Abstract
Heart failure (HF) with preserved ejection fraction (HFpEF) is a prevalent global condition affecting approximately 50% of the HF population. With the aging of the worldwide population, its incidence and prevalence are expected to rise even further. Unfortunately, until recently, no effective medications were available to reduce the high mortality and hospitalization rates associated with HFpEF, making it a significant unmet need in cardiovascular medicine. Although HFpEF is commonly defined as HF with normal ejection fraction and elevated left ventricular filling pressure, performing invasive hemodynamic assessments on every individual suspected of having HFpEF is neither feasible nor practical. Consequently, several clinical criteria and diagnostic tools have been proposed to aid in diagnosing HFpEF. Overall, these criteria and tools are designed to assist healthcare professionals in identifying and evaluating patients who may have HFpEF based on a combination of signs, symptoms, biomarkers, and non-invasive imaging findings. By employing these non-invasive diagnostic approaches, clinicians can make informed decisions regarding the best pharmacological and rehabilitation strategies for individuals with suspected HFpEF. This literature review aims to provide an overview of all currently available methods for diagnosing and monitoring this disabling condition.
Similar content being viewed by others
1 Introduction
Heart failure (HF) with preserved ejection fraction (HFpEF; defined as EF > 50%) represents half of the HF population, with rising incidence and prevalence as the world’s population ages [1]. Until recently [2], there was no medication available to reduce these patients' high mortality and hospitalization rates, making HFpEF one of the most significant unmet needs in cardiovascular (CV) medicine. Measuring left ventricular EF, typically through echocardiography, is fundamental to phenotype HF and selecting appropriate treatments. However, there are shortcomings in the clinical use of EF, which are elaborated on further. Yet, EF remains the main factor in defining HF and is the principal criterion for enrolling patients in clinical HF trials.
HF with reduced ejection fraction (HFrEF), identified when EF is below 40%, is well-understood, and there are effective treatments for patients with HFrEF. On the other hand, the term HFpEF has traditionally referred to patients showing HF symptoms with a “not reduced” EF. This has been variously defined over the years in different guidelines as being more than 40%, 45%, and now 50%. However, EF alone is not enough to define HFpEF. This clearly emerges from the different definitions and characteristics of patients enrolled for trial in HFpEF over the years. With HF being mostly a clinical diagnosis, novel strategies are needed to better identify patients with HFpEF. This paper aims to review the latest literature on the vast arsenal of diagnostic tools available to detect HFpEF.
2 Epidemiology
HFpEF was initially defined as HF occurring in individuals with a left ventricular (LV) EF exceeding 40% [3]. Cardiological organizations and societies later formalized various EF-based HF phenotypes, including HF with mid-range or mildly reduced EF (HFmrEF), which encompasses EF values between 40 and 50% [4]. HFpEF is currently defined as HF with an EF greater than 50%, in the absence of prior reduced EF to distinguish it from HF with improved ejection fraction (HFimpEF), a novel HF phenotype that is emerging as HF therapies progressively ameliorate reverse remodeling and systolic function in patients with HFrEF and HFmrEF [5, 6] (Table 1).
Previous studies have demonstrated that HF medications are mostly ineffective in lowering cardiovascular mortality in HF patients with EF of 50% and hospitalization for HF over the EF cut-off of 55%. More recently sodium glucose co-transporter 2 inhibitors (SGLT2i) have demonstrated efficacy in treatment of HFpEF reducing morbidity and mortality [7]. Moreover, recent meta-analysis including patients with HFpEF supports the beneficial effect of neurohormonal inhibitors, ACE inhibitors, angiotensin receptor inhibitors, and angiotensin receptor/neprilysin inhibitor (ARNi) on a composite outcome of death and HF hospitalizations [8, 9].
With hypertension being the most represented comorbidity in randomized clinical trials in HFpEF, the treatment of hypertension remains the only therapeutic intervention that has a class 1 recommendation in the recent ACC/AHA guidelines [6]. Etiology and therapy response in HFrEF and HFmrEF are similar, as established by meta-analyses and molecular research [10, 11]. Hence, the concept of HFpEF will remain volatile and keen to change as new information about its phenotyping becomes available.
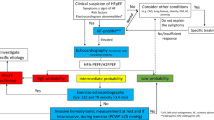
Invasive hemodynamic assessment, the gold standard for the diagnosis of HF, is neither feasible nor reasonable for all individuals with suspected HFpEF. Therefore, clinical scores and diagnostic tools for HFpEF have been developed [12, 13]. Combining clinical and diagnostic parameters, the H2FPEF [12] and HFA-PEFF [13] scores discriminate between HFpEF and non-cardiac dyspnea. Interestingly, the application of these scoring systems has shown limited concordance in diagnosis in those with elevated H2FPEF and HFA-PEFF scores, even though both scores correctly identified patients at high risk of HF hospitalization [14], with only 23% of patients being misclassified when compared to right heart catheterization [15].
3 Physical Examination
Precise acquisition of anamnestic information and physical examination constitute the foundational stages in assessing patients with HF, with a higher capacity to evaluate the congestive status than inadequate perfusion [16]. Addressing congestion is a focal point for adjusting medication regimens, as increased congestion is associated with reduced quality of life and a less favorable prognosis [17].
HFpEF is a heterogeneous syndrome; therefore, the clinical presentation may vary depending on the clinical context. A general physical examination may show signs of systemic or cardiac conditions that either cause or aggravate HF, like anemia, hyperthyroidism, alcoholism, hemochromatosis, high-rate atrial fibrillation, and mitral regurgitation.
Physical manifestations of HF, like heightened jugular venous pressure, pulmonary rales, and peripheral edema, might not be evident in a compensated HF state and are present across the whole spectrum of EF in HF [18, 19].
The determination of jugular venous pressure offers a means to evaluate volume status (about left-sided filling pressures) due to the observation that right-sided filling pressures frequently mirror left-sided filling pressures in HFpEF [20].
Among the predominant manifestations observed in patients with HFpEF, only orthopnea and rales have been identified as robust and autonomous predictors of CV mortality or hospitalization for HF [21].
As HFpEF advances from a compensated to a more unstable phase, dyspnea, and reduced exercise capacity become evident at lower levels of activity, and conspicuous signs of venous congestion may become observable, potentially resulting in hospital admittance [22]. Regarding the diagnosis of HF, dyspnea exhibits higher sensitivity and lower specificity, and orthopnea displays higher specificity but lower sensitivity. In comparison, exercise tolerance and fatigue may exceed the extent of evident cardiac anomalies, showing lower sensitivity and specificity [23].
4 Multimodality Imaging and Natriuretic Peptide
Multimodality imaging is a hybrid approach based on combining multiple imaging techniques to overcome the limitations of individual conventional imaging modalities. Multimodality cardiac imaging has demonstrated an emerging role in the etiological phenotyping, follow-up, and therapeutic management of HFpEF [24, 25].
Cardiac magnetic resonance imaging (CMR) is a non-invasive imaging technique for the morphological and functional characterization of the myocardium [26]. Moreover, emerging evidence has demonstrated CMR's pivotal role in assessing the pathophysiologic mechanisms underlying HFpEF, including myocyte hypertrophy, interstitial fibrosis, coronary microvascular and macrovascular diseases, and metabolic abnormalities [27].
The metabolic alterations in patients with HFpEF can also be evaluated by positron emission tomography (PET), a helpful imaging method to assess substrate changes in heart metabolism using isotope-labeled substrate compounds. Emerging PET tracers enable non-invasive assessment of coronary flow reserve in HFpEF subjects with documented or suspected CAD [28].
Iodine-123 metaiodobenzylguanidine (123I-MIBG) scintigraphy is a safe and helpful imaging technique to evaluate cardiac innervation [29]. Although this technique has been widely studied in HFrEF, current knowledge regarding cardiac sympathetic innervation in HFpEF is limited. However, a recent study conducted on 148 HFpEF patients with non-ischemic etiology showed that 123I-MIBG SPECT imaging can detect cardiac sympathetic nerve dysfunction more accurately than conventional planar imaging, thus providing useful prognostic information in patients with HFpEF [30].
Together with clinical examination and multimodality imaging, measurement of plasmatic levels of natriuretic peptides (NPs), especially B-type natriuretic peptide (BNP) and the N-terminal fragment of proBNP (NT-proBNP), are valuable biomarkers for HF diagnosis, prognosis, and therapeutic management [31]. However, the role of NPs in HFpEF is controversial, and their levels should be carefully evaluated on a case-by-case basis and corroborated by other imaging parameters [32]. Limited data are available regarding NPs in patients with HFpEF [33], and recent studies suggested that elevated circulating levels of NPs are strong predictors of adverse and fatal CV events in these patients [34, 35].
According to current European Society of Cardiology (ESC) guidelines, increased levels of NPs (BNP > 35 pg/mL and/or NT-proBNP > 125 pg/mL) represent a diagnostic criterion for HF [5]. Nevertheless, several studies have reported that NP levels are often normal or even reduced in many patients with HFpEF (20–30%) [36, 37].
There is an ongoing debate regarding the best specificity/sensibility threshold for NPs in diagnosing HFpEF. A very informative diagnostic meta-analysis [38] showed that NPs have reasonable diagnostic performance for detecting HFpEF, with lower sensitivity than specificity and adequate ability to rule out HFpEF, albeit with a shallow positive predictive value.
In a sub-analysis conducted within the I-PRESERVE trial, encompassing a cohort of 2162 patients, Jhund et al. explored the correlation between alterations in NT-proBNP levels over a 6-month follow-up period and subsequent clinical outcomes, including CV death or hospitalization due to HF, as well as all-cause mortality, HF-related death, or HF-related hospitalization. A significant association was found between changes in NT-proBNP levels and the risk of these clinical outcomes, with a notable emphasis on HF-related events. Specifically, an increase of 1000 ng/L in NT-proBNP over 6 months was linked to a two-fold rise in the risk of experiencing cardiovascular death or HF-related hospitalization [39]. The prognostic significance of NT-proBNP was further assessed through a recent analysis conducted within the EMPEROR-Preserved trial, which involved the enrollment and randomization of 5988 patients with EF greater than 40% and New York Heart Association (NYHA) class II-IV symptoms to either receive empagliflozin or a placebo [40]. Among these participants, patients with higher NT-proBNP concentrations tended to be older and exhibited more severe manifestations of HF, characterized by lower EF and more pronounced clinical symptoms, as reflected in health status measurements using the Kansas City Cardiomyopathy Questionnaire (KCCQ) scores.
Furthermore, as baseline NT-proBNP levels increased across quartiles, there was a corresponding rise in the risk of CV death. In the placebo group, this risk exceeded four-fold compared to the lowest to the highest quartile, with a substantial increase in HF-related hospitalizations, totaling five-fold more hospitalizations than in the highest quartile. Additionally, an increase in NT-proBNP levels from baseline to the 12-week assessment was correlated with an increased risk of CV death, both in the placebo (HR 1.88) and in the empagliflozin (HR 1.57) groups. Importantly, treatment with empagliflozin demonstrated the ability to improve clinical outcomes across NT-proBNP quartiles without displaying an interaction with baseline NT-proBNP levels, contributing to a mild reduction in NT-proBNP levels [40].
This lack of consensus regarding NPs' diagnostic and prognostic thresholds in HFpEF has affected the results of large clinical trials, leading to heterogeneous enrollment criteria and the under-use of NPs [41].
In this context, a recent study by Verbrugge and colleagues showed that, although adverse events were correlated to NP levels, patients with HFpEF and normal NPs showed higher mortality or HF hospitalizations compared with patients without HF, thus underlying the importance of the diagnosis of HFpEF to prevent adverse outcomes [42].
These findings suggest that differential levels of NPs could reflect distinct stages of HFpEF, which might be linked to different morphological and functional abnormalities of the heart and variable prognosis. Therefore, more studies are needed to better investigate the prognostic potential of NP levels in patients with HFpEF.
5 Echocardiography
Reduced EF is the benchmark to identify patients with HFrEF. At the same time, recognizing the HFpEF pattern implies a stepwise diagnostic approach based on a combined assessment of clinical characteristics and cardiac imaging [13]. Echocardiography provides critical information in case HF symptoms are present, as these symptoms are clinical manifestations of increased LV filling pressure generated to maintain adequate diastolic filling within the LV with impaired relaxation and increased LV mass [5, 43, 44]. The evaluation of LV geometry, by measuring LV wall thickness and diameters, can stratify morbidity and mortality by tracking structural changes of the LV from remodeling to concentric hypertrophy [45]. Pathological LV thickening can occur not only as a compensative hypertrophic response caused by chronically increased afterload, as observed in the case of arterial hypertension but also as a result of storage disorders (e.g., Fabry disease) or infiltrative disease (amyloidosis) [46]. However, even though LV hypertrophy is considered one of the main characteristics of HFpEF, a recent trial demonstrates that almost one-third of the HFpEF population may not express this feature [47]. This observation highlights that structural changes represent a late phase of adaptation that follows functional alteration (delayed relaxation) and mechanical remodeling (myocardial deformation, atrial dysfunction). These changes occur as a cascade of events resulting in elevated LV filling pressure and, eventually, HF [48]. Multiparametric assessment of LV filling pressure is necessary to identify better individuals needing intensive medical treatment. Diastolic function is assessed by measuring the trans-mitral pulsed wave velocity of the early filling (E wave), its deceleration time, and the ratio between E wave and A wave (E/A ratio). Considering the U-shape relationship between the E/A ratio and LV filling pressure, additional parameters are demanded to define the degree of diastolic dysfunction [49]. However, the E/A ratio might be applicable in an emergency setting for rapidly identifying acute HF [50]. Peak early diastolic annular e’ velocity by tissue Doppler imaging (TDI) represents LV relaxation velocity and is reduced when the LV is less compliant and filling pressure increases. The ratio between trans-mitral E wave velocity and e′ velocity (E/e′ ratio) is considered a robust index of LV filling pressure [51].
Peak systolic pulmonary artery pressure (sPAP) represents a non-invasive esteem of the Pulmonary capillary wedge pressure (PCWP), which helps identify increased LV filling pressure when causes of precapillary pulmonary hypertension can be excluded. Reddy et al. proposed the H2FPEF score that combines echocardiographic derived sPAP and E/e′ ratio with clinical characteristics to discriminate HFpEF from non-cardiac causes of dyspnea [12]. The left atrium (LA) is considered a valid litmus test for chronic increased LV filling pressure, as it contributes to preserving LV filling at the expense of volumetric changes. LA volume, either measured by 2D or 3D echocardiography, should, therefore, be routinely measured in patients with suspected HFpEF, and it is also helpful in the emergency department setting [52]. The recommendation of LV diastolic function proposed an algorithm that incorporates all echocardiographic parameters to estimate LV filling pressure [53]. However, analysis of the accuracy of the 2016 recommended algorithm for diastolic function assessment showed that up to 30% of the exams could be inconclusive [54]. In contrast, strain deformation analysis at rest and diastolic stress echo [55] could help to prevent invasive assessment through heart catheterization. Strain analysis allows quantitative assessment of myocardial deformation throughout the cardiac cycle.
LV longitudinal strain analysis strongly predicts systolic dysfunction, representing an early marker of subendocardial impaired contraction [56]. It has been reported that despite preserved ejection fraction, abnormalities of longitudinal strain may occur early in patients with HFpEF, thus helping to differentiate this condition from asymptomatic hypertensive heart disease [57, 58] with significant differences in prognostic risk stratification [59]. Additionally, strain polar maps assessed by LV strain analysis may provide visual hints to suggest possible HFpEF etiology [60] (Fig. 1).
Bullseye depicts longitudinal strain analysis of the LV in a polar regional map in which red represents normal contraction that lightens as the contraction is impaired. A Patients show reduced GLS (− 16.7%) with marked reduction of longitudinal contraction of the basal segment, suggesting infiltrative pathology (“apical sparing”). B Similar reduction of GLS as in the former case (− 16.6%) but with a balanced reduction of longitudinal function in all the LV segments
Also, strain analysis allows for studying the LA mechanism during the cardiac cycle, supplying information about reservoir, conduit, and pump function [61]. LA reservoir function represents the LA filling before the mitral valve’s opening, thus expressing the compliance of the LA wall, and is inversely related to the degree of fibrosis [62]. It is considered an early marker of LV diastolic dysfunction, having an incremental value to detect elevated LV filling pressure independently from LA geometric measurement [63] due to a significant correlation to pulmonary wedge pressure measured invasively [64], being usually reduced in patients with HFpEF [65]. A recent consensus paper recommends including LA reservoir strain (cut-off value < 18%) in patients with suspected HFpEF, especially in those where LV filling pressure results are undetermined [24]. Recently, a novel method to create a noninvasive P/V loop and its centroid has been tested using standard echocardiography, providing useful pathophysiologic and prognostic information in patients with HF [66].
6 Endothelial Dysfunction Assessment
In the context of HFpEF, systemic cardiovascular changes, including endothelial dysfunction, play a pivotal role in its pathophysiology and progression [67]. To better understand the vascular aspects of HFpEF, non-invasive vascular diagnostic tools have emerged as valuable resources for clinicians and researchers. Flow-mediated dilation (FMD) measures the ability of the brachial artery to dilate in response to increased blood flow, reflecting the endothelial-dependent vasodilatory response. Recent studies, such as the FMD-J study [68], have highlighted the relevance of FMD, especially in HFpEF hypertensive patients, in detecting increased LV diastolic stiffness.
Arterial stiffness, as assessed by pulse wave velocity, has also gained prominence as another non-invasive parameter of interest in HFpEF. Arterial stiffness reflects the rigidity of the arterial walls, which can affect cardiac afterload and ventricular-arterial coupling. As described in the IDENTIFY-HF study [69], arterial stiffness increases as vascular comorbidities accumulate, regardless of age, renal function, hemoglobin levels, obesity, smoking status, or hypercholesterolemia. Notably, HFpEF demonstrates the highest levels of arterial stiffness, while HFrEF displays nearly normal levels. This assessment provides valuable insights into the interaction between the heart and the arterial system, shedding light on the complex mechanisms underlying HFpEF.
7 Lung Ultrasound
Lung ultrasonography (LUS) is a straightforward and expeditious technique for evaluating pulmonary congestion among patients diagnosed with HF [70]. It is widely accessible, particularly within acute care environments [71].
However, in outpatient primary care settings, its feasibility is limited due to the constrained proficiency of general practitioners and the uneven dissemination of ultrasound equipment. LUS involves the measurement of vertical hyperechoic reverberation artifacts originating from the pleural line, extending uninterrupted to the screen's base, and exhibiting synchronous motion with lung sliding [72]. These phenomena are categorized as “B-lines,” they are identified through scanning along the intercostal spaces, preferably utilizing a curvilinear transducer. Different epidemiological studies have demonstrated its diagnostic utility in assessing extravascular lung congestion and correctly identifying patients with HF [73, 74]. B-lines and pleural effusion are the diagnostic hallmarks of increased extra-vascular lung congestion. Compared to chest X-rays, lung ultrasound demonstrated higher sensitivity, specificity, and accuracy for identifying lung congestion in HF regardless of EF [75].
Cogliati et al. show that pulmonary congestion at hospital admission and after discharge is more severe in patients with HFrEF compared to those with HFpEF [76]. In another study, including patients admitted to the cardiology ward, LUS was a fundamental diagnostic tool for detecting subclinical congestions in patients with HFpEF with the number of B-lines as an added predictive value compared to standard echocardiographic parameters [77]. The diagnostic ability of increased B-lines during exercise to identify HFpEF was tested in ambulatory patients, demonstrating a significant diagnostic incremental value over the established H2FPEF score and LA reservoir strain [78]. Systemic venous hypertension occurs due to disrupted coupling between the right ventricle and pulmonary artery, potentially impeding fluid clearance processes [79]. Thus, using LUS to measure pulmonary congestion at rest or with exercise in patients with HFpEF is both timely and attractive. Of course, B-lines can also be associated with other medical conditions, including pulmonary fibrosis and other interstitial lung diseases. In this regard, it is crucial to integrate ultrasound imaging modalities for the correct diagnosis [80]. Different LUS protocols have been validated, ranging from four to 28 chest zones. Naturally, a greater scanning area leads to higher LUS accuracy but also increases time requirements, suggesting using simplified protocols [81].
8 Role of Cardiopulmonary Stress Test
Exercise limitation and dyspnea on efforts are common findings in patients with suspected or defined HFpEF. Currently, the cardiopulmonary exercise test (CPET) is the gold standard technique for assessing functional capacity and exercise tolerance while also facilitating the characterization of exercise limitation. This allows for the differentiation of cardiogenic, pulmonary, and vascular etiologies, thus directing the diagnosis and determining the priority of therapeutic targets for healthcare decisions [82]. Several fundamental criteria must be met to ensure precise testing assessing exercise limitations during maximal efforts. These include maintaining stable clinical conditions, opting for a ramp CPET protocol specifically designed to last between 8 and 12 min, attaining a respiratory exchange ratio equal to or greater than 1.05 to account for the progressive changes in gas exchange parameters (such as peak oxygen uptake [VO2] and work rate [WR]), and accurately identifying ventilatory thresholds and exercise slopes (VO2/WR and ventilation/CO2 production [VE/VCO2] slope) [83].
Although there is no precise algorithm for the ramp protocol to choose in HF patients, both in HFrEF and in HFpEF, gender, age, CV risk factors, degree of routine exercise, symptom status, and comorbidities should be considered. While used to determine patients’ prognosis and to guide pharmacological treatment and timing for cardiac transplantation in HFrEF, CPET also plays its primary role in differentiating HFpEF from non-cardiac causes of exertional dyspnea. In this context, the HFA-PEFF score algorithm includes CPET in the initial work-up (pre-test assessment) of suspected HFpEF, with the only scope to rule out non-cardiac causes of exercise limitation [13]. The primary concern is that in HFpEF, the decrease in peak VO2 is sensitive but non-specific, meaning that in most patients, it is not possible to reliably distinguish HFpEF from non-cardiac forms of dyspnea unless it is at the bottom of the peak VO2 scale [84]. Although a peak VO2 below 14 ml/kg/min strongly suggests the possibility of HFpEF, and a peak VO2 above 20 ml/kg/min makes HFpEF highly unlikely, the range between these two values still requires additional diagnostic tests such as stress echocardiography or right heart catheterization for a more conclusive evaluation [85]. However, when all CPET parameters are combined, a distinct HF pattern with cardiac reserve limitation emerges a decreased O2 pulse, a downward VO2/WR relationship shift, and chronotropic incompetence [86]. Beyond VO2, a substantial increase in the VE/VCO2 slope should lead to further testing for the assessment of a ventilation-perfusion mismatch in a picture of pulmonary vascular involvement, leading to a differential diagnosis between HFpEF with coexisting pre-capillary pulmonary hypertension or chronic thromboembolic pulmonary hypertension [87].
Moreover, it is also essential to pay attention to the presence of interstitial pulmonary diseases, which could be initially revealed by gas exchange anomalies, such as a lower O2 saturation (95% at rest or > 5% reduction during the test), an increased dead volume (VD)/tidal volume (VT), consistent reduction in both Forced Expiratory Volume in the first second (FEV1) and forced vital capacity (FVC) with a preserved ratio during spirometry, and a decrease in diffusing capacity for carbon monoxide (DLCO) [88].
The value of a combined CPET and ecochardiography stress test protocol has also been evaluated to further rule-in patients with HFpEF, especially in the earliest stage of the disease when other diagnostic methodologies may have low specificity or not be indicated due to invasiveness [89].
HFpEF patients may confound diagnosis, frequently having a modest restrictive impairment on spirometry in addition to a decline in DLCO [90]. In these cases, chest tomography or right ventricular catheterization may be required to identify or rule out HFpEF [5]. However, despite its great usefulness, CPET remains only widely available in large tertiary/university centers.
9 Invasive Diagnostic Tools
The diagnosis of HFpEF remains challenging. Indeed, clinical presentation, echocardiographic features, and NP levels may increase the likelihood of diagnosing HFpEF, even though they have a low negative predictive value. This is due to their poor sensitivity and many HFpEF patients having normal resting filling pressures [91, 92]. Stress echocardiography or invasive hemodynamic test is indicated in dubious cases or when confirmation is needed [13]. Therefore, cardiac catheterization plays a crucial role in the differential diagnosis of many patients. Right heart catheterization (RHC) remains the gold standard test to establish the diagnosis of HFpEF [93, 94]. It is generally performed using a thermodilution catheter (Swan-Ganz catheter) inserted in the femoral, antecubital, or internal jugular vein through a 7 Fr sheath. The zero-reference level for the pressure transducer is placed at the mid-thoracic line with the patient in the supine position. After 15 min of catheter insertion, resting hemodynamic parameters can be assessed. Measurements should be recorded at the passive end-expiration phase. Right atrial pressure (RAP), pulmonary arterial pressure (PAP), and pulmonary capillary wedge pressure (PCWP) measurements should be averaged over three cardiac cycles in patients with sinus rhythm (more cycles in those with atrial fibrillation). Cardiac output (CO) should be determined by thermodilution or the direct Fick method [95]. An invasively measured PCWP ≥ 15 mmHg or LV end-diastolic pressure (LVEDP) ≥ 16 mmHg is generally considered diagnostic of HFpEF (5) while concurrently ruling out Pulmonary Hypertension group 1 (characterized by mPAP > 20 mmHg, PCWP ≤ 15 mmHg, and pulmonary vascular resistance > 2 Wood units) [96].
Left heart catheterization (LHC) is relevant to concomitantly measure LV pressures (LVEDP and LVESP) and elastance using pressure-volume loops. In HFpEF, there is a greater LVEDP (at rest and even greater during exercise), and generally, the PV loop shows an upward and leftward shift [97]. However, LHC is also helpful in assessing or excluding concomitant coronary artery disease (CAD) or other conditions that can simulate HFpEF (i.e., constrictive pericarditis).
In particular, invasive coronary angiography enables the detection of obstructive epicardial CAD but also assesses coronary microvascular dysfunction (MVD) by evaluating abnormalities of coronary flow reserve (CFR) and index of microvascular resistance (IMR). Indeed, the mechanisms underlying HFpEF are complex, and MVD has been hypothesized to be a potentially relevant driver in its pathophysiology. However, these patients also show a high prevalence of epicardial CAD due to the burden of comorbidities associated with atherosclerosis and HFpEF; therefore, since epicardial CAD affects myocardial perfusion, it is challenging to clarify the relationship between them and HFpEF and MVD in cohorts with highly prevalent epicardial CAD. Invasive studies show that patients with HFpEF have a high prevalence of MVD, ranging between 70 and 85% depending on the diagnostic thresholds used, which vary between studies: CFR ≤ 2 to ≤ 2.5, IMR ≥ 23 to ≥ 25 [98].
Many HFpEF patients have regular non-invasive and invasive tests at rest [93]. Indeed, in most patients with HFpEF, due to increased chamber stiffness, volume changes lead to more significant increases in LVEDP during exercise. Furthermore, the exercise-induced increase of CO is reduced due to poor contractile reserve and chronotropic incompetence [99, 100]. In such cases, exercise tests are recommended, and exercise RHC has been claimed as the gold-standard diagnostic test for HFpEF [82]. However, exercise RHC is expensive, time-consuming, has limited availability, and requires operator expertise with difficulties of interpretation due to wide swings in intrathoracic pressures.
Moreover, the procedural approach has not been widely standardized, including patients’ body position, exercise protocol, and hemodynamic measurements and interpretation. These non-negligible limitations could impact the reproducibility and generalizability of the results. Protocols differ slightly between sites but generally include increased supine or standing workload, RAP, PAP, and PCWP; measurement of CO using either direct Fick or thermodilution. It is still debated whether pressures should be measured at the end of expiration or using the average of the respiratory cycle [101, 102]. Patients with peak exercise PCWP ≥ 25 mmHg are classified as having HFpEF. Notably, patients with normal or high PCWP at rest and a pathological increase in PCWP during exercise have poor outcomes with increased mortality [103,104,105]. Some data show that healthy individuals could exceed these “normal” LV filling pressure parameters during exercise [106], and with advancing age, there is an increase in PCWP during exercise [107]. The PCWP/CO slope has been developed and validated to overcome these limitations as a novel method to evaluate LV performance during the exercise RHC [102, 108]. This continuous variable showed better diagnostic sensitivity and specificity compared with the peak PCWP criteria for diagnosis of HFpEF and in risk prediction, as it considers the whole workload spectrum [105]. An exercise PCWP/CO slope > 2 mmHg/L/min has been associated with poor functional capacity and adverse clinical outcomes in HFpEF patients. The Fick method has only validated this diagnostic value [109].
The diagnostic information can be further implemented by analyzing biventricular interaction and changes in RAP versus PCWP, providing the pressure-induced unfavorable RV to LV interaction mechanisms intended to reduce the LV/RV pressure gradient. A change in the septum becoming less convex toward the right ventricle is documented even at earlier stages of HFpEF [110, 111]. In advanced cases, a strong signal of abnormalities in RV to pulmonary circulation coupling can be provided by accurately assessing the pressure-flow relationship during exercise through plotting mPAP versus CO. An mPAP/CO relationship > 3 mmHg/L/min represents a pulmonary hypertensive response, indicating abnormalities in pulmonary vascular reserve, generally associated with a high VE/VCO2 slope [112]. Yet, RV to pulmonary circulation uncoupling is responsible for a delayed VO2 on kinetics during early exercise [113].
Alternative methods simulating exercise have been proposed due to the limitations of exercise RHC mentioned above and for patients unable to exercise, i.e., saline loading (fluid challenge test), stress echocardiography, and passive leg raises (PLR). Yet, dobutamine infusion during RHC may be more practical than exercise stress but needs additional validation studies to define its utility. Saline load is more straightforward to perform and reaching a PCWP > 18 mmHg immediately after rapid infusion (7 ml/kg) is considered diagnostic of diastolic LV dysfunction, even if its sensitivity is inferior to the exercise test [114, 115]. PLR determines an increase of venous return and thus can simulate exercise and an increase in the PCWP. Van de Bovenkamp et al. demonstrated that PCWP ≥ 19 mmHg (24% of cases) has a specificity of 100% for the diagnosis of occult-HFpEF, irrespective of diuretic use. A PCWP (PLR) < 11 mmHg has a 100% sensitivity and negative predictive value for diagnosing occult-HFpEF. According to this data, PLR might be helpful in those patients with normal PCWP at rest to unmask occult-HFpEF or rule it out to restrict the need for exercise RHC in those with intermediate values after PLR (PCWP 11–18 mmHg) [116]. However, these tests are less physiological as they reproduce exercise less well with increased heart rate, contractility, and loading conditions [115]. Regarding stress echo the correlation between E/e′ and invasive PCWP or LV End Diastolic Pressure (LVEDP) is poor [117]. More recently, the role of invasive diagnostics has been clarified by the HFA-PEFF and H2PEFF scores [91, 92] (Fig. 2).
Among the invasive diagnostic tools for evaluating patients with HFpEF, endo-myocardial biopsy has been helpful in correctly characterizing myocardial structure and identifying patients with unknown amyloidosis [118, 119]. Endomyocardial biopsy demonstrated that cardiomyocyte inflammation contributes to the development of diastolic dysfunction triggered by the accumulation of collagen and myocardial fibrosis [120]. As shown by Hahn et al. in a cohort of 108 patients with HFpEF, endomyocardial biopsy was helpful in the correct identification of myocardial fibrosis and myocyte hypertrophy, CD68+ monocyte infiltration, and even more importantly, the presence of cardiac amyloidosis, which was often unsuspected [121]. Post-mortem studies have also been performed, contributing to the understanding of the close link between coronary microvascular impairment and myocardial fibrosis [122].
10 Perspectives
Despite its limitations in predicting cardiac functional reserve and symptoms, the diagnosis of HF continues to rely on EF, partly due to historical reasons. Preserved EF holds no diagnostic value for HFpEF except for excluding HFrEF. Future non-invasive real-time measurements of chamber volumes, stroke volumes, CO, and filling pressures, coupled with innovative systolic and diastolic function indices, will significantly diminish the significance of EF in identifying HF (Fig. 3, Table 2).
Moreover, HFpEF patients may encounter hemodynamic limitations in raising stroke volume adequately during exercise, yet specific cut-off points for diagnosing reduced CO reserve have not been established. Unfortunately, reliable data on LV diastolic properties, stroke volume, and CO are obtainable only invasively, ideally via conductance catheterization. While pressure-volume loops and stroke volumes can now be acquired noninvasively through 2D/3D echocardiography and CMR, these measurements require validation within larger HFpEF cohorts. Hence, integrating diverse diagnostic tools is of utmost significance for a precise diagnostic approach to HFpEF.
The relationship between HFpEF and atrial fibrillation is closely intertwined. Overlapping symptoms, indicators, echocardiographic findings, and natriuretic peptide levels exist between the two conditions, and a notable portion of patients in HFpEF registries and trials exhibit atrial fibrillation. Diagnostic thresholds for natriuretic peptides and left atrial volume in sinus rhythm versus atrial fibrillation are primarily derived from existing literature and consensus, necessitating further prospective research for validation.
Controversies persist regarding optimal non-invasive indicators of elevated LV filling pressures and PCWP. The E/e′ index has gained prominence in clinical practice, although its universal support still needs to be completed across all clinical investigations. The diagnostic utility of alternative indices such as retrograde pulmonary venous flow, estimated LV stiffness (diastolic pressure-volume quotient), and left atrial strain rate during atrial contraction in patients with sinus rhythm, as well as the L wave of mitral inflow and left atrial strain warrants additional investigation.
Modern imaging techniques yield a vast amount of digital data related to global and regional LV morphology and function throughout the cardiac cycle, alongside arterial and endothelial function and myocardial perfusion. This data can be amalgamated with comprehensive demographic information, encompassing traditional risk factors, novel biomarkers, and proteomic, metabolomic, and genomic data.
11 Clinical Implications
When all previously reported data are combined, different phenotypes of HFpEF emerge [123]. Left ventricular hypertrophy with concentric remodeling, arterial hypertension, renal disease, and advanced age are typical of the “Older” phenotype. Elevated right ventricular systolic pressure, obesity, and type II diabetes mellitus are characteristic of the “Metabolic” phenotype. Conversely, low values of NP and younger age are typical of the “Younger” phenotype. It should be noted that these phenotypes are frequently over-lapping and, therefore, a careful multiparametric evaluation should be performed to carefully evaluate the best treatment strategies for these patients, considering that treatment of HFpEF should not be consequently based only on EF but also on signs, symptoms, and comorbidities, with hypertension being the most relevant comorbidity.
Based on the aforementioned HFpEF phenotypes, treatment of comorbidities becomes imperative to reduce cardiovascular events beyond the lone prescription of SGLT2i. Hypertension needs to be treated giving preferences to ACE inhibitors, angiotensin receptor blockers, mineralocorticoid receptor agonist and beta-blockers, drugs that have been neutral for their primary outcome in trials on HFpEF but have shown a small beneficial advantage in HF hospitalization or meta-analysis. A strict control of risk factor like obesity may also prove beneficial, especially considering the high prevalence of obesity in the general population and especially in the hypertensive population, this is even more true in the light of the recent results of the use of GLP-1 receptor agonist in the STEP-HFpEF [124] and SELECT [125] trials. Based on the aforementioned bulk of evidences, a tailored treatment beyond SGLT2i should be offered to all patients with HFpEF based on their profiles and respective comorbidities [126].
12 Conclusions
HFpEF represents a multifaceted clinical syndrome characterized by many contributing elements, etiologies, and pathophysiological presentations. Subsequent research endeavors should focus on assessing and enhancing the suggested diagnostic algorithm while categorizing HFpEF individuals into discrete subgroups. Ideally, a comprehensive investigation would encompass an extensive and diverse patient cohort encountering breathlessness alongside appropriately matched control groups, subjected to the entire spectrum of procedures examined in this manuscript.
References
Seferović PM, Vardas P, Jankowska EA, et al. The Heart Failure Association Atlas: heart failure epidemiology and management statistics 2019. Eur J Heart Fail. 2021;23(6):906–14.
Vaduganathan M, Docherty KF, Claggett BL, et al. SGLT-2 inhibitors in patients with heart failure: a comprehensive meta-analysis of five randomized controlled trials. Lancet. 2022;400(10354):757–67.
Dickstein K, Cohen-Solal A, Filippatos G, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Eur J Heart Fail. 2008;10(10):933–89.
Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Eur J Heart Fail. 2016;18(8):891–975.
McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for diagnosing and treating acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–726.
Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: a Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18):e895–1032.
Vaduganathan M, Docherty KF, Claggett BL, et al. SGLT-2 inhibitors in patients with heart failure: a comprehensive meta-analysis of five randomised controlled trials. Lancet. 2022;400(10354):757–67.
Gallo G, Tocci G, Fogacci F, Battistoni A, Rubattu S, Volpe M. Blockade of the neurohormonal systems in heart failure with preserved ejection fraction: a contemporary meta-analysis. Int J Cardiol. 2020;316:172–9.
Basile C, Paolillo S, Gargiulo P, et al. Sacubitril/valsartan reduces cardiac decompensation in heart failure with preserved ejection fraction: a meta-analysis. J Cardiovasc Med (Hagerstown). 2023;24(1):44–51.
Savarese G, Stolfo D, Sinagra G, Lund LH. Heart failure with mid-range or mildly reduced ejection fraction. Nat Rev Cardiol. 2022;19(2):100–16.
Stolfo D, Fabris E, Lund LH, Savarese G, Sinagra G. From mid-range to mildly reduced ejection fraction heart failure: a call to treat. Eur J Intern Med. 2022;103:29–35.
Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation. 2018;138(9):861–70.
Pieske B, Tschöpe C, de Boer RA, et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J. 2019;40(40):3297–317.
Amanai S, Harada T, Kagami K, et al. The H2FPEF and HFA-PEFF algorithms for predicting exercise intolerance and abnormal hemodynamics in heart failure with preserved ejection fraction. Sci Rep. 2022;12(1):13.
Churchill TW, Li SX, Curreri L, et al. Evaluation of 2 existing diagnostic scores for heart failure with preserved ejection fraction against a comprehensively phenotyped cohort. Circulation. 2021;143(3):289–91.
Thibodeau JT, Drazner MH. The role of the clinical examination in patients with heart failure. JACC Heart Fail. 2018;6(7):543–51.
Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, Fang JC, Fedson SE, Fonarow GC, Hayek SS, Hernandez AF, Khazanie P, Kittleson MM, Lee CS, Link MS, Milano CA, Nnacheta LC, Sandhu AT, Stevenson W. AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18):e895–1032.
Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC, Investigators. ADHERE Scientific Advisory Committee and Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006;47(1):76–84.
Solomon SD, Rizkala AR, Lefkowitz MP, Shi VC, Gong J, Anavekar N, Anker SD, Arango JL, Arenas JL, Atar D, Ben-Gal T, Boytsov SA, Chen CH, Chopra VK, Cleland J, Comin-Colet J, Duengen HD, Echeverría Correa LE, Filippatos G, Flammer AJ, Galinier M, Godoy A. Baseline characteristics of patients with heart failure and preserved ejection fraction in the PARAGON-HF trial. Circ Heart Fail. 2018;11(7): e004962.
Drazner MH, Prasad A, Ayers C, Markham DW, Hastings J, Bhella PS, Shibata S, Levine BD. The relationship of right- and left-sided filling pressures in patients with heart failure and a preserved ejection fraction. Circ Heart Fail. 2010;3(2):202–6.
Jering K, Claggett B, Redfield MM, Shah SJ, Anand IS, Martinez F, Sabarwal SV, Seferović PM, Kerr Saraiva JF, Katova T, Lefkowitz MP, Pfeffer MA. Burden of heart failure signs and symptoms, prognosis, and response to therapy: the PARAGON-HF trial. JACC Heart Fail. 2021;9(5):386–97.
Pfeffer MA, Shah AM, Borlaug BA. Heart failure with preserved ejection fraction in perspective. Circ Res. 2019;124(11):1598–617.
Pieske B, Tschöpe C, de Boer RA, Fraser AG, Anker SD, Donal E, Edelmann F, Fu M, Guazzi M, Lam CSP, Lancellotti P, Melenovsky V, Morris DA, Nagel E, Pieske-Kraigher E, Ponikowski P, Solomon SD, Vasan RS, Rutten FH, Voors AA, Ruschitzka F, Paulus WJ, Sefer P. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J. 2019;40(40):3297–317.
Smiseth OA, Morris DA, Cardim N, et al. Multimodality imaging in patients with heart failure and preserved ejection fraction: an expert consensus document of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2022;23(2):e34–61.
Perrone-Filardi P, Paolillo S, Agostoni P, et al. Renin-angiotensin-aldosterone system inhibition in patients affected by heart failure: efficacy, mechanistic effects and practical use of sacubitril/valsartan. Position Paper of the Italian Society of Cardiology. Eur J Intern Med. 2022;102:8–16.
Elmadi J, Satish Kumar L, Pugalenthi LS, Ahmad M, Reddy S, Barkhane Z. Cardiovascular magnetic resonance imaging: a prospective modality in the diagnosis and prognostication of heart failure. Cureus. 2022;14(4): e23840.
Quarta G, Gori M, Iorio A, et al. Cardiac magnetic resonance in heart failure with preserved ejection fraction: myocyte, interstitium, microvascular, and metabolic abnormalities. Eur J Heart Fail. 2020;22(7):1065–75.
Del Torto A, Guaricci AI, Pomarico F, et al. Advances in multimodality cardiovascular imaging in the diagnosis of heart failure with preserved ejection fraction. Front Cardiovasc Med. 2022;9: 758975.
Gargiulo P, Acampa W, Asile G, et al. 123I-MIBG imaging in heart failure: impact of comorbidities on cardiac sympathetic innervation. Eur J Nucl Med Mol Imaging. 2023;50(3):813–24.
Seo M, Yamada T, Tamaki S, et al. Prognostic significance of cardiac 123I-MIBG SPECT imaging in heart failure patients with preserved ejection fraction. JACC Cardiovasc Imaging. 2022;15(4):655–68.
Volpe M, Gallo G, Rubattu S. Endocrine functions of the heart: from bench to bedside. Eur Heart J. 2023;44(8):643–55.
Goetze JP, Bruneau BG, Ramos HR, Ogawa T, de Bold MK, de Bold AJ. Cardiac natriuretic peptides. Nat Rev Cardiol. 2020;17(11):698–717.
Gallo G, Rubattu S, Autore C, Volpe M. Natriuretic peptides: it is time for guided therapeutic strategies based on their molecular mechanisms. Int J Mol Sci. 2023;24(6):5131.
Grewal J, McKelvie RS, Persson H, et al. Usefulness of N-terminal pro-brain natriuretic Peptide and brain natriuretic peptide to predict cardiovascular outcomes in patients with heart failure and preserved left ventricular ejection fraction. Am J Cardiol. 2008;102(6):733–7.
Dal Canto E, Scheffer M, Kortekaas K, Driessen-Waaijer A, Paulus WJ, van Heerebeek L. Natriuretic peptide levels and stages of left ventricular dysfunction in heart failure with preserved ejection fraction. Biomedicines. 2023;11(3):867.
Kasahara S, Sakata Y, Nochioka K, et al. Comparable prognostic impact of BNP levels among HFpEF, borderline HFpEF and HFrEF: a report from the CHART-2 Study. Heart Vessels. 2018;33(9):997–1007.
Anjan VY, Loftus TM, Burke MA, et al. Prevalence, clinical phenotype, and outcomes associated with normal B-type natriuretic peptide levels in heart failure with preserved ejection fraction. Am J Cardiol. 2012;110(6):870–6.
Remmelzwaal S, van Ballegooijen AJ, Schoonmade LJ, Dal Canto E, Handoko ML, Henkens MTHM, van Empel V, Heymans SRB, Beulens JWJ. Natriuretic peptides for the detection of diastolic dysfunction and heart failure with preserved ejection fraction-a systematic review and meta-analysis. BMC Med. 2020;18(1):290.
Hund PS, Anand IS, Komajda M, Claggett BL, McKelvie RS, Zile MR, Carson PE, McMurray JJ. Changes in N-terminal pro-B-type natriuretic peptide levels and outcomes in heart failure with preserved ejection fraction: an analysis of the I-Preserve study. Eur J Heart Fail. 2015;17(8):809–17.
Januzzi JL Jr, Butler J, Zannad F, Filippatos G, Ferreira JP, Pocock SJ, Sattar N, Verma S, Vedin O, Iwata T, Brueckmann M, Packer M, Anker SD, EMPEROR-Preserved Trial Study Group. Prognostic implications of N-Terminal Pro-B-type natriuretic peptide and high-sensitivity cardiac troponin T in EMPEROR-preserved. JACC Heart Fail. 2022;10(7):512–24.
Tanase DM, Radu S, Al Shurbaji S, et al. Natriuretic peptides in heart failure with preserved left ventricular ejection fraction: from molecular evidences to clinical implications. Int J Mol Sci. 2019;20(11):2629.
Verbrugge FH, Omote K, Reddy YNV, Sorimachi H, Obokata M, Borlaug BA. Heart failure with preserved ejection fraction in patients with normal natriuretic peptide levels is associated with increased morbidity and mortality. Eur Heart J. 2022;43(20):1941–51.
Messerli FH, Rimoldi SF, Bangalore S. The transition from hypertension to heart failure: contemporary update. JACC Heart Fail. 2017;5(8):543–51.
Inciardi RM, Galderisi M, Nistri S, Santoro C, Cicoira M, Rossi A. Echocardiographic advances in hypertrophic cardiomyopathy: three-dimensional and strain imaging echocardiography. Echocardiography. 2018;35(5):716–26.
Erma A, Meris A, Skali H, et al. Prognostic implications of left ventricular mass and geometry following myocardial infarction: the VALIANT (VALsartan In Acute myocardial iNfarcTion) Echocardiographic Study. JACC Cardiovasc Imaging. 2008;1(5):582–91.
Mancusi C, Lembo M, Manzi MV, Basile C, Fucile I, Morisco C. From structural to functional hypertension mediated target organ damage—a long way to heart failure with preserved ejection fraction. J Clin Med. 2022;11(18):5377.
Zile MR, Gottdiener JS, Hetzel SJ, et al. Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation. 2011;124(23):2491–501.
Tadic M, Cuspidi C, Marwick TH. Phenotyping the hypertensive heart. Eur Heart J. 2022;43(38):3794–810.
Guide, Nagueh SF. Diastology: 2020—a practical. Echocardiography. 2020;37(11):1919–25.
Arnone MI, Sforza A, Carlino MV, Guarino M, Candido R, Bertolone D, Fucile I, De Luca N, Mancusi C. Assessment of E/A ratio helps emergency clinicians in the management of patients with acute dyspnea. Intern Emerg Med. 2023;18:1823–30.
Galderisi M, Rapacciuolo A, Esposito R, et al. Site-dependency of the E/e’ ratio in predicting invasive left ventricular filling pressure in patients with suspected or ascertained coronary artery disease. Eur Heart J Cardiovasc Imaging. 2013;14(6):555–61.
Carlino MV, Paladino F, Sforza A, Serra C, Liccardi F, de Simone G, Mancusi C. Assessment of left atrial size in addition to focused cardiopulmonary ultrasound improves diagnostic accuracy of acute heart failure in the Emergency Department. Echocardiography. 2018;35(6):785–91.
Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29(4):277–314.
Sorrentino R, Esposito R, Santoro C, et al. Practical impact of new diastolic recommendations on noninvasive estimation of left ventricular diastolic function and filling pressures. J Am Soc Echocardiogr. 2020;33(2):171–81.
Schiano-Lomoriello V, Santoro C, de Simone G, Trimarco B, Galderisi M. Diastolic bicycle stress echocardiography: normal reference values in a middle age population. Int J Cardiol. 2015;91:181–3.
Contaldi C, Imbriaco M, Alcidi G, et al. Assessment of the relationships between left ventricular filling pressures and longitudinal dysfunction with myocardial fibrosis in uncomplicated hypertensive patients. Int J Cardiol. 2016;202:84–6.
Kraigher-Krainer E, Shah AM, Gupta DK, et al. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63(5):447–56.
Pastore MC, Mandoli GE, Aboumarie HS, et al. Basic and advanced echocardiography in advanced heart failure: an overview. Heart Fail Rev. 2020;25(6):937–48.
Morris DA, Ma XX, Belyavskiy E, et al. Left ventricular longitudinal systolic function analysed by 2D speckle-tracking echocardiography in heart failure with preserved ejection fraction: a meta-analysis. Open Heart. 2017;4(2): e000630.
D’Andrea A, Radmilovic J, Ballo P, et al. Left ventricular hypertrophy or storage disease? The incremental value of speckle tracking strain bull’s-eye. Echocardiography. 2017;34(5):746–59.
Galderisi M, Santoro C, Esposito R. Left atrial function and dyssynchrony: main characters and not actor appearances in heart failure with preserved ejection fraction. Int J Cardiol. 2018;257:222–3.
Lisi M, Mandoli GE, Cameli M, et al. Left atrial strain by speckle tracking predicts atrial fibrosis in patients undergoing heart transplantation. Eur Heart J Cardiovasc Imaging. 2022;23(6):829–35.
Cameli M, Sparla S, Losito M, et al. Correlation of left atrial strain and Doppler measurements with invasive measurement of left ventricular end-diastolic pressure in patients stratified for different values of ejection fraction. Echocardiography. 2016;33(3):398–405.
Inoue K, Khan FH, Remme EW, et al. Determinants of left atrial reservoir and pump strain and use of atrial strain for evaluation of left ventricular filling pressure. Eur Heart J Cardiovasc Imaging. 2021;23(1):61–70.
Obokata M, Negishi K, Kurosawa K, et al. Incremental diagnostic value of la strain with leg lifts in heart failure with preserved ejection fraction. JACC Cardiovasc Imaging. 2013;6(7):749–58.
Cioffi G, Battiston R, Mancusi C, Di Lenarda A, Faganello G, Aurigemma GP, Tarantini L, Pulignano G, Cioffi V, de Simone G. Prognostic stratification of clinically stable patients with heart failure by echocardiographic pressure/volume loop model. J Am Soc Echocardiogr. 2023;36(7):746–59.
Capone F, Sotomayor-Flores C, Bode D, Wang R, Rodolico D, Strocchi S, Schiattarella GG. Cardiac metabolism in HFpEF: from fuel to signalling. Cardiovasc Res. 2023;118(18):3556–75.
Takei Y, Tomiyama H, Higashi Y, Yamashina A, Chikamori T. Association between endothelial dysfunction and left ventricular diastolic stiffness—subanalysis of the flow-mediated dilation Japan (FMD-J) Study. Circ J. 2023;87(9):1203–11.
Ali D, Tran P, Ennis S, Powell R, McGuire S, McGregor G, Kimani PK, Weickert MO, Miller MA, Cappuccio FP, Banerjee P. Rising arterial stiffness with accumulating comorbidities associates with heart failure with preserved ejection fraction. ESC Heart Fail. 2023;10(4):2487–98.
Gargani L, Girerd N, Platz E, Pellicori P, Stankovic I, Palazzuoli A, Pivetta E, Miglioranza MH, Soliman-Aboumarie H, Agricola E, Volpicelli G, Price S, Donal E, Cosyns B, Neskovic AN. Lung ultrasound in acute and chronic heart failure. A Clinical Consensus Statement of the European Association of Cardiovascular Imaging (EACVI). Eur Heart J Cardiovasc Imaging. 2023;24:jead169.
Mancusi C, Serra C, Muiesan ML, Cogliati C. Lung ultrasound national report (LUNARE PROJECT) by the Italian Society of Internal Medicine (SIMI). Intern Emerg Med. 2023;18(2):685–8.
Demi L, Wolfram F, Klersy C, et al. New international guidelines and consensus on the use of lung ultrasound. J Ultrasound Med. 2023;42(2):309–44.
Sforza A, Mancusi C, Carlino MV, Buonauro A, Barozzi M, Romano G, Serra S, de Simone G. Diagnostic performance of multi-organ ultrasound with pocket-sized device in the management of acute dyspnea. Cardiovasc Ultrasound. 2017;15(1):16.
Sforza A, Carlino MV, Albano G, Arnone MI, De Stefano G, D’Amato A, De Pisapia F, De Simone G, Mancusi C. A challenging diagnosis of dyspnea: a case report of contralateral reexpansion pulmonary edema. Monaldi Arch Chest Dis. 2018;88(1):900.
Maw AM, Hassanin A, Ho PM, et al. Diagnostic accuracy of point-of-care lung ultrasonography and chest radiography in adults with symptoms suggestive of acute decompensated heart failure: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(3): e190703.
Cogliati C, Ceriani E, Gambassi G, et al. Phenotyping congestion in patients with acutely decompensated heart failure with preserved and reduced ejection fraction: the Decongestion duRing therapY for acute decOmpensated heart failure in HFpEF vs HFrEF-DRY-OFF STUDY. Eur J Intern Med. 2022;97:69–77.
Gargani L, Pugliese NR, Frassi F, et al. Prognostic value of lung ultrasound in patients hospitalized for heart disease irrespective of symptoms and ejection fraction. ESC Heart Fail. 2021;8(4):2660–9.
Kagami K, Obokata M, Harada T, et al. Incremental diagnostic value of post-exercise lung congestion in heart failure with preserved ejection fraction. Eur Heart J Cardiovasc Imaging. 2023;24(5):553–61.
Reddy YNV, Obokata M, Wiley B, et al. The haemodynamic basis of lung congestion during exercise in heart failure with preserved ejection fraction. Eur Heart J. 2019;40(45):3721–30.
Mancusi C, Carlino MV, Sforza A. Point-of-care ultrasound with pocket-size devices in emergency department. Echocardiography. 2019;36(9):1755–64.
Sforza A, Carlino MV, Guarino M, et al. Anterior vs lateral symmetric interstitial syndrome in the diagnosis of acute heart failure. Int J Cardiol. 2019;280:130–2.
Guazzi M, Wilhelm M, Halle M, et al. Exercise testing in heart failure with preserved ejection fraction: an appraisal through diagnosis, pathophysiology and therapy—a clinical consensus statement of the Heart Failure Association and European Association of Preventive Cardiology. Eur J Heart Fail. 2022;24(8):1327–45.
Agostoni P, Dumitrescu D. How to perform and report a cardiopulmonary exercise test in patients with chronic heart failure. Int J Cardiol. 2019;288:107–13.
Reddy YNV, Olson TP, Obokata M, Melenovsky V, Borlaug BA. Hemodynamic correlates and diagnostic role of cardiopulmonary exercise testing in heart failure with preserved ejection fraction. JACC Heart Fail. 2018;6(8):665–75.
Nayor M, Houstis NE, Namasivayam M, et al. Impaired exercise tolerance in heart failure with preserved ejection fraction: quantification of multiorgan system reserve capacity. JACC Heart Fail. 2020;8(8):605–17.
Corrà U, Agostoni PG, Anker SD, et al. Role of cardiopulmonary exercise testing in clinical stratification in heart failure. A position paper from the Committee on Exercise Physiology and Training of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2018;20(1):3–15.
Klaassen SHC, Liu LCY, Hummel YM, et al. Clinical and hemodynamic correlates and prognostic value of VE/VCO2 slope in patients with heart failure with preserved ejection fraction and pulmonary hypertension. J Card Fail. 2017;23(11):777–82.
Alenezi F, Covington TA, Mukherjee M, Mathai SC, Yu PB, Rajagopal S. Novel approaches to imaging the pulmonary vasculature and right heart. Circ Res. 2022;130(9):1445–65.
Del Punta L, De Biase N, Balletti A, et al. Arterial hypertension and cardiopulmonary function: the value of a combined cardiopulmonary and echocardiography stress test. High Blood Press Cardiovasc Prev. 2022;29(2):145–54.
Reddy YNV, Kaye DM, Handoko ML, et al. Diagnosis of heart failure with preserved ejection fraction among patients with unexplained dyspnea. JAMA Cardiol. 2022;7(9):891–9.
Borlaug BA, Sharma K, Shah SJ, Ho JE. Heart failure with preserved ejection fraction: JACC scientific statement. J Am Coll Cardiol. 2023;81(18):1810–34.
Kittleson MM, Panjrath GS, Amancherla K, Davis LL, Deswal A, Dixon DL, Januzzi JL Jr, Yancy CW. 2023 ACC expert consensus decision pathway on management of heart failure with preserved ejection fraction: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2023;81(18):1835–78.
Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3(5):588–95.
Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1):1801913.
Rosenkranz S, Preston IR. Right heart catheterisation: best practice and pitfalls in pulmonary hypertension. Eur Respir Rev. 2015;24(138):642–52.
Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43(38):3618–731.
Nair N. Invasive hemodynamics in heart failure with preserved ejection fraction: importance of detecting pulmonary vascular remodeling and right heart function. Heart Fail Clin. 2021;17(3):415–22.
Bilak JM, Alam U, Miller CA, McCann GP, Arnold JR, Kanagala P. Microvascular dysfunction in heart failure with preserved ejection fraction: pathophysiology, assessment prevalence and prognosis. Card Fail Rev. 2022;8: e24.
Prasad SB, Holland DJ, Atherton JJ. Diastolic stress echocardiography: from basic principles to clinical applications. Heart. 2018;104(21):1739–48.
Hsu S, Fang JC, Borlaug BA. Hemodynamics for the heart failure clinician: a state-of-the-art review. J Card Fail. 2022;28(1):133–48.
Jain CC, Borlaug BA. Performance and interpretation of invasive hemodynamic exercise testing. Chest. 2020;158(5):2119–29.
Eisman AS, Shah RV, Dhakal BP, et al. Pulmonary capillary wedge pressure patterns during exercise predict exercise capacity and incident heart failure. Circ Heart Fail. 2018;11(5): e004750.
Pandey A, Khera R, Park B, et al. Relative impairments in hemodynamic exercise reserve parameters in heart failure with preserved ejection fraction: a study-level pooled analysis. JACC Heart Fail. 2018;6(2):117–26.
Obokata M, Olson TP, Reddy YNV, Melenovsky V, Kane GC, Borlaug BA. Haemodynamics, dyspnoea, and pulmonary reserve in heart failure with preserved ejection fraction. Eur Heart J. 2018;39(30):2810–21.
Dorfs S, Zeh W, Hochholzer W, et al. Pulmonary capillary wedge pressure during exercise and long-term mortality in patients with suspected heart failure with preserved ejection fraction. Eur Heart J. 2014;35(44):3103–12.
Finet JE, Van Iterson EH, Wilson Tang WH. Invasive hemodynamic and metabolic evaluation of HFpEF. Curr Treat Options Cardiovasc Med. 2021;23(5):32.
Wolsk E, Bakkestrøm R, Thomsen JH, et al. The influence of age on hemodynamic parameters during rest and exercise in healthy individuals. JACC Heart Fail. 2017;5(5):337–46.
Naeije R, Vanderpool R, Dhakal BP, et al. Exercise-induced pulmonary hypertension: physiological basis and methodological concerns. Am J Respir Crit Care Med. 2013;187(6):576–83.
Ho JE, Zern EK, Wooster L, et al. Differential clinical profiles, exercise responses, and outcomes associated with existing HFpEF definitions. Circulation. 2019;140(5):353–65.
Parasuraman SK, Loudon BL, Lowery C, et al. Diastolic ventricular interaction in heart failure with preserved ejection fraction. J Am Heart Assoc. 2019;8(7): e010114.
Gorter TM, Obokata M, Reddy YNV, Melenovsky V, Borlaug BA. Exercise unmasks distinct pathophysiologic features in heart failure with preserved ejection fraction and pulmonary vascular disease. Eur Heart J. 2018;39(30):2825–35.
Lewis GD, Bossone E, Naeije R, et al. Pulmonary vascular hemodynamic response to exercise in cardiopulmonary diseases. Circulation. 2013;128(13):1470–9.
Dhakal BP, Malhotra R, Murphy RM, et al. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction: the role of abnormal peripheral oxygen extraction. Circ Heart Fail. 2015;8(2):286–94.
D’Alto M, Romeo E, Argiento P, et al. Clinical relevance of fluid challenge in patients evaluated for pulmonary hypertension. Chest. 2017;151(1):119–26.
Andersen MJ, Olson TP, Melenovsky V, Kane GC, Borlaug BA. Differential hemodynamic effects of exercise and volume expansion in people with and without heart failure. Circ Heart Fail. 2015;8(1):41–8.
van de Bovenkamp AA, Wijkstra N, Oosterveer FPT, et al. The value of passive leg raise during right heart catheterization in diagnosing heart failure with preserved ejection fraction. Circ Heart Fail. 2022;15(4): e008935.
Hummel YM, Liu LCY, Lam CSP, Fonseca-Munoz DF, Damman K, Rienstra M, van der Meer P, Rosenkranz S, van Veldhuisen DJ, Voors AA, Hoendermis ES. Echocardiographic estimation of left ventricular and pulmonary pressures in patients with heart failure and preserved ejection fraction: a study utilizing simultaneous echocardiography and invasive measurements. Eur J Heart Fail. 2017;19(12):1651–60.
Reddy YNV, Kaye DM, Handoko ML, van de Bovenkamp AA, Tedford RJ, Keck C, Andersen MJ, Sharma K, Trivedi RK, Carter RE, Obokata M, Verbrugge FH, Redfield MM, Borlaug BA. Diagnosis of heart failure with preserved ejection fraction among patients with unexplained dyspnea. JAMA Cardiol. 2022;7(9):891–9.
van Heerebeek L, Hamdani N, Handoko ML, Falcao-Pires I, Musters RJ, Kupreishvili K, Ijsselmuiden AJ, Schalkwijk CG, Bronzwaer JG, Diamant M, Borbély A, van der Velden J, Stienen GJ, Laarman GJ, Niessen HW, Paulus WJ. Diastolic stiffness of the failing diabetic heart: importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation. 2008;117(1):43–51.
Westermann D, Lindner D, Kasner M, Zietsch C, Savvatis K, Escher F, von Schlippenbach J, Skurk C, Steendijk P, Riad A, Poller W, Schultheiss HP, Tschöpe C. Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ Heart Fail. 2011;4(1):44–52.
Hahn VS, Yanek LR, Vaishnav J, Ying W, Vaidya D, Lee YZJ, Riley SJ, Subramanya V, Brown EE, Hopkins CD, Ononogbu S, Perzel Mandell K, Halushka MK, Steenbergen C Jr, Rosenberg AZ, Tedford RJ, Judge DP, Shah SJ, Russell SD, Kass DA, Sharma K. Endomyocardial biopsy characterization of heart failure with preserved ejection fraction and prevalence of cardiac amyloidosis. JACC Heart Fail. 2020;8(9):712–24.
Mohammed SF, Hussain S, Mirzoyev SA, Edwards WD, Maleszewski JJ, Redfield MM. Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation. 2015;131(6):550–9.
Peters AE, Tromp J, Shah SJ, Lam CSP, Lewis GD, Borlaug BA, Sharma K, Pandey A, Sweitzer NK, Kitzman DW, Mentz RJ. Phenomapping in heart failure with preserved ejection fraction: insights, limitations, and future directions. Cardiovasc Res. 2023;118(18):3403–15 (Erratum in: Cardiovasc Res. 2023 Jan 21).
Kosiborod MN, Abildstrøm SZ, Borlaug BA, et al. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N Engl J Med. 2023;389(12):1069–84.
Lincoff AM, Brown-Frandsen K, Colhoun HM, et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med. 2023;389(24):2221–32.
Sevre K, Rist A, Wachtell K, Devereux RB, Aurigemma GP, Smiseth OA, Kjeldsen SE, Julius S, Pitt B, Burnier M, Kreutz R, Oparil S, Mancia G, Zannad F. What is the current best drug treatment for hypertensive heart failure with preserved ejection fraction? Review of the totality of evidence. Am J Hypertens. 2023;37:hpad073.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
None to declare.
Funding
Open access funding provided by Università degli Studi di Napoli Federico II within the CRUI-CARE Agreement.None to declare. FM was supported by Fondazione Umberto Veronesi.
Ethical approval
Not applicable.
Availability of Data
Not applicable.
Author Contributions
Conceptualization, C.M. and C.B.; methodology, C.S.; validation, G.G., I.F. and S.F.; writing—original draft preparation, C.M.; writing—review and editing, S.P., L.M., F.M., P.A., N.L. and G.E.; visualization, P.G.; supervision, C.S.; All authors have read and agreed to the published version of the manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Mancusi, C., Basile, C., Spaccarotella, C. et al. Novel Strategies in Diagnosing Heart Failure with Preserved Ejection Fraction: A Comprehensive Literature Review. High Blood Press Cardiovasc Prev 31, 127–140 (2024). https://doi.org/10.1007/s40292-024-00629-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40292-024-00629-1