Abstract
Background
The purpose of this prospective study was to evaluate the clinical diagnostic value of iodine-124 (124I)-positron emission tomography (PET) in patients with advanced differentiated thyroid carcinoma (DTC) and to compare the 124I-PET imaging results with the 131I whole-body scan (WBS).
Materials and methods
Twenty patients with histologically proven advanced DTC (including T4, extra-nodal tumour growth, or distant metastases) underwent diagnostic 131I-WBS, 124I-PET scan, and post-treatment 131I-WBS 4 months after ablation. The findings on the 124I-PET were compared with the findings on the diagnostic and post-therapeutic 131I-WBS and were also correlated with radiologic and/or cytological investigations.
Results
124 I-PET vs diagnostic 131 I-WBS. Eleven patients showed uptake on the 124I-PET. Only 3 of these 11 patients also showed uptake on the diagnostic 131I scan, but the uptake was more clearly visible and the abnormalities were more extensive on the 124I-PET. 124 I-PET vs post-treatment 131 I-WBS. Eleven patients showed uptake on the 124I-PET, which was also visible on the post-treatment scan in nine patients; in the other two patients, no uptake was observed on the post-treatment scan and no anatomical localisation could be confirmed. Two patients showed only uptake on the post-treatment scan without uptake on the 124I-PET: in one, the uptake was confirmed by MRI, and in the other, no anatomical localisation was found. In seven patients, no uptake was observed on both the scans.
Conclusion
124I-PET proved to be a superior diagnostic tool as compared to low-dose diagnostic 131I scans and adequately predicted findings on subsequent high-dose post-treatment 131I scans.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Papillary and follicular thyroid cancer are the most frequent histological types of thyroid cancer (85-90%) [1, 2]. Total thyroidectomy with or without lymph node dissection is the initial therapy, followed by radioactive iodine therapy.
In the routine follow-up of low-risk patients, diagnostic 131I whole-body scanning (WBS) is not recommended in the recently published guidelines [3] (http://www.british-thyroid-association.org/draft_thyca_23.12.06.pdf; http://www.oncoline.nl/uploaded/docs/Schildkliercarcinoom/schildklier%20ebro/ijn%20schildklier.pdf), but is still considered to be valuable in the follow-up of the high-risk patients. Measurement of the serum level of thyroglobulin (Tg), under recombinant human thyroid-stimulating hormone (rhTSH) stimulation or suppression, and ultrasonography have gained a more central role in monitoring for recurrent thyroid cancer [3, 4-7] (http://www.british-thyroid-association.org/draft_thyca_23.12.06.pdf). In patients with increasing or elevated Tg, a blind treatment with high-dose 131I can be applied, followed by a post-treatment 131I scan which also serves as a diagnostic tool [21]. However, with this strategy, unnecessary high radiation exposure and high TSH level must be taken into account especially in patients who subsequently have no 131I uptake on their post-treatment scan. Improvement of diagnostic imaging for the detection of recurrent or metastatic disease and a better (anatomical) localisation, e.g. using advanced imaging technique such as 124I-positron emission tomography (PET)/CT would allow more selective application of 131I therapy and might avoid unnecessary high-dose treatments.
Several iodine isotopes such as 123I, 125I, 131I play an important role in nuclear medicine, both for diagnostic purposes and for therapy. Iodine-124 is a positron emitting isotope and therefore suitable PET imaging. Its half-life is 4.2 days with a very complex decay scheme leading to extra non- or partially annihilation radiation coincidence detection [8, 9]. Approximately 23% of the desintegrations results in positron emissions.
While the radioisotopes 123I and especially 131I are used on a wide scale in diagnosis and treatment of all thyroid disorders, 124I has received little attention. This isotope would allow thyroid cancer imaging using the high-resolution PET technique [8, 10]. Iodine-124 has so far mainly been used for dosimetry or thyroid volume measurements [10-16]. However, the recent development of combined PET/CT scanners may increase clinical application in thyroid cancer patients, as detailed anatomical information is combined with the location of iodine positive tissue [17]. Iodine-124 has recently been applied for staging of differentiated thyroid cancer, as reported in a few case reports and small series, with promising results [17-19]. However, the diagnostic value of 124I-PET imaging as compared to (low- and high-dose) 131I scintigraphy has to be further investigated.
Iodine-124 PET may be able to detect recurrent or residual disease in differentiated thyroid carcinoma (DTC) with a higher sensitivity than the conventional (diagnostic) 131I scans because of the higher spatial resolution. In this way, 124I-PET imaging could possibly be a useful diagnostic tool during the follow-up of DTC patients. Therefore, we have performed a pilot study in the early treatment phase of thyroid cancer to get an impression of the diagnostic potential of 124I-PET for detection and staging of advanced DTC. We compared the 124I-PET imaging results with the diagnostic and post-treatment 131I-WBS.
Materials and methods
Twenty patients with histologically proven advanced DTC, including extrathyroidal tumour growth (T4), extra-nodal tumour growth or distant metastasis (M1), were examined in this study. These patients had undergone (near) total thyroidectomy. Four to 6 weeks after surgery, diagnostic 131I-WBS after 37 MBq of 131I was obtained, followed by an ablative dose of 5,550 MBq 131I. Post-treatment WBS was obtained after 10 days. At the time of ablation, serum Tg and Tg antibodies (TgAb) levels were also determined.
All 20 studied patients underwent diagnostic 131I-WBS, 124I-PET scan and post-treatment 131I-WBS 4 months after ablation therapy (Fig. 1). The Tgoff levels (under TSH stimulation after thyroid hormone withdrawal or after rhTSH injection) were also measured just before the administration of the diagnostic low-dose 131I. The Medical Ethics Committee of University Medical Center Groningen approved the study protocol, and all patients gave written informed consent.
Tg was measured by immunoradiometric assay (Brahms, Henningsdorf, Germany), with a functional sensitivity (i.e. the Tg concentration with a vital capacity of 20%) of 0.3 ng/ml as determined by the laboratory. Thyroglobulin antibodies (TgAb) were detected by both immunoradiometric assay (Brahms, Henningsdorf, Germany) with a cutoff of 60 U/ml and by immunoluminometric assay (Abbott, Hoofddorp, Netherlands) on the Architect i 2000 platform (Abbott) with a cutoff of 4.1 U/ml. Both cutoff concentrations were determined by Brahms and Abbott, respectively. In 19 patients, the Tg level was determined after thyroid hormone withdrawal and in one patient after rhTSH due to poor physical condition (Table 1).
Tracer
124I-sodium iodide solution was obtained from Ritverc Isotope Products, St. Petersburg, Russia and was imported by I.D.B. Holland BV (Baarle-Nassau, The Netherlands). Radiochemical purity using instant thin layer chromatography and radionuclide purity using germanium (HP-Ge) detector were tested before release according to standard radiopharmaceutical procedures. Before use, the solution was filtered using a Millipore bacterial filter (0.22 μm) and diluted with sterile saline. A sterility test was performed on each batch, but data were only available after administration due to the length of the test procedure (7 days).
PET scanning
Diagnostic 124I-PET imaging was performed 24 h after intravenous administration of 74 MBq of 124I [17]. A Siemens PET camera (Exact HR+, Knoxville, TN, USA) was used for imaging. The patient was positioned in the scanner, and a standard clinical whole-body PET study was performed in 2D ETTE mode over seven to eight positions (from the upper thigh up until the top of the skull) of 5-min emission and 3-min transmission, standard energy window setting of 350-650 keV. Images were reconstructed with attenuation-weighted OSEM, two iterations and eight subsets. The total time needed for the scan was approximately 60 min.
In the initial four patients, the 124I-PET images were repeated 96 h after 124I administration (thus, 72 h after therapeutic dose of 131I) using the same acquisition settings as after 24 h. Narrowing of the energy window (425-650 or 460-562 keV, 3D mode) for 124I-PET imaging during high-dose 131I therapy to reduce the effects of γ-rays (364, 637 keV) of 131I and 124I (602 keV) and to improve image quality, as described in the phantom study (and clinical application in one thyroid cancer patient) by Lubberink et al. [20], was not possible in our institution due to technical reasons, e.g. camera characteristics. The 96-h 124I-PET images were very noisy and blurred and were excluded from the study. This poor image quality could partially be explained by the poor statistics due to the very low radioactivity left in the body after 96 h and partially by technical reasons as described above.
Data analysis
The 131I-WBS and 124I-PET scans were visually interpreted by two independent, experienced nuclear medicine physicians (HTP, PLJ). The findings on the 124I-PET scans were compared with the findings on the diagnostic and post-therapeutic 131I-WBS. Correlation with radiologic imaging (US, CT, MRI) and/or cytological (fine needle aspiration cytology, FNAC) investigation was done to confirm the findings or in case of discordant findings on the 124I-PET scan and 131I-WBS. If no abnormal uptake was seen on the PET scan and 131I-WBS, additional radiologic imaging (MRI, US), with or without FNAC, and fluorodeoxyglucose (FDG) PET were also performed to detect local or metastatic disease and/or used as a follow-up diagnostic tool.
Results
From December 2005 until April 2007, 20 consecutive patients with advanced DTC were included in this prospective study. The group consisted of ten women and ten men, median age of 67 years (range 18-85 years). Individual patient characteristics and the findings on the 124I PET scan and 131I-WBS are summarised in Table 1.
Physiological uptake of 124I was observed in the salivary glands, esophagus, gastrointestinal tract and bladder as it is also normally seen on the 131I scans.
124I-PET vs diagnostic 131I-WBS
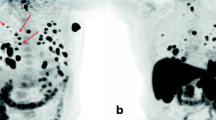
Eleven patients (no. 1-11) showed uptake on the 124I-PET scan. In only three of them (no. 1-3) was the uptake also observed on the diagnostic 131I scan, but the uptake was clearer and the abnormalities were more extensive on the 124I-PET scan (Fig. 2). In nine patients (no. 12-20), no uptake was observed on both the scans. Results of additional radiologic imaging (MRI and/or US-FNAC) are also listed in Table 1.
This patient (no. 1) showed a clearly visible lesion in the cervical vertebrae on the 124I-PET (a, arrow), comparable with the lesion visible on the post-treatment 131I-WBS (c, arrow). This lesion was vaguely visible on the diagnostic 131I-WBS (b, arrow). Physiologic uptake in the esophagus, gastrointestinal tract and bladder is observed on the 124I-PET
It has also been noticed that the Tgoff level (after endogenous TSH stimulation) was not detectable (<0.30 ng/ml, TSH >30 mU/L) without the presence of Tg antibodies in five (Table 1, no. 5, 8, 12, 13, 20) of the 20 patients. However, two of these five patients showed lesions on the 124I-PET (and post-treatment 131I scan) confirmed by MRI. Diagnostic 131I-WBS was negative in all five.
124I-PET vs post-treatment 131I-WBS
Nine (no. 1-9) out of the 11 patients with uptake on the 124I-PET scan had lesions which were also visible on the post-treatment scan (Fig. 3). In two patients (no. 10, 11), no uptake was observed on the post-treatment 131I scan and no anatomical localisation could be confirmed. FDG PET showed, however, lesions in the costae and pelvis in patient no. 11 (Fig. 4).
Clearly, uptake in the right costae (arrow) and left hip is seen on the FDG-PET (c) in patient no. 11, whereas the post-treatment 131I-WBS (a) was negative. The 124I-PET (b) showed only uptake around the femur prosthesis on the right side, which is also observed on the FDG-PET, which could be explained by reactive tissue on the MRI. The complementary uptake of radioiodine and FDG is also known as the flip-flop phenomenon, which was first described by Joensuu and Ahonen [27]
Two patients (no. 12, 13) showed uptake on the post-treatment 131I scan, which was not visible on the 124I-PET scan: In one, MRI confirmed the 131I uptake in the skull, and in the other, no anatomical localisation could be found for the 131I uptake in the pelvis region. In seven patients (no. 14-20), no uptake was observed on both the scans. In four of seven patients (no. 14-17), no lesions could be found with MRI and/or US-FNAC; in one of these four (no. 17), FDG-PET showed uptake in the lungs which was confirmed by CT. One patient (no. 18) showed slightly enlarged lymph node in the superior mediastinum on the MRI which was not accessible for US-guided FNAC. In two patients (no. 19, 20), MRI/US of the neck will be obtained (due to patient delay). Results of additional radiologic imaging are listed in Table 1.
Discussion
This pilot study showed that 124I-PET detected more abnormalities in comparison to the diagnostic 131I-WBS, but showed comparable findings with the post-treatment 131I-WBS. Eleven patients had positive 124I-PET scanning, and only three had visible abnormalities with the low-dose 131I scan. Moreover, 124I-PET also showed abnormalities in two of the five patients with undetectable Tg, while the low-dose diagnostic 131I scan was negative in all five. These findings suggest that 124I-PET better predicts the outcome of high-dose 131I treatment and would be better suited as a diagnostic tool to base clinical decisions on such as additional surgery or application of high dose 131I. Therefore, 124I-PET should be performed before considering high-dose 131I treatment. A negative 124I-PET could mean omitting 131I treatment, and further additional imaging should be performed to detect (non-iodine avid) metastatic disease.
Two patients (no. 12, 13) showed uptake in the skull and pelvis region, respectively, on the post-treatment 131I-WBS which was not visible on the 124I-PET. Possible explanations for these findings might be the low dose of 124I in which (small) lesions in the skull might be missed and false-positive uptake of 131I in the bowel which could be mistaken for abnormality.
Our results are in agreement with the study by Freudenberg et al. [17]. In their study, 12 patients with advanced DTC underwent high-dose 131I-WBS, 124I-PET, CT, combined 124I-PET/CT, FDG-PET and US post-thyroidectomy during routine clinical staging. The overall lesion detectability for high-dose 131I-WBS was comparable with the 124I-PET, 83 vs 87%, respectively. Moreover, combined 124I-PET/CT modality, which showed an overall lesion detectability of 100%, resulted in a change of staging in two patients and a change in management in one.
It has been questioned why 124I-PET would be a suitable diagnostic tool when blind treatment is given anyway in the clinical practice. Blind treatment with high-dose 131I in patients known with negative low-dose diagnostic 131I scanning but with elevated Tg has been used as a diagnostic, therapeutic and prognostic tool. Patients without iodine accumulation on the post-treatment WBS, which could be an indication for tumour dedifferentiation, had a worse prognosis compared to those with a positive post-treatment WBS [21]. Repeating high doses of 131I may bear the risks in terms of long-term risk of secondary malignancies. In addition, repeated long-lasting TSH stimulation might have adverse effects on tumour growth. This aspect of long-lasting TSH stimulation may be prevented when performing the 124I-PET after rhTSH stimulation before the decision of giving additional high-dose 131I. However, comparative studies are needed to evaluate the yield of the 124I-PET under endogenous TSH stimulation and after rhTSH stimulation.
Additional advantages of 124I-PET would include the better resolution of this tomographic method and the ease of combining this with CT data to increase the diagnostic value, as shown by Freudenberg et al. [17]. Moreover, 124I-PET would allow more precise dosimetric calculations [25, 26, 28, 29], although that was not a focus of this study.
Another aspect which favours performing of 124I-PET before blind high-dose 131I is the radiation dose. The effective dose of 124I is in the same order of magnitude as 131I [10, 22-24]. The effective dose of 124I-iodide is 0.095 mSv/MBq with a thyroid uptake of 0% and increases to1.5 mSv/MBq with a thyroid uptake of 35% [23]. The total radiation exposure has been calculated at 7.0 mSv for a dose of 74 MBq 124I. The administered dose is comparable to the dose administered for routine nuclear medicine scans. However, the total radiation exposure of 124I is just a fraction compared to the total radiation exposure of the therapeutic dose of 131I (340 mSv for 5,550 MBq [23], which was also mentioned in the study by Freudenberg et al. [17].
The issue whether stunning is a real phenomenon and its clinical relevance/consequence is debatable. Stunning effect after (higher) diagnostic dose of 131I has been described and discussed in the literature. The applied diagnostic 131I dose could impair the ability of the residual thyroid carcinoma tissue to accumulate the subsequently applied high-dose 131I dose. The degree of stunning probably depends on the absorbed radiation dose and the time between the diagnostic and therapeutic 131I dose.
Stunning was not seen with diagnostic 131I doses of 185 MBq (5 mCi) or lower [30, 31], whereas stunning was frequently observed after a diagnostic dose of 370 MBq (10 mCi) 131I [32]. There is also evidence that a short time interval between the administration of the diagnostic and therapeutic 131I dose may diminish the effect of stunning [31]. Most authors who have described stunning administered the therapeutic 131I dose several days after the completion of the diagnostic 131I scan [32-35]. Stunning is not seen when the therapeutic dose is administered within several hours. Furthermore, the time interval between the administration of a high dose of 131I and the performance of a post-therapy WBS may influence the observation of stunning [30, 36]. A longer time interval allows more time for soft tissue clearance of 131I, which results in a higher sensitivity of the post-therapy WBS. No stunning was seen at the post-therapy 131I-WBS, performed 5-10 days after doses of 1,110-3,700 MBq (30-100 mCi) 131I after 74 MBq (2 mCi)) and 370 MBq (mCi) diagnostic scan [37].
In our study, we used a rather a low dose of 124I (74 MBq) and a short interval (1 day) between the administration of the diagnostic dose of 124I and the administration of the therapeutic 131I dose and a long interval (10 days) between the therapeutic 131I dose and the post-therapy 131I-WBS. If stunning does exist after 124I, it is therefore unlikely to have reduced the efficacy of 131I treatment in our study.
Iodine-124 is, however, poorly available with high costs, but the advantages of 124I-PET could outweigh these disadvantages and can lead to more clinical application in the follow-up of DTC patients when 124I will become more available.
Conclusions
In this study, 124I-PET proved to be a superior diagnostic tool as compared to low-dose diagnostic 131I scans and adequately predicted findings on subsequent high-dose post-treatment 131I scans. In combination with the high resolution and the possibilities to combine with CT, this could lead to improved clinical decision making.
References
Sherman SI. Thyroid carcinoma. Lancet 2003;361:501-11.
Ringel MD, Ladenson PW. Controversies in the follow-up and management of well-differentiated thyroid cancer. Endocr Relat Cancer 2004;11:97-116.
Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, et al. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. The American Thyroid Association Guidelines Taskforce. Thyroid 2006;16:109-42.
Cailleux AF, Baudin E, Travagli JP, Ricard M, Schlumberger M. Is diagnostic iodine-131 scanning useful after total thyroid ablation for differentiated thyroid cancer? J Clin Endocrinol Metab 2000;85:175-8.
Torlontano M, Attard M, Crocetti U, Tumino S, Bruno R, Costante G, et al. Follow-up of low-risk patients with papillary thyroid cancer: role of neck ultrasonography in detecting lymph node metastases. J Clin Endocrinol Metab 2004;89:3402-7.
Baudin E, Do Cao C, Cailleux AF, Leboulleux S, Travagli JP, Schlumberger M. Positive predictive value of serum thyroglobulin levels, measured during the first year of follow-up after thyroid hormone withdrawal, in thyroid cancer patients. J Clin Endocrinol Metab 2003;88:1107-11.
Menendez Torre E, Lopez Carballo MT, Rodriguez Erdozain RM, Forga Llenas L, Goni Iriarte MJ, Barberia Layana JJ. Prognostic value of thyroglobulin serum levels and 131I whole body scan after initial treatment of low-risk differentiated thyroid cancer. Thyroid 2004;14:301-6.
Pentlow KS, Graham MC, Lambrecht RM, Daghighian F, Bacharach SL, Bendriem B, et al. Quantitative imaging of iodine-124 with PET. J Nucl Med 1996;37:1557-62.
Lambrecht RM, Woodhouse N, Phillips R, Wolczak D, Qureshi A, Reyes ED, et al. Investigational study of iodine-124 with a positron camera. Am J Physiol Imaging 1988;3:197-200.
Eschmann SM, Reischl G, Bilger K, Kupferschlager J, Thelen MH, Dohmen BM, et al. Evaluation of dosimetry of radioiodine therapy in benign and malignant thyroid disorders by means of iodine-124 and PET. Eur J Nucl Med Mol Imaging 2002;29:760-7. DOI 10.1007/s00259-002-0775-8.
Crawford DC, Flower MA, Pratt BE, Hill C, Zweit J, McCready VR, et al. Thyroid volume measurement in thyrotoxic patients: comparison between ultrasonography and iodine-124 positron emission tomography. Eur J Nucl Med 1997;24:1470-8.
Flower MA, al-Saadi A, Harmer CL, McCready VR, Ott RJ. Dose-response study on thyrotoxic patients undergoing positron emission tomography and radioiodine therapy. Eur J Nucl Med 1994;21:531-6.
Flower MA, Irvine AT, Ott RJ, Kabir F, McCready VR, Harmer CL, et al. Thyroid imaging using positron emission tomography-a comparison with ultrasound imaging and conventional scintigraphy in thyrotoxicosis. Br J Radiol 1990;63:325-30.
Ott RJ, Batty V, Webb BS, Flower MA, Leach MO, Clack R, et al. Measurement of radiation dose to the thyroid using positron emission tomography. Br J Radiol 1987;60:245-51.
Frey P, Townsend D, Flatted A, De Gautard R, Widgren S, Jeavons A, et al. Tomographic imaging of the human thyroid using 124I. J Clin Endocrinol Metab 1986;63:918-27.
Frey P, Townsend D, Jeavons A, Donath A. In vivo imaging of the human thyroid with a positron camera using 124I. Eur J Nucl Med 1985;10:472-6.
Freudenberg LS, Antoch G, Jentzen W, Pink R, Knust J, Gorges R, et al. Value of 124I-PET/CT in staging of patients with differentiated thyroid cancer. Eur Radiol 2004;14:2092-8.
Freudenberg LS, Antoch G, Gorges R, Knust J, Pink R, Jentzen W, et al. Combined PET/CT with iodine-124 in diagnosis of spread metastatic thyroid carcinoma: a case report. Eur Radiol 2003;13 Suppl:L19-23.
Georges R, Antoch G, Brandau W, Freudenberg LS, Knust J, Dutschka K, et al. Kombinierte PET/CT mit dem Positronenstrahler 124I bei metastasierten follikularen Schilddrusenkarzinom. Nuklearmedizin 2002;5:N69-71.
Lubberink M, van Schie A, de Jong HW, van Dongen GA, Teule GJ. Acquisition settings for PET of 124I administered simultaneously with therapeutic amounts of 131I. J Nucl Med 2006;47:1375-81.
Van Tol KM, Jager PL, de Vries EG, Piers DA, Boezen HM, Sluiter WJ, et al. Outcome in patients with differentiated thyroid cancer with negative diagnostic whole-body scanning and detectable stimulated thyroglobulin. Eur J Endocrinol 2003;148:589-96.
Glaser M, Luthra S, Brady F. Applications of positron-emitting halogens in PET oncology (review). Int J Oncol 2003;22:253-67.
Johansson L, Mattsson S, Nosslin B, Leide-Svegborn. Effective dose from radiopharmaceuticals. Eur J Nucl Med 1992;19:933-8 (Erratum in: Eur J Nucl Med 1993;20:570).
Berman M, Braverman LE, Burke J, et al. Report No.5 I-123, I-124, I-125, I-126, I-130, I-131, I-132 as sodium iodide. In: Loevinger R, Budinger TF, Watson EE, editors. MIRD primer for absorbed dose calculations. New York: The Society of Nuclear Medicine; 1991. p. 49-54.
Sgouros G, Kolbert KS, Sheikh A, Pentlow KS, Mun EF, Barth A, et al. Patient-specific dosimetry for 131I thyroid cancer therapy using 124I PET and 3-dimensional-internal dosimetry (3D-ID) software. J Nucl Med 2004;8:1366-72.
Erdi YE, Macapinlac H, Larson SM, Erdi AK, Yeung H, et al. Radiation dose assessment for I-131 therapy of thyroid cancer using I-124 PET imaging. Clin Positron Imaging 1999;2:41-6.
Joensuu H, Ahonen A. Imaging of metastases of thyroid carcinoma with fluorine-18 fluorodeoxyglucose. J Nucl Med 1987;28:910-4.
Freudenberg LS, Jentzen W, Görges R, Petrich T, Marlowe RJ, Knust J, et al. 124I-PET dosimetry in advanced differentiated thyroid cancer: therapeutic impact. Nuklearmedizin 2007;46:121-8.
Jentzen W, Weise R, Kupferschläger J, Freudenberg L, Brandau W, Bares R, et al. Iodine-124 PET dosimetry in differentiated thyroid cancer: recovery coefficient in 2D and 3D modes for PET(/CT) systems. Eur J Nucl Med Mol Imaging 2007 (in press); DOI 10.1007/s00259-007-0554-7.
Pacini F, Lippi F, Formica N, Elisei R, Anelli S, Ceccarelli C, et al. Therapeutic doses of iodine-131 reveal undiagnosed metastases in thyroid cancer patients with detectable serum thyroglobulin levels. J Nucl Med 1987;28:1888-91.
Cholewinski SP, Yoo KS, Klieger PS, O’Mara RE. Absence of thyroid stunning after diagnostic whole-body scanning with 185 MBq 131I. J Nucl Med 2000;41:1198-202.
Park HM, Perkins OW, Edmondson JW, Schnute RB, Manatunga A. Influence of diagnostic radioiodines on the uptake of ablative dose of iodine-131. Thyroid 1994;4:49-54.
Jeevanram RK, Shah DH, Sharma SM, Ganatra RD. Influence of initial large dose on subsequent uptake of therapeutic radioiodine in thyroid cancer patients. Int J Rad Appl Instrum B 1986;13:277-9.
Muratet JP, Daver A, Minier JF, Larra F. Influence of scanning doses of iodine-131 on subsequent first ablative treatment outcome in patients operated on for differentiated thyroid carcinoma. J Nucl Med 1998;39:1546-50.
Leger FA, Izembart M, Dagousset F, Barritault L, Baillet G, Chevalier A, et al. Decreased uptake of therapeutic doses of iodine-131 after 185-MBq iodine-131 diagnostic imaging for thyroid remnants in differentiated thyroid carcinoma. Eur J Nucl Med 1998;25:242-6.
Morris LF, Waxman AD, Braunstein GD. The nonimpact of thyroid stunning: remnant ablation rates in 131I-scanned and nonscanned individuals. J Clin Endocrinol Metab 2001;86:3507-11.
Waxman A, Ramanna L, Chapman N, Chapman D, Brachman M, Tanasescu D, et al. The significance of I-131 scan dose in patients with thyroid cancer: determination of ablation: concise communication. J Nucl Med 1981;22:861-5.
Acknowledgements
The authors thank the “Innovatie Fonds UMCG”, The Netherlands, for their grant support.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Phan, H.T.T., Jager, P.L., Paans, A.M.J. et al. The diagnostic value of 124I-PET in patients with differentiated thyroid cancer. Eur J Nucl Med Mol Imaging 35, 958–965 (2008). https://doi.org/10.1007/s00259-007-0660-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-007-0660-6