrease recurrence, but the evidence is not still conclusive. The American Thyroid Association [3] recommends radioiodine ablation for selected patients with 1–4 cm thyroid cancers confined within the gland, who have documented lymph node metastases, other high-risk features (age, tumor size, lymph node status, histology), worrisome histological subtypes (tall cell, columnar, insular and solid variants and poorly differentiated thyroid cancer), vascular invasion or gross or microscopic multifocal disease.
Correct pre-ablation staging constitutes an important instrument from the perspective of patient management, as it provides a basis for deciding whether or not to use other therapies before or after RAIT, and allows the radioiodine dose to be tailored to the single patient.
Moreover, there is still no general consensus on the amount of radioactivity that can be administered for ablation [2]. Several years ago a fixed empirical activity of 3.7 GBq was administered, but subsequent studies demonstrated that the same success rate could be reached with lower activities, both after thyroid hormone withdrawal and after rhTSH stimulation [4–9]. Fixed empirical activities are also used in cases of local relapse and/or lymph node metastases (5.55–7.4 GBq) and distant metastases (7.4 GBq or more) [3].
According to the only available dosimetric studies, by Maxon et al. [10, 11], the minimum absorbed dose necessary to obtain a high probability of therapeutic success is 300 Gy for ablation of DTC remnants, and 80–100 Gy for complete eradication of metastases, while below 40 Gy no metastatic complete response was found. The activity must, in any case, be chosen in order to avoid exceeding a blood dose of 2 Gy, the threshold above which there is a high risk of serious hematological complications. As the second organ at risk is the lung, when pulmonary metastases are present, the retention of therapeutic activity at 48 h should not exceed 3.0 GBq [10–12]. Thus, there has emerged a need to modulate the amount of radiation in order to improve the efficacy of the treatment while at the same time reducing the associated radiation exposure risks.
In view of these considerations, many centers have adopted a pre-therapeutic dosimetric approach [13–18]. This preliminary assessment should be recommended in pluri metastatic patients, in order to obtain precise individualized dosimetry [2]. Nowadays, diagnostic imaging with 131I SPECT/CT is prescribed before the therapeutic dose of 131I, but this could lead to the stunning effect (for activities of 37–74 MBq) [19–22]. In addition, radionuclide imaging with 131I shows poor spatial resolution and image quality as a result of the effects of high-energy 364 keV gamma emissions leading to septal penetration artifacts; nevertheless, it is used in clinical practice [23–28].
The alternative radiopharmaceutical for staging and/or dosimetry, 123I, is not readily available and has a short half-life. In fact, late imaging with 123I is not possible when using a reasonable amount of 123I activity, a finding that led to questions over whether dosimetry was worth the effort [29].
New horizons have recently been opened up by the PET tracer 124I. The literature contains studies on 124I that date back to 1960 [30], and we have known about the radiotoxicity of 124I for half a century [31], but it is only recently that 124I has actually become available, thanks in part to the spread of PET scanners; in particular, this development was prompted by a phantom study by Erdi et al. [32], who concluded that quantification of 124I is possible. This situation has obviously created new perspectives for 124I PET, especially in relation to the scope for hybrid PET/CT studies.
Imaging with 124I PET/CT
124I is a positron-emitting isotope with a half-life of 4.2 days. It has a cascade gamma (602 keV) with 60 % abundance, and positron emission (23 % decays) suitable for PET/CT imaging [33]. Despite the many high-energy gamma rays (723 keV 10 % abundance and 1691 keV 10 % abundance) associated with the cascade gamma photons, which may create false triple coincidence events, as demonstrated by Pentlow et al. [34], 124I PET imaging is feasible, offering better spatial resolution and diagnostic sensitivity than 123I and 131I SPECT [29], but showing a slightly worse resolution (a difference of about 1 mm), less contrast and more scatter than traditional PET imaging with 18F-FDG [35–37]. Using particular scanner settings, it is also possible to obtain good quality images from 124I PET/CT simultaneously with therapeutic activities of 131I; these acquisitions are suitable for quantitative dosimetric evaluation [38].
In order to obtain visible and evaluable images it is necessary to acquire about 5 min/bed in a 3D PET scanner, after oral or intravenous administration of 25–76 MBq of 124I [39, 40], using hybrid scanners, in order to have attenuation correction and better localization from the anatomical CT study [41]. In accordance with what has long been established [42], the first scan is usually taken at 24 h. It is possible to acquire images for 120 h or more after the radiopharmaceutical administration, which in turn allows better dosimetric studies [39, 43].
Even though 2D PET imaging does not require corrections for spurious events, 3D imaging is the most widespread modality, partly because almost all the available commercial scanners offer only this PET configuration.
The estimated total radiation exposure of diagnostic 124I PET is 5 mSv from the administration of 50 MBq [44] as compared with a whole-body effective dose of 11 mSv from 185 MBq of 131I [45].
To date, no cases of thyroid tissue “stunning” after 124I PET/CT have been reported [35].
Clinical studies
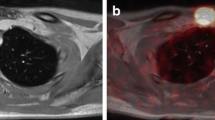
Many clinical studies have shown the superiority of 124I PET/CT versus 131I conventional imaging (planar images and/or SPECT/CT), which is attributable to the possibility of combining morphological imaging and highly specific functional imaging, avoiding most of the known pitfalls of 131I scanning. This approach could also lead to improved clinical decision-making. See Figs. 1 and 2.
Phan et al. [46] studied the feasibility of 124I PET in 24 patients with histologically proven advanced DTC and compared the findings with those of diagnostic and post-therapy 131I whole-body scans (WBSs). The 124I PET adequately predicted findings on subsequent post-therapy scans, and was shown to be superior to diagnostic 131I and equivalent to post-therapy 131I planar imaging, with abnormal uptake better depicted on 124I PET/CT.
Capoccetti et al. [41] studied 69 patients with DTC (67 recruited for ablation of thyroid remnants and two affected by pluri metastatic disease) and remarked that the findings of 124I PET/CT and 131I WBS matched in 58 out of 67 patients (87 %). In 12 % of these 67, 124I PET/CT revealed previously unknown lymph node metastases, which changed the N stage, and in a further 4 % unknown distant metastases were found.
In fact, 124I PET/CT imaging can also be used to verify the correct assignment of thyroid remnants and lymph node metastases, as shown by Rosenbaum-Krumme et al. [39], who analyzed kinetic quantities by determining the maximum activity concentration and effective half-life of each lesion.
Van Nostrand et al. [47] compared the ability of diagnostic 124I PET images and 131I WBSs to detect residual thyroid tissue and/or metastases. Confirming the results of other studies, they showed that 124I PET allowed them to identify 50 % more sites of radioiodine uptake in 32 % of 25 patients with DTC suggestive of additional residual thyroid tissue and/or metastatic disease.
Recurrence is found in up to 30 % of patients with DTC, although some cases can show elevated thyroglobulin values and negative 131I conventional imaging. In this situation, in which 131I imaging displays poor sensitivity and low yield in detecting metastases in patients who have undergone thyroidectomy [48], some authors [49] have proposed 18F-FDG PET/CT as an instrument allowing selection of patients for surgery, which may be curative.
Freudenberg et al. [50] compared 124I PET/CT and 18F-FDG PET/CT in the detection of recurrent DTC lesions in patients with increasing serum thyroglobulin, thyroglobulin antibodies, or both, but without cervical pathology on ultrasonography. They studied 21 patients who had previously undergone thyroidectomy and RAIT. The sensitivities for DTC lesion detection were: 49 % for 124I PET, 67 % for CT, 80 % for 124I PET/CT, 70 % for 18F-FDG PET and 91 % for combined modalities. For recurrences the sensitivities were: 60 % for 124I PET, 20 % for CT, and 65 % for 18F-FDG PET. One-third of lesions showed pathological uptake with both 124I and 18F-FDG PET, while the other two-thirds were positive with only one technique. In conclusion, the combination of the two imaging modalities improves restaging in recurrent DTC by enabling detection, on WBSs, of local recurrence or metastases that are often not found if only one technique is applied.
Even though it has been demonstrated that 124I PET/CT is a useful tool, this technique may suffer from other problems or pitfalls: Abdul-Fatah et al. [51] reported that the activity of 124I near the trachea can result in annihilations in the opposite wall of this organ, incorrectly suggesting activity at that location. They found this artifact in 17 % of 29 patients. A different problem may occur in patients with disseminated iodine-avid lung metastases. The lungs are one of the most common sites of distant spread of DTC [52]; indeed, the prevalence of pulmonary metastases at the initial diagnosis is reported to range from 2 to 20 % [53, 54]. Freudenberg et al. [55] demonstrated that visual analysis of 124I PET scans was sufficient in only one out of seven patients affected by disseminated pulmonary disease. On the contrary, quantitative analysis of 124I PET data, using a lung-to-background ratio, allows patients with suspected pulmonary disease to be differentiated from those in whom it is not suspected; however, this criterion cannot be used alone, and must instead be correlated with additional diagnostic tests, such as serum thyroglobulin levels.
124I PET/MRI may also be a useful instrument: in a study of 33 high-risk DTC patients, Nagarajah et al. [56] intra-individually and prospectively compared the lesion detection ability of 124I PET alone and 124I PET used in combination with CT and MRI, considering their ability to characterize a lesion as thyroid remnant tissue or lymph node metastasis, and the consequences of higher detectability on lesion dosimetry. They found that MRI detected more morphological correlates to PET foci than CT did, and 65 % of these lesions were <10 mm in diameter. They concluded that PET/MRI is superior to PET/CT in pre-therapeutic imaging, particularly in lesions measuring <10 mm in diameter, resulting in more precise and individually tailored 124I dosimetry.
Dosimetric studies with 124I PET/CT
Dosimetry using 124I PET is a complex technique whose application needs to be evaluated on an individual, case-by-case basis. It can be used to stage patients with remnants, to determine the optimal administrable activity for remnant ablation, and, in selected pluri metastatic patients, to establish the maximum dose that can be delivered to lesions while avoiding side effects on organs at risk.
The aforementioned problems related to the 124I spectrum can be addressed by the use of a correction factor called recovery coefficient (RC), studied by Jentzen et al. [36] in 2D and 3D modes and evaluated taking into account all the factors affecting this value, such as object shape, background activity spill-in and iterative image reconstruction parameters [57]. These authors concluded that the RC value is not significantly influenced by changes in object shape, whereas reconstruction parameters do have a large effect, especially when an OSEM algorithm with too small a number of iterations is chosen. In addition, 124I PET quantification of lesions smaller than 1 ml, containing very low activity concentrations, was found to be associated with large uncertainties, as confirmed by Capoccetti et al. [41]. Thanks to the long half-life of the tracer, it is possible to acquire images of 124I for up to 120 h after its administration [39]. This advantage makes it possible to structure data acquisition protocols that could be delayed for up to 5 days from 124I administration. The most widely used protocol for pre-therapy dosimetry was developed around serial data collection, for both red marrow and lesion analysis. This model involves serial blood sampling, whole-body counting and serial imaging acquisition for the localization and quantification of lesion activity see Fig. 3.
Freudenberg et al. [40] first applied this model using 124I and a protocol of five PET images (4,24,48,72,96 h), one PET/CT acquisition (25 h), six blood samples (2,4,24,48,72,96 h) and six whole-body counts performed following the same schedule used for the blood samples. The image acquisition makes it possible to study the estimated absorbed lesion dose per GBq of administered 131I, and thus to calculate the minimum effective activity. Through blood collection it is possible to determine the critical threshold that avoids marrow toxicity see Fig. 4.
If there is no anatomical information from CT data, lesion volume can be estimated using a segmentation method [58].
In the case of lesions receiving <80 Gy, the efficacy is reduced [40] and in the event of doses below 40 Gy, an alternative therapeutic option must be chosen [10] see Table 1; see Fig. 5.
Thus, the EANM dosimetry committee has described a pre-therapeutic model for standard operational procedures [59] and explained all the mathematical calculations and the data management in another guideline publication [60].
It is also possible, for the convenience of patients and staff and to reduce healthcare costs, to use a simplified protocol with only two PET points. This option could also help to make 124I PET/CT dosimetry more widely available and user-friendly [43]. The calculation that obviously follows the data collection can be performed using a standard method (MIRD), or it could be simplified through the use of 3-D software, developed by Sgouros et al. [61] and tested by Kolbert et al. [62]. It is also possible to use more sophisticated methods such as 3-D voxel calculations by either convolution with a dose-point Kernel or Monte Carlo simulation [60].
Evaluation of rhTSH versus thyroid hormone withdrawal in RAIT
As an alternative to thyroid hormone withdrawal, rhTSH stimulation has been shown to be an effective tool for thyroid remnant ablation in patients with DTC [63–67]. The success rate of rhTSH-stimulated remnant ablation using 1–3 GBq of 131I is comparable to that of remnant ablation after thyroid hormone withdrawal, despite the shorter duration of TSH elevation and the faster renal clearance of radioiodine in the euthyroid state [68, 69].
Potzi et al. [70] reported low effective delivered doses to thyroid metastases with rhTSH stimulation compared to thyroid hormone withdrawal, using dosimetric calculations based on tumor and whole-body uptake from 123I scans performed 0, 4, 24 and 48 h after the administration of 123I, and cautioned against empirical radioiodine 131I treatments using exogenous stimulation.
Freudenberg et al. [71], using 124I PET lesion-based dosimetry, delivered adequate radiation doses >300 Gy to remnant thyroid tissue treated using standard therapeutic activities of 131I. They concluded that there were no differences between rhTSH stimulation and thyroid hormone withdrawal, and therefore that no modifications of prescribed therapeutic activities were necessary. Regarding distant metastases, the same authors [72], retrospectively comparing mean absorbed doses between groups of consecutive patients receiving 124I PET/CT aided by rhTSH stimulation or thyroid hormone withdrawal, found some indications but no statistically significant evidence that rhTSH administration resulted in a lower radiation dose to DTC metastases than thyroid hormone withdrawal did.
Hartung-Knemeyer et al. [73] studied 198 patients with locally advanced or metastasized DTC, candidates to receive a high-activity therapy, who underwent pre-therapeutic blood dosimetry using 124I. They showed that exogenous, as opposed to endogenous, TSH stimulation could result in a lower blood dose, and that blood doses seemed to be generally higher at first RAIT compared to subsequent RAITs. They concluded that blood dosimetry should become standard practice, as the range of absorbed doses was rather wide.
Evaluation of salivary glands and other organs
Salivary dysfunction is the most common side effect of RAIT, leading to sialoadenitis and occasionally some degree of xerostomia, both of which have a potentially negative impact on quality of life [74]. Stimulation of saliva flow soon after administration of 131I using lemon juice for example is a popular approach for reducing radiogenic damage [75], but only starting 24 h after 131I therapy [76]. Dosimetric studies using 131I and 124I and comparing the well-known dose–effects relationships in external radiation therapy showed that the average absorbed doses in the salivary glands per administered 131I and 124I are too low to induce the observed radiogenic damage. This could suggest an inhomogeneous distribution of iodine in this organ; in fact, Jentzen et al. [77, 78] assessed the effect of chewing lemon slices on the parotid and submandibular glands in patients who underwent pre-therapy 124I PET/CT dosimetry by examining the organ absorbed doses per administered 131I activity. The mean absorbed dose was found to be similar both in the submandibular glands and in the parotid glands, and lower in the non-stimulation group than in the stimulation one. This reduction in the non-stimulation group was significant for the parotid glands, but not for the submandibular glands. These results might demonstrate that lemon juice stimulation shortly after 131I administration increases the absorbed dose to salivary glands.
Conclusions and future perspectives
In a group of patients affected by DTC, a fixed activity-based approach may offer benefits due to its simplicity, with regimens that in most cases remain below toxicity limits. The main disadvantage in using this approach is the failure to consider the individuality of each patient. There may, in fact, be evidence of occasional over- or under-treatment; in addition, the efficacy of each successive treatment in an individual patient might be degraded.
The results of all the considered studies demonstrate that 124I PET/CT is a powerful tool, which can be used both to perform an excellent pre-therapy staging (allowing earlier multimodal interventions than standard empirical protocols do) and to study patients with suspected local relapses and/or metastases, with limited stunning risk thanks to the lower activity injected and the shorter effective half-life of the tracer. PET/MRI is superior to PET/CT in pre-therapy imaging, particularly in lesions measuring <10 mm in diameter, resulting in more precise and individually tailored 124I dosimetry.
124I has not yet been registered, and is currently available only for experimental studies; its cost is comparable to that of many radiopharmaceuticals routinely used in clinical practice. Once registered, it is likely to become more readily available and more widespread.
In conclusion, dosimetric studies calculate individually tailored 131I therapy for the ablation of thyroid remnants with the same success rate as the empirical fixed dose approach, and optimize treatment for patients in the high-risk group, for those with established metastatic disease, and even for cases with measurable thyroglobulin values and negative 131I imaging, reducing the radiation exposure risks and avoiding the administration of unnecessary activities. By estimating absorbed doses to lesions, it is possible not only to avoid side effects, but also to calculate the probability of cure; on the other hand, it is also possible to choose therapeutic activities in order to avoid exceeding absorbed dose limits not only for organs at risk, but also for the salivary glands and probably other organs too, such as the lacrimal glands, stomach and breast.
This approach, which could be routinely performed in order to obtain fine dosimetry in patients with thyroid cancer, could in the future be used to drive the development of new technologies and techniques in the detection and treatment of other malignancies that express NIS, such as stomach and breast cancer.
References
Dohan O, De la Vieja A, Carrasco N (2000) Molecular study of the sodium-iodide symporter (NIS): a new field in thyroidology. Trends Endocrinol Metab 11(3):99–105
Luster M, Clarke SE, Dietlein M, Lassmann M, Lind P, Oyen J, Tennvall J et al (2008) Guidelines for radioiodine therapy of differentiated thyroid cancer. Eur J Nucl Med Mol Imaging 35:1941–1959
Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, Pacini F, Schlumberger M, Cooper DS, Sherman SI, Steward DL, Tuttle RM, (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer (2009) Revised American thyroid association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19(11):1167–1214
Pacini F, Molinaro E, Castagna MG, Lippi F, Ceccarelli C, Agate L, Elisei R, Pinchera A (2002) Ablation of thyroid residues with 30 mCi (131)I: a comparison in thyroid cancer patients prepared with recombinant human TSH or thyroid hormone withdrawal. J Clin Endocrinol Metab 87(9):4063–4068
Barbaro D, Boni G, Meucci G, Simi U, Lapi P, Orsini P, Pasquini C, Piazza F, Caciagli M, Mariani G (2003) Radioiodine treatment with 30 mCi after recombinant human thyrotropin stimulation in thyroid cancer: effectiveness for postsurgical remnants ablation and possible role of iodine content in l-thyroxine in the outcome of ablation. J Clin Endocrinol Metab 88(9):4110–4115
Pilli T, Brianzoni E, Capoccetti F, Castagna MG, Fattori S, Rossi G et al (2007) A comparison of 1850 MBq (50 mCi) and 3700 MBq (100 mCi) 131-iodine administered doses for recombinant ablation in differentiated thyroid cancer. J Clin Endocrinol Metab 92:3542–3546
Mallick U, Harmer C, Hackshaw A (2008) The HiLo trial: a multicentre randomised trial of high- versus low-dose radioiodine, with or without recombinant human thyroid stimulating hormone, for remnant ablation after surgery for differentiated thyroid cancer. Clin Oncol (R Coll Radiol) 20:325–326
Schlumberger M, Catargi B, Borget I, Deandreis D, Zerdoud S, Bridji B, Bardet S, Leenhardt L, Bastie D, Schvartz C, Vera P, Morel O, Benisvy D, Bournaud C, Bonichon F, Dejax C, Toubert ME, Leboulleux S, Ricard M, Benhamou E (2012) Tumeurs thyroïde de la refractaires network for the essai stimulation ablation equivalence trial. Strategies of radioiodine ablation in patients with low-risk thyroid cancer. N Engl J Med 3366(18):1663–1673
Mallick U, Harmer C, Yap B, Wadsley J, Clarke S, Moss L, Nicol A, Clark PM, Farnell K, McCready R, Smellie J, Franklyn JA, John R, Nutting CM, Newbold K, Lemon C, Gerrard G, Abdel-Hamid A, Hardman J, Macias E, Roques T, Whitaker S, Vijayan R, Alvarez P, Beare S, Forsyth S, Kadalayil L, Hackshaw A (2012) Ablation with low-dose radioiodine and thyrotropin alfa in thyroid cancer. N Engl J Med 366:1674–1685
Maxon HR, Englaro EE, Thomas SR et al (1992) Radioiodine 131 therapy for well-differentiated thyroid cancer: a quantitative radiation dosimetric approach: outcome and validation in 85 patients. J Nucl Med 33:1132–1136
Maxon HR, Thomas SR, Samaratunga RC (1997) Dosimetric considerations in the radioiodine treatment of macro metastases and micro metastases from differentiated thyroid cancer. Thyroid 7:183–187
Benua R, Cicale N, Sonemberg M et al (1962) The relation of radioiodine dosimetry to results and complications in the treatment of metastatic thyroid cancer. Am J Roentgenol Radium Ther Nucl Med 87:171–179
Van Nostrand D, Atkins F, Yeganeh F, Acio E, Bursaw R, Wartofsky L (2002) Dosimetrically determined doses of radioiodine for the treatment of metastatic thyroid carcinoma. Thyroid 12:121–134
Tuttle RM, Leboeuf R, Robbins RJ, Qualey R, Pentlow K, Larson SM et al (2006) Empiric radioactive iodine dosing regimens frequently exceed maximum tolerated activity levels in elderly patients with thyroid cancer. J Nucl Med 47:1587–1591
Lee JJ, Chung JK, Kim SE, Kang WJ, do Park J, Lee DS, Cho BY, Lee MC (2008) Maximal safe dose of I-131 after failure of standard fixed dose therapy in patients with differentiated thyroid carcinoma. Ann Nucl Med. 22(9):727–734
Verburg FA, Hänscheid H, Biko J, Hategan MC, Lassmann M, Kreissl MC, Reiners C, Luster M (2010) Dosimetry-guided high-activity (131)I therapy in patients with advanced differentiated thyroid carcinoma: initial experience. Eur J Nucl Med Mol Imaging 37(5):896–903
Dorn R, Kopp J, Vogt H, Heidenreich P, Carroll RG, Gulec SA (2003) Dosimetry-guided radioactive iodine treatment in patients with metastatic differentiated thyroid cancer: largest safe dose using a risk-adapted approach. J Nucl Med 44(3):451–456
Klubo-Gwiezdzinska J, Van Nostrand D, Atkins F, Burman K, Jonklaas J, Mete M, Wartofsky L (2011) Efficacy of dosimetric versus empiric prescribed activity of 131I for therapy of differentiated thyroid cancer. J Clin Endocrinol Metab 96(10):3217–3225
McMenemin RM, Hilditch TE, Dempsey MF, Reed NS (2001) Thyroid stunning after (131)I diagnostic whole-body scanning. J Nucl Med 42(6):986–987
Shapiro B, Freitas J, Gross M. (2005) Follow-up of patients with well-differentiated thyroid cancer. In: Biersack H-J, Grunwald F. (ed) Thyroid cancer, 2nd edn. Springer-Verlag Berlin Heidelberg, pp199–219
Silberstein EB (2007) Comparison of outcomes after (123)I versus (131)I pre-ablation imaging before radioiodine ablation in differentiated thyroid carcinoma. J Nucl Med 48(7):1043–1046
Verburg FA, Verkooijen RB, Stokkel MP, Van Isselt JW (2009) The success of I131 ablation in thyroid cancer is significantly reduced after a diagnostic activity of 40 MBq I131. Nuklearmedizin 48(4):138–142
BRAITish Thyroid Association RCOP. (2009) Guidelines for the management of thyroid cancer 2nd edn. cited 2011 October 3 Available at: http://www.bRAITish-thyroidassociation.org/guidelines/Accessed 24 March
Wang H, Fu HL, Li JN, Zou RJ, Gu ZHWuJC (2009) The role of single-photon emission computed tomography/computed tomography for precise localization of metastases in patients with differentiated thyroid cancer. Clin Imaging 33:49–54
Mustafa M, Kuwert T, Weber K, Knesewitsch P, Negele T, Haug A et al (2010) Regional lymph node involvement in T1 papillary thyroid carcinoma: a bicentric prospective SPECT/CT study. Eur J Nucl Med Mol Imaging 37:1462–1466
Schmidt D, Linke R, Uder M, Kuwert T (2010) Five months’ follow-up of patients with and without iodine-positive lymph node metastases of thyroid carcinoma as disclosed by 131I-SPECT/CT at the first radioablation. Eur J Nucl Mol Imaging 37:699–705
Wong KK, Sisson JC, Koral KF, Frey KA, Avram AM (2010) Staging of differentiated thyroid carcinoma using diagnostic 131-I SPECT-CT. AJR Am J Roentgenol 195(3):730–736
Grewal RK, Tuttle RM, Fox J, Borkar S, Chou S, Gonen M et al (2010) The effect of post therapy 131I SPECT/CT on risk classification and management of patients with differentiated thyroid cancer. J Nucl Med 51:1361–1367
Rault E, Vandenberghe S, Van Holen R, De Beenhouwer J, Staelens S, Lemahieu I (2007) Comparison of image quality of different iodine isotopes (I-123, I-124 and I-131). Cancer Biother Radiopharm 22:423–430
Phillips AF, Haybittle JL, Newberry GR (1960) Use of iodine-124 for the treatment of carcinoma of the thyroid. Acta Unio Int Contra Cancrum 16:1434–1438
Technical reports Series No 15, “A basic toxicity. Classification of Radionuclides” (1963). IAEA Publications, Vienna
Erdi YE, Macapinlac H, Larson SM et al (1999) Radiation dose assessment for I-131 therapy of thyroid cancer using I-124 PET imaging. Clin Positron Imaging 2:41–46
Lubberink M, Herzog H (2011) Quantitative imaging of 124I and 86Y with PET. Eur J Nucl Med Mol Imaging. 38(Suppl 1):S10–S18. doi:10.1007/s00259-011-1768-2
Pentlow KS, Graham MC, Lambrecht RM, Daghighian F, Bacharac SL, Bendriem B, Finn RD, Jordan K, Kalaigian H, Robeson WR, Larson SM (1996) Quantitative imaging of iodine-124 with PET. J Nucl Med 37:1557–1562
Eschmann SM, Reischl G, Bilger K, Kupferschlager J, Thelen MH, Dohmen BM, Besenfelder H, Bares R (2002) Evaluation of dosimetry of radioiodine therapy in benign and malignant thyroid disorders by means of iodine-124 and PET. Eur J Nucl Med 29:760–767
Jentzen W, Weise R, Kupferschlager J, Freudenberg L, Brandau W, Bares R, Burchert W, Bockisch A (2008) Iodine-124 PET dosimetry in differentiated thyroid cancer: recovery coefficient in 2D and 3D modes for PET/(CT) system. Eur J Nucl Med Mol Imaging 35:611–623
Lubberink M, Abdul Fatah S, Brans B, Hoekstra OS, Teule GJJ (2008) The role of 124I PET in diagnosis and treatment of thyroid carcinoma. Q J Nucl Med Mol Imaging 52:30–36
Lubberink M, van Schie A, de Jong HW, van Dongen GA, Teule GJ (2006) Acquisition settings for PET of 124I administered simultaneously with therapeutic amounts of 131I. J Nucl Med 47(8):1375–1381
Rosenbaum-Krumme S, Nagarajah J, Ruhlmann M, Bockisch A, Jentzen W (2012) 124-I PET/CT images of differentiated thyroid cancer patients. Nuklearmedizin 51:1–4
Freudenberg LS, Jentzen W, Gorges R, Petrich T, Marlowe RJ, Knust J, Bockisch A (2007) 124I-PET dosimetry in advanced differentiated thyroid cancer: therapeutic impact. Nuklearmedizin 46:121–128
Capoccetti F, Criscuoli B, Rossi G, Ferretti F, Manni C, Brianzoni E (2009) The effectiveness of 124I PET/CT in patients with differentiated thyroid cancer. Q J Nucl Med Mol Imaging 53(5):536–545
Frey P, Townsend D, Flattet A et al (1986) Tomographic imaging of the human thyroid using I-124. J Clin Endocrinol Metab 63:918–927
Jentzen W, Freudenberg L, Eising EG, Sonnenschein W, Knust J, Bocksisch A (2008) Optimized 124I PET dosimetry protocol for radioiodine therapy of differentiated thyroid cancer. J Nucl Med 49:1017–1023
International commission on radiological protection (1998) ICRP publication 80. Addendum to publication 53-radiation dose to patients from radiopharmaceuticals. Ann ICRP 28
Johansson L, Mattsson S, Nosslin B, Leide-Svegborn S (1993) Effective dose from radiopharmaceuticals. Eur J Nucl Med. 19(11):933–8. Erratum in. Eur J Nucl Med 20(6):570
Phan HT, Jager PL, Paans AM, Plukker JT, Sturkenboom MG, Sluiter WJ et al (2008) The diagnostic value of 124I PET in patients with differentiated thyroid cancer. Eur J Nucl Med Mol Imaging 35(5):958–965
Van Nostrand D, Moreau S, Bandaru VV, Atkins F, Chennupati S, Mete M et al (2010) (124)I positron emission tomography versus (131)I planar imaging in the identification of residual thyroid tissue and/or metastasis in patients who have well-differentiated thyroid cancer. Thyroid 20:879–883
Lubin E, Machlis-Frish S, Zatz S, Shimoni A, Segal K, Avraham A, Levy R, Feinmesser R (1994) Serum thyroglobulin and iodine 131 whole body scan in diagnosis and assessment of treatment for metastatic differentiated thyroid carcinoma. J Nucl Med 35:257–262
Schluter B, Bohuslavizki KH, Beyer W, Plotkin M, Buchert R, Clausen M (2001) Impact of FDG PET on patients with differentiated thyroid cancer who present with elevated thyroglobulin and negative 131I scan. J Nucl Med 42:71–76
Freudenberg LS, Antoch G, Frilling A, Jentzen W, Rosenbaum SJ, Kuhl H et al (2008) Combined metabolic and morphologic imaging in thyroid carcinoma patients with elevated serum thyroglobulin and negative cervical ultrasonography: role of 124 I PET/CT and FDG PET. Eur J Nucl Med Mol Imaging 35(5):950–957
Abdul-Fatah SB, Zamburlini M, Halders SG, Brans B, Teule GJ, Kemerink GJ (2009) Identification of a shine-through artefact in the trachea with (124I) PET/CT. J Nucl Med 50(6):909–911
Stewart BW, Kleihues P (2003) Thyroid cancer. In: Stewart BW, Kleihues P (eds) World cancer report. IARC Press, Lyon, pp 257–260
Casara D, Rubello D, Saladini G et al (1993) Different features of pulmonary metastases in differentiated thyroid cancer: natural history and multivariate statistical analysis of prognostic variables. J Nucl Med 34:1626–1631
Sisson JC, Giordano TJ, Jamadar DA et al (1996) 131I treatment of micronodular pulmonary metastases from papillary thyroid carcinoma. Cancer 78:2184–2192
Freudenberg LS, Jentzen W, Muller SP, Bocksisch A (2008) Disseminated iodine-avid lung metastases in differentiated thyroid cancer: a challenge to 124I PET. Eur J Nucl Med Mol Imaging 35(3):502–508
Nagarajah J, Jentzen W, Hartung V, Rosenbaum-Krumme S, Mikat C, Heusner TA, Antoch G, Bockisch A, Stahl A (2011) Diagnosis and dosimetry in differentiated thyroid carcinoma using 124I PET: comparison of PET/MRI vs PET/CT of the neck. Eur J Nucl Med Mol Imaging 38(10):1862–1868
Jentzen W (2012) Experimental investigation of factors affecting the absolute recovery coefficients in Iodine-124 PET lesion imaging. Phys Med Biol 55:2365–2398
Jentzen W, Freudenberg LS, Eising EG, Heinze M, Brandau W, Bockisch A (2007) Segmentation of PET volumes by iterative image thresholding. J Nucl Med 48:108–114
Lassmann M, Hanscheid H, Chiesa C, Hindorf C, Flux G, Luster M (2008) EANM Dosimetry Committee series on standard operational procedures for pre-therapeutic dosimetry I: blood and bone marrow dosimetry in differentiated thyroid cancer therapy. Eur J Nucl Med Mol Imaging 35:1405–1412
Hindorf C, Glatting G, Chiesa C, Lindén O, Flux G (2010) EANM dosimetry committee. EANM dosimetry committee guidelines for bone marrow and whole-body dosimetry. Eur J Nucl Med Mol Imaging 37(6):1238–1250
Sgouros G, Kolbet KS, Sheikh A, Pentlow KS, Mun EF, Barth A, Robbins RJ, Larson SM (2004) Patient-specific dosimetry for 131 I thyroid cancer therapy using 124I PET and 3-dimensional-internal dosimetry (3D-ID) software. J Nucl Med 45:1366–1372
Kolbert KS, Pentlow KS, Pearson JR, Sheikh A, Finn RD, Humm JL, Larson SM (2007) Prediction of absorbed dose to normal organs in thyroid cancer patients treated with 131I by use of 124I PET and 3-Dimensional Internal Dosimetry software. J Nucl Med 48:143–149
Robbins RJ, Larson SM, Sinha N, Shaha A, Divgi C, Pentlow KS et al (2002) A retrospective review of the effectiveness of recombinant human TSH after a preparation for radioiodine thyroid remnant ablation. J Nucl Med 43:1482–1488
Luster M, Sherman SI, Skarulis MC, Reynolds JR, Lassmann M, Hanscheid H et al (2003) Comparison of radioiodine biokinetics following the administration of recombinant human thyroid stimulating hormone and after thyroid hormone withdrawal in thyroid carcinoma. Eur J Nucl Med Mol Imaging 30:1371–1377
Hanscheid H, Lassmann M, Luster M, Thomas SR, Pacini F, Ceccarelli C et al (2006) Iodine biokinetics and dosimetry in radioiodine therapy of thyroid cancer: procedures and results of a prospective international controlled study of ablation after rhTSH or hormone withdrawal. J Nucl Med 47:648–654
Pacini F, Landerson PW, Schlumberger M, Driedger A, Luster M, Kloos RT et al (2006) Radioiodine ablation of thyroid remnants after preparation with recombinant human thyrotropin in differentiated thyroid carcinoma: results of an international, randomized, controlled study. J Clin Endocrinol Metab 91:926–932
Vaiano A, Traino AC, Boni G, Grosso M, Lazzeri P, Colato C et al (2007) Comparison between remnant and red-marrow absorbed dose in thyroid cancer patients submitted to 131I ablative therapy after rhTSH stimulation versus hypothyroidism induced by L-thyroxine withdrawal. Nucl Med Commun 28:215–223
Montenegro J, González O, Saracho R, Aguirre R, González O, Martínez I (1996) Changes in renal function in primary hypothyroidism. Am J Kidney Dis 27(2):195–198
Dietlein M, Busemeyer S, Kobe C, Schmidt M, Theissen P, Schicha H (2010) Recombinant human TSH versus hypothyroidism. Cost-minimization-analysis in the follow-up care of differentiated thyroid carcinoma. Nuklearmedizin. 49(6):216–224
Potzi C, Moameni A, Karanikas G, Knust J, Pink R, Jentzen W et al (2006) Comparison of iodine uptake in tumour and nontumour tissue under thyroid hormone deprivation and with recombinant human thyrotropin in thyroid cancer patients. Clin Endocrinol (Oxf) 65:519–523
Freudenberg LS, Fromke C, Petrich T, Marlowe RJ, Koska WW, Brandau W et al (2010) Thyroid remnant dose: 124I PET/CT dosimetric comparison of rhTSH versus thyroid hormone withholding before radioiodine remnant ablation in differentiated thyroid cancer. Exp Clin Endocrinol Diabetes 118:393–399
Freudenberg LS, Jentzen W, Petrich T, Frömke C, Marlowe RJ, Heusner T, Brandau W, Knapp WH, Bockisch A (2010) Lesion dose in differentiated thyroid carcinoma metastases after rhTSH or thyroid hormone withdrawal: 124I PET/CT dosimetric comparisons. Eur J Nucl Med Mol Imaging 37(12):2267–2276. doi:10.1007/s00259-010-1565-3
Hartung-Knemeyer V, Nagarajah J, Jentzen W, Ruhlmann M, Freudenberg LS, Stahl AR, Bockisch A, Rosenbaum-Krumme SJ (2012) Pre-therapeutic blood dosimetry in patients with differentiated thyroid carcinoma using 124-iodine: predicted blood doses correlate with changes in blood cell counts after radioiodine therapy and depend on modes of TSH stimulation and number of preceding radioiodine therapies. Ann Nucl Med 26:723–729
Hyer S, Kong A, Pratt B, Harmer C (2007) Salivary gland toxicity after radioiodine therapy for thyroid cancer. Clin Oncol (R Coll Radiol) 19(1):83–86
Dietlein M, Dressler J, Farahati F et al (2004) Procedure guidelines for radioiodine therapy of differentiated thyroid cancer (version 2). Nuklearmedizin 43:115–120
Nakada K, Ishibashi T, Takei T, Hirata K, Shinohara K, Katoh S, Zhao S, Tamaki N, Noguchi Y, Noguchi S (2005) Does lemon candy decrease salivary gland damage after radioiodine therapy for thyroid cancer? J Nucl Med 46(2):261–266
Jentzen W, Balschuweit D, Schmidtz J, Freudenberg L, Eising E, Hilbel T et al (2010) The influence of saliva flow stimulation on the absorbed radiation dose to the salivary glands during radioiodine therapy of thyroid cancer using 124I PET/(CT) imaging. Eur J Nucl Med Mol Imaging 37:2298–2306
Jentzen W, Hobbs RF, Stahl A, Knust J, Sgouros G, Bockisch A (2010) Pre-therapeutic (124)I PET/(CT) dosimetry confirms low average absorbed dose per administered 131I activity to the salivary glands in radioiodine therapy of differentiated thyroid cancer. Eur J Nucl Med Mol Imaging 37(5):884–895
Conflict of interest
The authors F. Capoccetti, E. Biggi, G. Rossi, E. Brianzoni declare that they have no conflict of interest.
Human and Animal Studies
This article does not contain any studies with human or animal subjects performed by the any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Capoccetti, F., Biggi, E., Rossi, G. et al. Differentiated thyroid carcinoma: diagnosis and dosimetry using 124I PET/CT. Clin Transl Imaging 1, 185–193 (2013). https://doi.org/10.1007/s40336-013-0021-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40336-013-0021-3