Abstract
Purpose
HtrA1, HtrA2, HtrA3 and HtrA4 appear to be involved in the development of pathologies such as cancer. This systematic review reports the results of a literature search performed to compare the expression of HtrA family genes and proteins in cancer versus non-cancer tissues and cell lines, assess relationships between HtrA expression and cancer clinical features in cancer, and analyse the molecular mechanism, by which HtrA family affects cancer.
Methods
The literature search was conducted according to the PRISMA statement among four databases (PubMed, Web of Science, Embase and Scopus).
Results
A total of 38 articles met the inclusion criteria and involved the expression of HtrA family members and concerned the effect of HtrA expression on cancer and metastasis development or on the factor that influences it. Additionally, 31 reports were retrieved manually. Most articles highlighted that HtrA1 and HtrA3 exhibited tumour suppressor activity, while HtrA2 was associated with tumour growth and metastasis. There were too few studies to clearly define the role of the HtrA4 protease in tumours.
Conclusion
Although the expression of serine proteases of the HtrA family was dependent on tumour type, stage and the presence of metastases, most articles indicated that HtrA1 and HtrA3 expression in tumours was downregulated compared with healthy tissue or cell lines. The expression of HtrA2 was completely study dependent. The limited number of studies on HtrA4 expression made it impossible to draw conclusions about differences in expression between healthy and tumour tissue. The conclusions drawn from the study suggest that HtrA1 and HtrA3 act as tumour suppressors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
HtrA1 and HtrA3 mRNA/protein expression is downregulated in most cancers and these proteins act as tumour suppressors, HtrA2 expression level depends on the tumour type and might be associated with tumour growth and metastasis progression, while HtrA4 expression and role in cancer is still unknown. |
HtrA family genes are involved in EMT-related processes, degradation of the anti-apoptotic protein XIAP, cytoskeletal dynamics, and EGFR/Akt, PI3K/Akt and TGF-β1 signalling pathways. |
HtrA1 and HtrA3 loss or decreased expression is associated with chemoresistance and decreased anticancer drugs cytotoxicity, while increased expression with chemosensitivity and increased cytotoxicity. |
1 Introduction
The HtrA (High temperature requirement A) family of serine proteases present in prokaryotic and eukaryotic organisms is composed of four proteins—HtrA1 (L56 or PRSS11), HtrA2/Omi, HtrA3 (PRSP) (with its long and short isoforms—HtrA3-L and HtrA3-S) and HtrA4 [1,2,3,4]. Overall, the proteins are involved in protein quality control, regulate many processes in the cell and play a role in the development of pathologies such as neurodegenerative disorders, arthritis and cancer [1, 2, 4].
The structure of HtrA1, HtrA3 and HtrA4 is similar, but the structure of HtrA2 differs from other proteases in the family [2, 3]. The proteins are composed of the N-terminal region, protease domain, postsynaptic density protein domain (PDZ-domain) (except HtrA3-S) and C-terminal region [1, 2]. N-terminal regions in HtrA1, HtrA3 and HtrA4 contain a signal peptide, insulin-like growth factor (IGF) binding domain and protease inhibitor motif. In contrast, the HtrA2 N-terminal region is almost removed through processing and contains a transmembrane domain [2, 3]. Protease domains are composed of the catalytic triad and are responsible for the proteolytic activity of proteins [2], while the PDZ-domain participates in protein-protein interactions [5].
HtrA1 gene is localised on chromosome 10q26.13 and has nine exons and three defined transcripts [6]. The protein has a cytoplasmic location in the tissue and plasma membrane subcellular location and is proposed to be both intracellular and secreted. HtrA2 gene is on chromosome 2p13.1, has eight exons, three defined transcripts (like HtrA1) and its protein is proposed to be localised in the mitochondria membrane [7]. HtrA3 is on chromosome 4p16.1 with ten exons and two transcripts, and its protein also has a cytoplasmic expression with additional extracellular positivity and is proposed to be secreted in vesicles [8]. HtrA4 is on chromosome 8p11.22, with 11 exons [9]. Although the protein is not well characterised, it shows high expression in the placenta and is proposed to be secreted.
HtrA1 is involved in caspase-dependent and independent apoptosis, anoikis, cellular processes associated with transforming growth factor-β (TGF-β) signalling and reorganisation of extracellular matrix (ECM) [2,3,4]. HtrA2 participates in the maintenance of mitochondria homeostasis, apoptosis and anoikis induction, protein quality control and under stressful conditions converts from a protective factor into a proapoptotic one [2, 3, 10]. HtrA3, such as HtrA1, is involved in TGF-β signalling, apoptosis and cleaving ECM proteins [2, 3, 11]. In turn, HtrA4 protease is associated with pregnancy, embryo implantation, trophoblast invasion, placenta morphogenesis and preeclampsia [2, 3, 12].
This systematic review reports the results of a literature search that was performed: (1) to compare the expression of four HtrA family genes and proteins in cancer versus non-cancer tissues and cell lines; (2) to assess relationships between HtrA family genes/proteins expression and cancer clinical features or survival in different cancers; and (3) to analyse the molecular mechanism, by which HtrA family genes/proteins affect cancer. According to the existing literature, this review summarises the role of HtrA family genes in various cancer types and indicates their potential as therapeutic biomarkers and prognostic factors.
2 Methods
The systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Supplementary Information Table S1—PRISMA checklist and Supplementary Information Table S2—PRISMA abstract checklist) [13].
2.1 Patients and Public Involvement
No patient was involved in the study.
2.2 Search Strategy
A literature search was conducted in PubMed, Web of Science, Embase and Scopus (up to 4 December 2023). The keywords were (HtrA) AND (cancer). No restriction was applied in terms of date or study design. A manual search was also conducted.
2.3 Study Selection
The studies were selected using the following criteria: (I) involve gene/protein expression of HtrA family members (HtrA1, HtrA2, HtrA3, HtrA4, bacterial HtrA) and (II) concern the effect of HtrA gene/protein expression on any cancer and metastasis development or on the factor that influences it. During the selection, studies were excluded if (I) the expression of HtrA genes/proteins was studied to determine another parameter (e.g., apoptosis, virulence) and not directly the importance of expression in tumours; (II) they concerned Helicobacter pyloria-induced bacterial inflammation not related to cancer; (III) they concerned complications of pregnancy and preeclampsia; and (IV) they discussed the effect of HtrA genes on a factor that did not directly affect cancer.
2.4 Data Extraction
Two authors (M.A.R. and K.K.) collected and selected data. Any disagreements were solved by discussion and consensus with the third author (W.M.S.). The information collected from the articles included: the first author’s name, publication year, cancer type, gene name, study type (in vitro, in vivo, ex vivo, in silico), obtained results and conclusions.
3 Results
3.1 Search Results
The flow diagram (Fig. 1) shows the selection of studies included in the systematic review. A total of 600 studies were found through the initial search of the four databases, namely PubMed, Web of Science, Embase and Scopus. After removing duplicates, 351 studies were excluded. Then 70 reviews, conference material, comments, notes, erratum and short surveys were excluded. When reviewing the titles and abstracts, 129 studies were further excluded because they did not match the inclusion criteria, and the last 50 relevant studies underwent full-text screening.
After the full-text screening, 12 studies were excluded for ineligibility because of failure to meet the inclusion criteria or because the data in the studies could not be retrieved. For two articles, the full text was not found [14, 15], six studies were not related to cancer [10, 16,17,18,19,20], a further three studies did not directly address the effect of HtrA in cancer [21,22,23] and one study referred to a different gene/protein than the one searched for [24]. The manual search retrieved 31 additional studies. Overall, 69 studies met the inclusion criteria and are reviewed below (Fig. 1).
3.2 Study Characteristics
The main characteristics and details about retrieved studies are detailed in Table 1.
Table 1 Summary information of included studies
3.3 HtrA Expression Impact on Cancer Development
3.3.1 Breast Tumours
Breast cancer is the most common cancer in women. Despite many studies on the diagnosis and treatment of the disease, the mortality rate is still high [25, 26]. This may be because breast cancer is a heterogeneous disease – exhibiting different clinical, histopathological, and molecular features [25]. The discovery of new molecular, prognostic and predictive markers could lead to the development of novel anti-cancer therapies and thus reduce the number of deaths associated with breast cancer.
Genes and proteins of the HtrA family could be potential markers for breast cancer (Table 2). It has been shown that in most cases of breast cancer, HtrA1 expression is reduced or completely lost, i.e. in human epidermal growth factor receptor 2 (Her2)-enriched subtypes of breast cancer [25, 27, 28]. However, Franco et al. revealed that in luminal subtypes of breast cancer HtrA1 expression was increased [25]. Differences in HtrA1 expression between molecular subtypes of breast cancer may result from different expressions of oestrogen, progesterone and HER2 receptors; however, to date this thesis has not been fully clarified. HtrA1 expression was positively associated with oestrogen and progesterone receptor expression and affected breast cancer risk in breast cancer gene 1 (BRCA1) mutation carriers [25, 29]. Downregulation of HtrA1 was related to higher tumour stage, proliferation index and metastasis [25, 28]. HtrA1 loss in sentinel node-positive breast cancer was associated with metastasis of non-sentinel ones [25]. Conversely, HtrA1 upregulation was associated with favourable overall survival (OS) and disease-free survival (DFS) [28]. The gene’s mechanism in breast cancer is not fully known, but it was suspected that HtrA1 downregulation affected epithelial–mesenchymal transition (EMT), and thus led to cancer development and invasiveness [25, 27]. Reduced expression of HtrA1 also resulted in ataxia telangiectasia mutated (ATM) gene and DNA damage response activation (while the gene’s overexpression counteracts the response), which could be a potential chemotherapeutic response mechanism [27]. HtrA1 promoter hypermethylation could also be a possible mechanism of cancer development [28].
HtrA3 expression, like HtrA1, was downregulated in breast cancer [26, 30]. Moreover, both HtrA1 and HtrA3 were assumed to be tumour stroma-specific markers in situ [31]. Although no correlation between the oestrogen receptor (ER) and progesterone receptor (PR) status and overall HtrA3 expression has been proven, ER- and PR-positive tumours showed lower HtrA3 expression [26]. The presence of lymphatic metastases was also associated with lower HtrA3 expression [26]. Wenta et al. revealed that HtrA3 could influence cytoskeletal dynamics in cancer cells as well as act as a co-chaperone which promoted cell death and affected carcinogenesis [32]. To investigate the mechanisms of action of HtrA3, the researchers carried out a detailed analysis of the function and activity of the protein domains. HtrA3 occurs in two isoforms—short and long—and it was demonstrated that these forms differ in function [32]. Moreover, the HtrA3 N-terminal domain was responsible for its increased activity and formation of complexes with actin, β-tubulin, vimentin and T-complex protein 1 subunit α (TCP1α) [32]. The short form of the protein lacking the N-terminus exhibits greater proteolytic activity, while the long form of the protein lacking the N-terminus leads to more efficient proliferation [32].
HtrA4, the most recently characterised of the HtrA family, may also be involved in tumourigenesis. Kummari et al. investigated the potential mechanisms of HtrA4 action and the functions of its component domains. They revealed that HtrA4 interacted with the X-linked inhibitor of apoptosis protein (XIAP) in apoptotic processes, although HtrA4 was less able to catalyse than other family proteins, like HtrA2 [33]. HtrA4 existed in both a trimeric form and as a monomer, but the trimeric form was more dominant and beneficial [33]. A study of the HtrA4 domains’ function has demonstrated that the gene’s N-terminal region was essential for oligomerisation, stability and formation of functional enzymes [33]. In turn, Wenta et al. focussed on investigating the function and mechanism of HtrA4 (both full-length and N-terminal deleted forms) in the context of chemotherapeutic-dependent cancer cell death [34]. They revealed that N-terminally deleted HtrA4 was more efficient in apoptosis stimulation than other forms [34]. Moreover, they suspected that increased cell death following drug treatment and with increased HtrA4 expression was due to degrading antiapoptotic protein XIAP and proteolysis of pro-caspase 7, cytoskeletal proteins, actin and β-tubulin [34]. In contrast, the role of HtrA2 in breast cancer has not yet been thoroughly described; however, it has been indicated that its expression in breast cancer may be downregulated [35].
3.3.2 Female Gynaecological Tumours
3.3.2.1 Endometrial Cancer
Among tumours of the female genitals, endometrial cancer and ovarian cancer can be mentioned. The mechanisms leading to the development of both types of cancer are not clear. Most of the risk factors associated with the development of endometrial cancer are associated with high levels of oestrogen, which has a mitogenic effect on endometrial tissue [36]. HtrA family proteins play an important role in cell death and signalling, thus it was suspected that they might also be involved in endometrial cancer development (summarised in Table 2).
Most studies have shown that HtrA1 was downregulated in endometrial cancer [37,38,39], except Singh et al. who did not prove differences between HtrA1 expression in tumour and control tissue [30]. Moreover, the downregulation of HtrA1 expression in endometrioid-type cell lines resulted in increased invasive potential [39].
Narkiewicz et al. demonstrated that HtrA2 was involved in malignancy development in endometrial cancer [38], in contrast, Singh et al. revealed no difference in HtrA2 expression between tumour and healthy tissue [30].
HtrA3 expression, like HtrA1, was reduced in tumour samples [30, 36,37,38]. Moreover, HtrA1 and HtrA3 expression was negatively correlated with grade [36, 37, 39]. Also, HtrA3 decreased gradually from the premenopausal to the post-menopausal group [38]. Lv et al. revealed also that hypoxic conditions reduced HtrA3 gene expression in endometrial cancer cell lines [36].
Narkiewicz et al. suspected that genes of the HtrA family act as tumour suppressors because the expression of HtrA1, HtrA2 and HtrA3 negatively correlates with levels of TGF-β, which at advanced stages of tumourigenesis stimulates tumour progression, invasion and metastasis [38].
3.3.2.2 Ovarian Cancer
Another group of cancers of the female reproductive system are ovarian tumours. Ovarian cancer is described as one of the most aggressive types of tumours in women.
Similar to endometrial cancer, HtrA1 expression in ovarian cancer was decreased in most studies [40, 41]. Moreover, patients with higher HtrA1 expression showed a higher response rate compared with patients with lower gene expression [42]. This may be partly explained by the fact that HtrA1 expression also correlates with ovarian cancer malignancy [40]. As with previous cancers, the mechanism of HtrA1 in ovarian cancer is not well understood. It has been shown that stable knockdown of HtrA1 resulted in anoikis resistance, increased cell survival as a result of forced activation of the epidermal growth factor receptor/protein kinase B (EGFR/AKT) pathway [43] and also upregulation of XIAP [44]. In contrast, ectopic expression decreased XIAP levels in ovarian cancer cell lines and promoted cell sensitivity to cisplatin [44]. Sensitivity to cisplatin was further investigated in a study by Chien et al. who demonstrated that reduced HtrA1 expression attenuated cisplatin- and paclitaxel-induced cytotoxicity in ovarian cancer [42]. The search for new signalling pathways involving HtrA1 has shown that tuberous sclerosis complex 2 (TSC2) was one of the substrates for HtrA1 [45]. The TSC2 gene product was part of the mammalian target of the rapamycin (mTOR) signalling pathway and was involved in the control of cell growth and division.
HtrA2 expression was only slightly downregulated or even expressed without difference with normal tissue [30, 40]. Yang et al. revealed that lower HtrA2 expression was correlated with cisplatin resistance [46].
HtrA3 expression was downregulated in ovarian cancer, repeating the trend seen in endometrial cancer [30, 40, 47]. Some studies highlight that HtrA3 expression, like HtrA1, correlated with ovarian cancer malignancy [40, 47]. Narkiewicz et al. demonstrated that it is the short HtrA3 isoform that plays a key role in maintaining cell homeostasis and in ovarian oncogenesis [40]. All information about the ovarian cancer is summarised in the Table 2.
3.3.3 Male urogenital tumours
3.3.3.1 Prostate cancer and testes cancer
Genes encoding serine proteases of the HtrA family are not well studied in the context of prostate and testicular cancer (Table 2). Most reports have resulted from additional analyses performed within other cancers. Conducted studies showed that HtrA1 was detectable in testicular and prostate cancer cell lines [30]. The expression of HtrA2 was increased in prostate cancer, but cells isolated from normal prostate, benign prostatic hyperplasia, normal testes and testicular cancer showed weak HtrA2 expression [30, 48]. Moreover, HtrA2 expression negatively correlated with cancer differentiation [48].
HtrA3 expression was downregulated in most prostate cancer cell lines compared with the control, in contrast, normal testes and testicular cancer cell lines showed positive HtrA3 mRNA expression [30]. It was also shown that HtrA3 promotes cell death and affects cancerogenesis by the influence on the cytoskeleton and also protease coded by the HtrA3 gene may function as a co-chaperone [32].
Table 2 Characteristics of the studies included in the systematic review, related to breast, endometrial, ovarian, prostate and testes cancers
3.3.4 Head and neck tumours
3.3.4.1 Oral carcinoma
Among head and neck cancers, Oral carcinoma was screened for HtrA family gene expression (summarised in the Table 3). Moriya et al. demonstrated that HtrA3 may contribute to oral squamous cell carcinoma (OSCC) [49]. HtrA3 mRNA and protein expression were observed only in invasive cancer cells, not in normal epithelium and premalignant lesions [49]. High HtrA3 expression was associated with decreased DFS and OS. Researchers indicated that HtrA3 protein expression was a prognostic indicator of OS in OSCC [49].
3.3.4.2 Head and neck squamous cell carcinoma
Chen et al. studied head and neck cancer, precisely head and neck squamous cell carcinoma, using both databases and in vitro culture of human cancer cell lines (Table 3). The researchers demonstrated that the expression of all four HtrAs was upregulated in HNSCC patients, but the in vitro results confirmed that only HtrA3 expression was upregulated at the mRNA level in HNSC cell lines [50]. HtrA1–4 in vitro expression in HNSCC was increased at the protein level. Moreover, genes’ upregulation was associated with patient age, TNM stage, clinical stage and TP53 mutation status. High HtrA1 and HtrA3 expression was also associated with shorter OS as opposed to HtrA2 and HtrA4 [50]. The pathways analysis revealed that HtrA-related genes were enriched in the immune response processes and cell apoptosis. It was confirmed by in vitro study, where HtrA3 knockdown inhibited cancer cell proliferation and promoted apoptosis [50]. The HtrA family gene expression is also correlated with immune cell infiltration. Chen et al. in their study proved that the expression of all HtrAs differ between normal and tumour tissue and HtrAs, especially HtrA3, have significant potential as the prognostic marker and therapeutic target in HNSC [50].
3.3.5 Digestive Tumours
3.3.5.1 Gastric Cancer and Stomach Cancer
Gastric cancer (GC) is a disease characterised by a multistage process influenced by many factors. Surgery remains the predominant treatment for GC, but advances in molecular biology and bio-immunotherapy are leading to increasingly precise methods of diagnosing and treating patients [51]. The studies conducted on the HtrA family genes, and their protein products have shown that the expression of these genes in gastric cancer differs from that in healthy tissue (Table 3).
Wu et al. demonstrated that HtrA1 is downregulated in gastric cancer [52]. Moreover, the median overall survival for patients with high/medium HtrA1 expression was twice that of those with low expression, also higher response rate in patients was associated with the gene’s upregulation [42, 53]. What is more, loss of HtrA1 expression induced chemoresistance development in patients with gastric cancer [42, 53].
In contrast to HtrA1, expression of HtrA2 and HtrA3 in gastric cancer was enhanced [54, 55]. In addition, HtrA3 expression was associated with tumour type, grade, stage and tumour protein p53 (TP53) status, but not with an abundance of innate immunocytes [55]. HtrA3 overexpression was more often seen in samples with higher grade, higher stage and wild-type T53 status [55].
3.3.5.2 Colorectal Cancer/Carcinoma, Rectal Carcinoma and Colon Cancer
Colorectal cancer (CRC) is among the top three most common cancers and due to detection at a late stage, is one of the more frequent cancers leading to death. Investigating the mechanisms of development of this cancer will allow more accurate development of diagnosis and treatment. It is hypothesised that as with the previously discussed cancers, genes and proteins of the HtrA family may play a significant role in the tumourigenesis of colorectal cancer (Table 3).
Bioinformatics analysis of signalling pathways and molecular complexes showed that HtrA1 was one of seven key nodes in the protein–protein interaction network in rectal cancer [56]. However, reports of HtrA1 expression changes in colorectal cancer are inconclusive. Altobelli et al. revealed that HtrA1 expression in patients’ CRC tissue was generally reduced, but immunostaining in the stromal compartment showed no difference between adenoma with high- or low-grade dysplasia and healthy tissue [57]. In turn, Zurawa-Janicka et al. demonstrated that HtrA1 mRNA expression was increased in CRC tissue (especially in primary lesions of metastasizing cancer) compared with healthy mucosa, but expression at the protein level was reduced [58]. Differences in HtrA1 expression at the mRNA versus protein level may be due to post-translational modifications occurring. However, a common result of the studies presented here is a reduced expression of HtrA1 protein in patient material. Furthermore, the study performed by Singh et al. on colorectal adenocarcinoma cell lines showed that HtrA1 mRNA expression was not detected in one of the analysed colorectal adenocarcinoma cell lines, WiDr, but was detected in the second cell line, SW480. However, the HtrA1 expression did not differ between the tumour and the healthy cell line [30]. The results obtained by the researchers should be repeated on other adenocarcinoma cell lines to draw clear conclusions. Thus, the exact trend of changes in expression between healthy and cancerous tissue is unclear; however, it favours more towards reduced HtrA1 protein expression in colorectal cancer.
Analysis of the function performed by HtrA1 in colorectal adenocarcinoma has shown that this serine protease was involved in cellular protein homeostasis, cell cycle regulation, the mechanism of DNA repair and annexin- and caspase-dependent apoptosis [59]. Thus, HtrA1 was potentially implicated in G2- and M-phase-related cell cycle checkpoints and was probably associated with later stages of carcinogenesis rather than the first stages of cancer formation [57, 59]. Annexin A3 (ANXA1) was reported as a novel substrate for HtrA1, confirming a role for HtrA1 related to apoptosis [59].
A certain fraction of colorectal cancers show resistance to cisplatin treatment, which is associated with therapeutic failures. Xiong et al. focussed on the role of HtrA1 in cisplatin (CDDP) resistance to colon cancer. They revealed that HtrA1 was upregulated in CRC cells incubated with CDDP [60]. In addition, both mRNA and protein HtrA1 expression was reduced in CDDP-resistant colon cancer cell lines [60]. The relationship was also confirmed by the stable knockdown of the gene, which resulted in chemoresistance against cisplatin [60]. In contrast, HtrA1 ectopic expression reduced CDDP resistance [60]. Researchers highlighted that cisplatin resistance induced by HtrA1 downregulation was due to an increase in XIAP expression and activation of the phosphatidylinositol 3‑kinase/protein kinase B (PI3K/Akt) pathway [60].
When comparing HtrA2 expression in cancer and healthy cells, the results were different for mRNA and protein expression. Singh et al. showed that HtrA2 mRNA expression was upregulated in both tested cell lines (SW480 and WiDr) compared with normal colon cells [30]. However, the study conducted by Zurawa-Janicka et al. revealed that the HtrA2 protein level was reduced in cancer tissue [58]. As with HtrA1 expression, we suspect that differences in the HtrA2 expression between mRNA and protein may be due to post-translational modifications.
Furthermore, it was demonstrated that reduced expression of both HtrA1 and HtrA2 correlated with poorer patient survival [58]. Whereas, HtrA1 overexpression was associated with poor prognosis, and HtrA2 overexpression with poor survival in patients with colon or rectum cancer [35]. Transcript-level screening confirmed that samples with microsatellite instability showed reduced levels of HtrA1 and HtrA2. The microsatellite instability provides a potential explanation for the changes in HtrA1 and HtrA2 levels, which occurred during the development of colorectal cancer [58].
As in breast cancer, HtrA1 and HtrA3 were prediction markers for tumour stroma-specific in situ in colorectal cancer [31]. Similar to HtrA1, HtrA3 (both long and short isoform) expression was not detected in WiDr cells, and expression did not differ in SW480 and normal colon cells [30]. Immunohistochemistry (IHC) staining confirmed the presence of HtrA3 expression in tissues of patients with CRC, but no difference was found at mRNA or protein level between the tumour and control tissue [58]. In peritumoral stroma of patients with CRC, HtrA3 level was associated with decreased overall survival [61]. Moreover, HtrA3 expression in peritumoral stroma of patients with cancer stage II was related to high-grade tumour budding, which is the type of EMT [61].
3.3.6 Hepatocellular Carcinoma, Pancreatic Cancer and Renal Cancer
Hepatocellular carcinoma (HCC) accounts for 80–90% of all cases of liver cancer [62]. Early detection of HCC makes it possible to perform treatments to cure but the lack of symptoms at an early stage makes it difficult to identify and diagnose the disease quickly [63]. In turn, patients in advanced stages of HCC show a poor prognosis [64]. Pancreatic cancer is a common cause related to cancer death. It is suspected to become the second most common cause of death by 2030 [65]. It shows a low (< 1 year from diagnosis) median survival which is the main reason for increased research on this cancer [65].
The expression of HtrA family genes and proteins has also been studied in liver and pancreatic tumours, due to strong links to apoptosis, migration, and proliferation (Table 3). It was shown that HtrA1 expression in hepatocellular carcinoma cells was downregulated [66, 67]. Reduced expression or loss of HtrA1 correlated with a higher grade of hepatocellular carcinoma and with venous invasion [66]. In contrast, patients with higher gene expression had a better survival rate [66]. However, the mechanism explaining these relationships has not been thoroughly explained. Bao et al. explained that HtrA1 overexpression enhanced chemosensitivity in hepatocellular carcinoma, which could reverse the multidrug resistance of those cells [67]. They suspected that it was possible due to targeting XIAP for degradation [67]. Moreover, HtrA1 overexpression correlated with poor prognosis in patients with pancreatic cancer, which indicated that in individuals, HtrA1 might be targeted for cancer therapy [35].
For HtrA2, the level of expression in liver tumours appeared to be study-dependent. Zeng et al. treated rats with a carcinogen, namely diethyl nitrosamine, and revealed that HtrA2 expression in carcinogen-treated hepatic tissues was much lower than in normal tissues [68]. However, Xu et al. demonstrated that HtrA2 was overexpressed in hepatocellular carcinoma cell lines than in normal L02 hepatocellular cells [69]. HtrA2 was also correlated with tumour size and differentiation, clinical stage and metastasis in lymph nodes [69]. Moreover, enhanced HtrA2 expression induced apoptosis in certain carcinoma cells, in the HepG2 cell line probably due to its serine protease activity (caspase-independent pathway), while in PLC indirectly by inhibitor of apoptosis protein (IAP) binding (caspase-dependent pathway), and in the case of Hep3B by both IAP-binding and serine protease activity [69]. The difference in the HtrA2 pro-apoptotic marker ability was caused by phosphoprotein enriched in diabetes/phosphoprotein enriched in astrocytes (ped/pea-15) expression level [69]. Zeng et al. performed the study to investigate the effect of the herbal drug Hu Qisan (HQS), used in traditional Chinese medicine, on apoptosis in HCC and revealed that the drug inhibited XIAP by promoting HtrA2 expression and release in HepG2 carcinoma cells, but protease release was correlated with the induction of caspase-3 activation, which is contrary to the results of Xu et al., who suggested that apoptosis in HepG2 occurs in a caspase-independent manner [68, 69]. Moreover, HQS caused an increase in both mRNA and protein levels of HtrA2 in HepG2 cells [68]. HtrA2 upregulation was also associated with poor survival in patients with pancreatic cancer and can serve as a prognostic biomarker (Table 3) [35].
HtrA3 emerged as a possible candidate for a tumour suppressor gene. In pancreatic cancer cells (Capan-1), ectopic HtrA3 expression reduced cell viability and increased expression of apoptotic protein Bcl-2-associated X protein (Bax) [65]. In turn, decreased HtrA3 expression was associated with carcinogenesis [65]. HtrA3 expression was also upregulated after treatment of pancreatic cancer cells (Capan-1) with paeoniflorin, a glycoside known for its anticancer properties. However, this trend was not evident in MIA PaCa-2 cells, suggesting that paeoniflorin treatment inhibited the growth of pancreatic cancer cells of different lineages through other mechanisms [65].
Due to the rather benign symptoms of renal cancer, it is often only diagnosed at the metastatic stage. However, Reza et al. revealed that HtrA1 overexpression was positively associated with poor prognosis in patients with renal cancer [35]. This demonstrated the potential for HtrA1 to be used in targeted therapy for individual cases of kidney cancer (Table 3).
Zurawa-Janicka et al. investigated the association of proteins encoded by HtrA family genes with oestrogen-induced acute oxidative stress response and nephrocarcinogenesis using an in vivo hamster model [70]. The study hypothesised that oestradiol treatment increases protein oxidation in the hamster kidney and also contributes to nephrocarcinogenesis [70]. They revealed that short oestrogen administration resulted in increased mRNA and protein levels of HtrA1, while prolonged oestrogenisation (6 months) decreased both (mRNA and protein) HtrA1 levels [70]. Meanwhile, HtrA2 expression did not increase until the third month of treatment [70]. The conclusion drawn from the study was that both HtrA1 and HtrA2 proteins were involved in defence against oxidative stress and decreased expression of HtrA1 together with increased expression of HtrA2 were associated with nephrocarcinogenesis in the hamster model (Table 3) [70].
Table 3 Characteristics of the studies, included in the systematic review, related to oral carcinoma, head and neck squamous cell carcinoma, gastric, colon, rectal hepatocellular, pancreatic, and renal cancers
3.3.7 Endocrine Tumours
3.3.7.1 Thyroid Cancer
Studies on the effects of serine proteases of the HtrA family on endocrine tumours are not widespread (Table 4). The only study found was of thyroid cancer [71]. The study proved that, as in the previously discussed cancers, HtrA1, HtrA2 and HtrA3 are associated with oncogenesis.
Expression of HtrA2 and both HtrA3 isoforms (HtrA3-S and HtrA3-L) was elevated in thyroid cancer, but only HtrA2 and HtrA3-S show higher expression in thyroid malignant tumours compared with normal tissues and benign tumours, which indicated that these genes were correlated with thyroid cancer malignancy [71]. HtrA3-L expression was increased in malignant tumours compared with benign tumours and control tissues from patients with benign lesions [71]. HtrA3-L was also elevated in normal tissues from patients with carcinoma compared with normal tissues from patients with benign lesions [71]. This demonstrates that different HtrA3 isoforms may have different roles in thyroid cancer development.
Differences in the expression of HtrA family genes have been demonstrated not only for different tumour stages but also by subtypes [71]. HtrA1 expression was increased in follicular thyroid carcinoma compared with papillary thyroid carcinoma, in contrast, HtrA3-S was upregulated in papillary thyroid carcinoma compared with follicular thyroid carcinoma [71]. The study also examined the TGF-β1 relative protein levels and demonstrated no differences between healthy controls and thyroid benign or malignant tumour tissues [71]. Also, no correlation was proved between TGF-β1 expression and HtrA family genes indicating that regulation of the TGF-β1 signalling pathway by HtrA proteases may be tissue-specific and play different roles in tumour development [71].
3.3.8 Thoracic Tumours
3.3.8.1 Lung Cancer, Mesothelioma and Esophageal Carcinoma
Lung cancer is still one of the most common and lethal cancers in Western countries [11, 72]. Despite much research into new methods of treatment and diagnosis, the prognosis for cancer remains poor. Current treatment is based on surgery, radiation, chemotherapy, targeted drugs and immunotherapy [11, 73]. Among the first-line chemotherapy-based treatments, cisplatin, carboplatin plus etoposide, or topoisomerase II inhibitor, with or without radiation, were used [73, 74]. However, the difficulty of diagnosis at the early stage of the disease, as well as the development of chemoresistance to drugs, means that most patients with lung cancer die during the first 5 years after diagnosis [11, 72, 73]. The need to further understand the mechanisms underlying tumourigenesis and the search for new markers has prompted the development of research into the utility of HtrA family genes and proteins in lung cancer (available information summarised in the Table 4).
HtrA1 expression was detected in all analysed human lung mesothelioma cell lines (Ist-Mes1, Ist-Mes2, MSTO-211H, MPP89 and NCI-H2452); however, different cell lines demonstrated diverse expression levels [75]. Moreover, in the metastatic lymph node, the HtrA1 expression level was equal or lower compared with the primary tumour [72]. Analysis performed on patients' material showed that HtrA1 expression positively correlated with survival in patients with lung cancer, in contrast, EGFR expression correlated negatively [75]. This was proven by the outcome in which the median survival in patients with high HtrA1 or low EGFR expression was longer than for those patients with low HtrA1 or high EGFR expression [75]. However, after grouping the samples according to EGFR expression and evaluating OS based on HtrA1 expression, researchers revealed that a low HtrA1 score was associated with shorter OS but independently from EGFR expression [75]. A similar relationship has been demonstrated for relative risk (RR), where high HtrA1 expression was related to lower RR than in patients with low HtrA1 expression [75].
Due to the high risk of developing chemoresistance in patients with lung cancer, Xu et al. conducted a study to link cisplatin resistance to HtrA1 expression. They revealed that HtrA1 expression, both mRNA and protein level, was reduced in cisplatin-resistant lung cancer cells (A549/CDDP) compared with cisplatin-sensitive cells (A549) [76]. Moreover, exogenous expression of HtrA1 in A549/CDDP cells reversed cancer stem cell‐like properties and chemoresistance, promoting chemosensitivity [76]. In turn, they analysed stable knockdown of HtrA1 and demonstrated that it promoted cisplatin resistance, cancer stem cell-like properties and tumourigenesis [76]. The effects of HtrA1 knockdown were blocked by inhibition of PI3K/Akt pathway using LY294002 (AKT inhibitor) in vivo model – nude mice [76]. This demonstrated that HtrA1 acts as an inhibitor towards EGFR through direct interaction, resulting in suppression of the PI3K/Akt signalling pathway and inhibition of tumour cell alteration towards a stem cell-like phenotype.
HtrA2 was downregulated in non-small-cell lung cancer (NSCLC) compared with adjacent non-cancerous [77]. Similarly, HtrA2 expression was low in poorly differentiated specimens compared with well-differentiated specimens [77]. Study outcomes demonstrated that gene expression was associated with histological differentiation and clinical stage, but not with the patient’s age or gender and histological type or size of tumour [77]. In the case of survival, low HtrA2 expression was a prognostic factor for patients with worse survival, while high expression was associated with better survival [77]. HtrA2 deletion was negatively correlated with apoptosis in non-small cell lung cancer cells [77].
HtrA3 expression was downregulated or lost in most of oatients with lung cancer and cell lines [74, 78]. Patients with high HtrA3 expression were at a lower risk of postoperative recurrence than patients with low or negative HtrA3 expression [78]. HtrA3 was also expressed in the bronchial cell line (BEAS-2B) [74]. Moreover, HtrA3 expression may also have differed depending on the isoform studied. HtrA3-L (long isoform) expression was lower in NSCLC cells than in normal lung cells, while HtrA3-S (short isoform) expression did not differ [78].
Similar to the previously discussed cancers, HtrA3 influenced cytoskeleton dynamics, formed complexes and partially co-localised with the anti-apoptotic protein XIAP [11, 32]. HtrA3 might also be a mitochondrial cell death effector [73]. Exogenous HtrA3 expression attenuated the invasion of NSCLC cells, while HtrA3 knockdown had a converse effect, which indicated the involvement of the protein in tumour invasion [78]. Despite differences in expression, isoforms may also differ in function [32]. However, Wenta et al. revealed that both HtrA3 isoforms stimulated drug-induced apoptotic death of lung cancer cells, through XIAP cleavage under etoposide-induced apoptosis conditions, degradation of vimentin filaments and modulation of microtubules [11].
It was demonstrated that the removal of N-terminal domains resulted in increased HtrA3 activity, which revealed that N-terminal regions were not essential for protease activity [11, 32]. Both isoforms with N-terminal deleted domains (ΔN-HtrA3L/S) formed complexes with actin, β-tubulin, vimentin and TCP1α and co-localized with the actin and vimentin filaments, microtubules and TCP1α [32]. Exogenous ΔN-HtrA3L/S promoted apoptosis of lung cancer cells treated with etoposide and decreased XIAP levels [11]. Although both ΔN-HtrA3L and -S cleaved the cytoskeleton proteins, promoted tubulin polymerization and displayed chaperone-like activity, the short isoform was more efficient in proteolysis and the long isoform in polymerisation [32].
Although no correlation was found between HtrA3 expression and patient gender or tumour grade, stage or histology, the high expression of HtrA3 correlated with longer DFS and OS in patients with lung cancer [74, 78, 79]. The DFS was longer in patients with low expression than in those with negative HtrA3 expression but with no significance due to the limited sample size [78].
As with HtrA1, the association of chemoresistance with HtrA3 expression was analysed. HtrA3 upregulation attenuated cell survival of lung cancer cells treated with etoposide and cisplatin (H157 and A549 lines), while HtrA3 downregulation or loss promoted cell survival and attenuated cytotoxicity in cancer cell lines (Hop62 and HCC827 lines) [73, 74]. Low HtrA3 expression in primary lung tumours strongly correlated with heavy smoking history [74]. Beleford et al. demonstrated that HtrA3 methylation induced by cigarette smoke contributed to chemoresistance in lung cancer [74].
Despite the downregulation in lung tumour tissues, HtrA3 had decreased expression in metastatic subtypes of lung cancer [79]. Although there were no differences in TGF-β1 expression between lung cancer samples with and without metastases, decreased HtrA3 expression (especially HtrA3-L) was associated with increased TGF-β1 expression in metastatic lung cancer [79]. Zhao et al. emphasised that TGF-β1 activation elevated c-Jun expression, which is directly bound with the HtrA3-L promoter and inhibited transcription [79]. In turn, HtrA3-L overexpression attenuated TGF-β1-mediated invasion-metastasis cascades, through SMAD2/3 activation and cancer cell sensitisation to anti-programmed death-ligand 1 (PD-L1) treatment [79]. They summed up, that in the early stages of carcinogenesis, HtrA3 overexpression inhibited TGF-β1 and tumour metastasis, while in the later stages, the HtrA3 role was weakened and TGF-β1 was able to promote EMT [79].
HtrA4 and its N-terminal-deleted variant promoted cancer cell chemo-induced apoptosis [34]. However, ΔN-HtrA4 was more efficient in the cell death stimulation [34]. HtrA4 inhibited the clonogenic potential, and motility of cancer cells and raised cell cycle arrest at the G2/M phase [34]. In contrast, downregulation reversed – increased survival, clonogenic potential and motility of lung cancer cells [34]. Wenta et al. emphasised that HtrA4 and ∆N-HtrA4 degraded anti-apoptotic protein XIAP and cytoskeletal proteins, like actin and β-tubulin and also affected cell death by degrading procaspase 7 [34].
Similar changes were observed in oesophageal squamous cell carcinoma (ESCC) as in other cancers. HtrA1 expression (both mRNA and protein) was lower in ESCC than in normal oesophageal epithelial tissues and cells [80]. HtrA1 was associated with tumour/node/metastases (TNM) staging and lymph node metastasis, but not with patients’ age, gender or tumour differentiation [80]. The survival rate was lower in patients with low HtrA1 expression compared with patients with high expression [80]. HtrA1 overexpression inhibited proliferation in vitro and in vivo, reduced invasion and induced apoptosis by the blockade of the nuclear factor kappa B (NF-κB) signalling pathway and downregulation of downstream target genes [80].
3.3.8.2 Allergy-Related Cancer
Renke et al. in their work highlighted the unclear relationship between allergy and the presence of cancers, including colorectal cancer, acute lymphoblastic leukaemia, lung cancer and breast cancer [81]. In their study, they demonstrated that HtrA family protein synthesis was increased in patients with IgE-dependent allergy, but not mastocytosis [81]. Furthermore, the levels of HtrA1, HtrA2 and HtrA3 proteins did not depend on the number of mast cells but on their activity [81]. Considering the functions that HtrA proteins play in tumours, the results obtained by the research group demonstrate the need for further studies to determine the link between HtrA expression and the occurrence of cancer in patients diagnosed with allergy (Table 4) [81].
3.3.9 Skin tumours
3.3.9.1 Melanoma
Melanoma is the most aggressive type of skin cancer [82]. Although melanoma in the early stages of the disease is curable, the prognosis if it metastasises is very poor [83]. Hence, it is becoming important to identify new markers involved in melanoma metastasis, such as the HtrA family proteins (summarised in the Table 4). In metastatic melanoma cell lines, the gene encoding the HtrA1 protein – PRSS11 –was downregulated [83]. In the human melanoma tissue array, primary melanomas showed higher expression than unrelated metastatic lesions [83]. HtrA1 overexpression inhibited proliferation and invasion of cells in vitro, as well as melanoma growth in vivo [83]. In contrast, HtrA2 overexpression was positively associated with poor survival in skin cancer [35].
3.3.10 Central Nervous System Tumours
3.3.10.1 Glioblastoma and Neuroblastoma
The two most common central nervous system tumours are glioblastoma and neuroblastoma. Neuroblastoma (NB) affects mostly young children because it is initiated in the embryo and is characterised by high morbidity and mortality [84]. Glioblastoma occurs in the adult brain and is the most common malignant brain tumour [84, 85].
The search for new markers for nervous system tumours is becoming crucial to diagnose patients more quickly and efficiently. One potential marker is HtrA1, whose expression in neuroblastoma samples varied according to stage [86]. D’Angelo et al. revealed that the HtrA1 expression level was high in samples with stages 1 and 2 and some samples with stage 4, but low in the samples with stage 3 and sometimes 4 [86]. They assumed that HtrA1 overexpression was correlated with cellular differentiation grade and stage. Moreover, all samples with high HtrA1 expression came from patients in continuous complete remission [86]. However, patients in the early stage and with HtrA1 low levels or loss of expression relapsed and had a poor outcome [86]. HtrA1 downregulation was detected in advanced tumours with undifferentiated histology which was associated with a poor prognosis for patients [86]. They also showed that a higher tumour stage was related to a lower score [86]. The HtrA1 was also highly expressed in ganglioneuroblastoma [86].
In contrast, Minchenko et al. investigated the relationship between the expression of insulin-like growth factor binding proteins, HtrA1 and endoplasmic reticulum to nucleus 1 (ERN1)-mediated signalling and hypoxia regulation in glioblastoma cell lines and revealed that ERN1 inhibition led to strong HtrA1 upregulation [87]. Information about the expression, and role of the HtrAs in the central nervous system tumours is summarised in the Table 4.
Haematolymphoid tumours:
3.3.11 Lymphoma
Lymphomas are heterogeneous, malignant tumours originating from the lymphatic system [23]. They are characterised by a varied clinical picture and response to treatment [88]. The prognosis for a patient with lymphoma depends, among others, on molecular features [88]. Wiita et al. speculated that the detection of proteolytic fragments associated with apoptosis might serve as an indicator for chemo-induced cell death [89]. Such an indicator would be useful when monitoring patients with different types of lymphoma – acute myeloid leukaemia (AML), non-Hodgkin lymphoma (NHL) of diffuse large B-cell lymphoma subtype, B-acute lymphoblastic leukaemia (ALL), and multiple myeloma evolved to plasma cell leukaemia (PCL) – after chemotherapy. They identified 153 peptides in post-chemotherapy patient plasma, including HtrA2 [89]. However, a few years earlier, Li et al. demonstrated that HtrA2 was only weakly expressed in small lymphocytic lymphoma/chronic lymphocytic leukaemia (SLL/CLL) and diffuse large B-cell lymphoma (DLBCL) [90]. Thus HtrA2 did not appear to play a significant role in the apoptosis of DLBCL and SLL/CLL [90].
These results demonstrated that HtrA2 cannot be unequivocally regarded as a marker of apoptosis for lymphomas and their response to treatment. Although HtrA2 was involved in apoptosis, further, more detailed studies are needed to prove the use of the protein as a marker for specific lymphoma subtypes.
Most of the studies presented showed that genes of the HtrA family, particularly HtrA1 and HtrA3, play a role in processes related to tumourigenesis and metastasis. Differences in expression of the genes studied between healthy and cancer tissue were demonstrated (Fig.2), noting that the level of expression may vary depending on the tumour subtype. It was also shown that, in some tumours, expression of HtrA family genes correlates with tumour grade and stage, as well as with response to chemotherapy and survival of patients (Table 4).
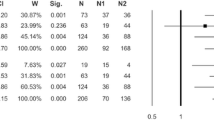
Expression status of HtrA family genes in different tumour types according to location. The ↓ sign indicates decreased expression in tumour tissue compared with expression in healthy tissue, the ↑ sign indicates increased expression in tumour compared with healthy tissue, and the Ø sign indicates no difference between expression in tumour and healthy tissue. Detailed information and references are given in Tables 2, 3 and 4.
Table 4 Characteristics of the studies, included in the systematic review, related to thyroid, lung and oesophageal cancers, melanoma, glioblastoma, neuroblastoma, haematolymphoid tumours and allergy-related cancer
3.4 Mechanism of Action
Understanding the mechanisms of the HtrA family genes and proteins functioning is fundamental for designing potential therapies for diseases related to their dysregulation. In turn, Cabrera et al. provided a mechanistic description of HtrA1 activation and regulation which will allow the design of therapies targeting defects associated with protease activity [91]. They demonstrated that the HtrA1 trimer was regulated by an allosteric mechanism in a PDZ-domain independent manner, in which monomer activation was associated with the signal from each other [91]. In the absence of communication between the monomers, inhibitor binding became impossible [91]. Moreover, it was demonstrated that HtrA1 trimerisation was fundamental for proteolytic activity (Fig. 3, Table 5) [91].
HtrA family proteins’ mechanisms of action. (A) HtrA1 monomers’ activation is necessary for trimerisation and is associated with signals from each other. Trimerisation is fundamental for proteolytic activity. (B) For HtrA2 the mechanism of action is not specified. (C) In HtrA3, inhibitory mAb blocks the catalytic site and stimulatory mAb binds with PDZ-domain. (D) For HtrA4 the mechanism of action is not specified. (E) Bacterial HtrA induces the kynurenine pathway directly by IDO1 stimulation and indirectly by induction of proinflammatory cytokines – IL1β and IL6.
Regulation of the proteases’ proteolytic activity is possible using monoclonal antibodies (mAbs) directed against the protease. Singh et al. analysed the effect of two antibodies against HtrA3 and showed that one of them had a blocking effect [92]. It blocked substrate access to the HtrA3 catalytic site [92]. The second one – with the stimulated effect – bound the PDZ domain (Fig. 3) [92]. Moreover, binding the inhibitory mAb increased cell migration and invasion in vitro which may translate into therapeutic applications in disease entities associated with HtrA3 dysregulation, like cancer (Table 5) [92].
Glaza et al. in their work presented the crystal structure of the HtrA3 protease and showed domain similarity between HtrA3, HtrA1 and HtrA2 [93]. Furthermore, they demonstrated that the PDZ domain was required for HtrA3 to form a trimer, but is not required for proteolytic activity [93]. They highlighted that HtrA3 had a unique combination of features among other HtrAs [93]. The mechanism of action for HtrA2 and HtrA4 has not yet been thoroughly elucidated and described in the literature (Fig. 3, Table 5).
Table 5 Characteristics of the studies, included in the systematic review, related to the HtrA mechanism of action in cancer
3.5 Bacterial HtrA
The HtrA protein family comprises serine proteases that are evolutionarily conserved. These peptides are homologues of the heat shock-induced high temperature requirement A/degradation of extracellular proteins (HtrA/DegP) protease originating from the bacterium Escherichia coli [2]. Eukaryotic proteins show a very similar domain organisation to prokaryotic proteins, exhibit chaperone activity and influence tumourigenesis [4]. For these reasons, their impact on gastric, colorectal and skin tumours is discussed in the article.
Helicobacter pylori infections are considered to be the risk factor (carcinogen class I) for the development and progression of gastric cancer [94,95,96]. H. pylori secrets HtrA protease, which interacts with E-calmodulin and E-cadherin – members of cell adhesion molecules – and provides the possibility of bacterial invasion [96, 97]. Protease activity of HtrA is also required for H. pylori to transmigrate and inject cytotoxin-associated gene A (CagA) – oncogenic protein [97].
Zawilak-Pawlik et al. revealed that deletion or mutation induction in HtrA was possible in one strain of H. pylori. In consequence, the catalytically active site in HtrA was destroyed, H. pylori exhibited reduced transmigration activity in human gastric adenocarcinoma cells and translocation of oncogenic protein CagA was decreased in colorectal adenocarcinoma cells [97].
Zhou et al. investigated the potentially beneficial effects of compounds isolated from Meehania fargesii plants on the virulence factor HtrA. Several of them demonstrated comparable docking capacity to standard drugs. The researchers emphasised that novel molecules capable of inhibiting HtrA proteases could become a potential treatment for H. pylori-induced gastric cancer [96].
Similar conclusions were reached by Mubarak Ali et al. who studied the effect of peptides from algae on virulence genes in H. pylori. A peptide derived from Tetradesmus sp. (green alga) had an inhibitory effect on the virulence factor HtrA, which presents another potential therapy to reduce the risk of gastric cancer caused by H. pylori infection [95].
In the case of melanoma, bacterial strain VNP20009 was an effective anti-cancer agent, which could target tumours and even inhibit their growth [98, 99]. To improve bacterial therapies, it was tested how knockout virulence-related genes (including HtrA) would interact with therapeutic strains and their anti-tumour abilities [98, 99].
Zhang et al. demonstrated that HtrA was required for bacterial virulence (survival within macrophages) and was essential for remodelling the tumour microenvironment [98]. In the case of immunoinfiltration, a bacterial strain lacking HtrA facilitated the migration of CD4+ T cells, macrophages and granulocytes into the tumour [98]. Moreover, tumour necrosis factor-α (TNF-α) and interleukin 1β (IL1β) were significantly downregulated in strains lacking HtrA than in those with gene [98]. The reduced anti-cancer abilities of strains lacking HtrA and two other genes (slyA and STM3120) were explained by the lack of possibility to activate the immune response [98]. The results highlighted that HtrA, slyA and STM3120 were vital for the anti-cancer ability of bacterial strains and mutants lacking HtrA had tumour-targeting abilities but reduced anti-cancer capacities [98].
In contrast, Xu et al. revealed that bacterial strain (VNP20009) lacking HtrA protein showed lower survival in macrophages so that was suspected to have therapeutic activity in cancer [99]. The strain also exhibited reduced colonisation in normal tissues and unchanged in tumour tissues [99]. This might indicate the potential for therapeutic use; however, the closer analysis indicated that strain lacking HtrA failed to suppress tumour growth [99]. Hence the conclusion that it only had a partial anticancer capacity [99].
For studies analysing the mechanism of action of HtrA, Clanchy et al. confirmed that the serine protease HtrA may be involved in processes leading to tumourigenesis [100]. They demonstrated that bacterial HtrA induced indoleamine-2,3-dioxygenase (IDO1), which is a component of the kynurenine pathway and a major checkpoint in tumourigenesis [100]. In addition, HtrA induced the expression of genes encoding the pro-inflammatory cytokines IL1β and interleukin 6 (IL6) (Fig. 3) [100], which demonstrates the involvement of HtrA in tumourigenesis (Table 5).
4 Discussion and Conclusion
The role of HtrA family genes and proteins has been described in many cancers. Most highlighted that HtrA1 and HtrA3 exhibited tumour suppressor activity [41, 55, 58, 79], while HtrA2 might be associated with tumour growth and metastasis [30, 58]. In the case of HtrA4, there were too few studies to clearly define the role of the protease in tumours.
The great strength of the conducted review is the general approach to the given subject. Despite the many reports, the review discusses the role of all four genes/proteins of the HtrA family – HtrA1, HtrA2, HtrA3 and HtrA4 – with a breakdown into 16 cancer types and a group of general studies explaining the mechanism of function of the genes.
Among the limitations of our review, it can be noted that despite the many studies on genes and proteins of the HtrA family, comparison of the results obtained is significantly hampered by differences in analysis methods, equipment used, reagents, the origin of the studied material, dissimilar detection methods, and a non-uniform system for concluding the studies obtained. The analysis methods employed in the cited studies only allow for comparing the results obtained within a given study, which makes it impossible to compare the results between studies. The results obtained by various research groups can be validated with data available in databases, such as The Cancer Genome Atlas (TCGA). However, this allows only the comparison of basic clinical data and results obtained at the molecular level.
4.1 HtrA Family Genes: Expression in Cancer
The present study highlights that the expression of genes and proteins from the HtrA family varies significantly depending on the type and subtype of cancer, the stage of the disease and the presence of metastases. Discrepancies in HtrA expression between types of tumours most likely result from distinct tissue structures and functions from which the analysed tumours originate. Diverse tumour origins can influence variations in active signalling pathways, leading to differential gene expression.
In most of the papers discussed, HtrA1 expression at both the mRNA and protein levels was downregulated compared with healthy tissues and cell lines [27, 37, 41]. The expression of HtrA2 was completely study-dependent, making it impossible to infer a relationship in its expression [30, 40, 48]. HtrA3 expression, like HtrA1, was downregulated in tumours in most papers [26, 30, 47], but we cited studies that detected elevated expression [49, 54]. The limited number of studies on HtrA4 expression makes it impossible to draw conclusions about differences in expression between healthy and tumour tissue.
4.2 HtrA Family Genes: Role in Cancer
The carried-out study strongly suggests a similar function of HtrA1 and HtrA3 in tumours. Both proteases act as tumour suppressors due to their downregulated expression in cancer and associations with tumour signalling [41, 55, 58, 79]. The cited articles make it very clear that the role of HtrA3 depends on the protein isoform studied – the long form of HtrA3 differs in function from the short form [32, 78]. Due to the large differences in reports depending on the study, the function of HtrA2 in tumours remains unexplained, although some reports indicated that HtrA2 functions differently from the other proteases in the family [68, 69]. It was pointed out that HtrA2 might be involved in tumour growth and progression of metastasis; however, the data obtained were contradictory [30, 58]. It is known that genes of the HtrA family are involved in signalling pathways, such as EGFR/Akt, PI3K/Akt, TGF-β1, and in processes related to EMT, degradation of the anti-apoptotic protein XIAP and cytoskeletal dynamics [25, 27, 32, 43, 44, 55, 76]. In addition, HtrA genes are associated with response to chemotherapy – loss or decreased gene expression was most commonly associated with chemoresistance and decreased cytotoxicity of anticancer drugs against tumour cells, while increased gene expression was associated with chemosensitivity and increased cytotoxicity [42, 73, 74]. Studies on bacterial HtrA have pointed out that it is a virulence gene, essential for the anticancer properties of bacteria used in cancer therapies [97,98,99].
4.3 Research Implications
The conclusions drawn from the study suggest that HtrA1 and HtrA3 act as tumour suppressors in most cancers; however, this is strongly dependent on the type of cancer. Their further analysis could lead to the design of new therapies targeting the modification of their expression in tumours, which could improve the response of patients with cancer to chemotherapy. As the expression of HtrA1 and HtrA3 is downregulated in most cancers, upregulation of these genes becomes a potential way to slow or inhibit cancer growth. Higher expression may lead to increased sensitivity of cancer cells to chemotherapy or even to their apoptosis. The use of HtrA family genes and proteins in cancer therapies as both prognostic biomarkers and therapeutic targets will only be possible once the mechanisms of action in cancer are well understood, the signalling pathways in which they are involved are thoroughly investigated and their substrates identified.
However, the current study does not clearly define the function of the HtrA family genes. To determine differences in HtrA gene expression between tumour and healthy tissue, a precise protocol specifying how the analyses were performed would be required so that the results could be compared with other analyses performed at other research centres following the same protocol. The information contained in the manuscript aims to summarise the current state of knowledge regarding the role of HtrA genes in cancer and to direct the researchers' attention towards a promising target for new anti-cancer therapies.
References
Oka C, Saleh R, Bessho Y, Reza HM. Interplay between HTRA1 and classical signalling pathways in organogenesis and diseases. Saudi J Biol Sci. 2022;29:1919–27. https://doi.org/10.1016/j.sjbs.2021.11.056.
Zurawa-Janicka D, Wenta T, Jarzab M, et al. Structural insights into the activation mechanisms of human HtrA serine proteases. Arch Biochem Biophys. 2017;621:6–23. https://doi.org/10.1016/j.abb.2017.04.004.
Wu L, Li X, Li Z, et al. HtrA serine proteases in cancers: a target of interest for cancer therapy. Biomed Pharmacother. 2021;139: 111603. https://doi.org/10.1016/j.biopha.2021.111603.
Skorko-Glonek J, Zurawa-Janicka D, Koper T, et al. HtrA protease family as therapeutic targets. Curr Pharm Des. 2013;19:977–1009. https://doi.org/10.2174/1381612811319060003.
Wang Y, Nie G. Overview of human HtrA family proteases and their distinctive physiological roles and unique involvement in diseases, especially cancer and pregnancy complications. Int J Mol Sci. 2021. https://doi.org/10.3390/ijms221910756.
HTRA1 HtrA serine peptidase 1 [Homo sapiens (human)] - Gene - NCBI. https://www.ncbi.nlm.nih.gov/gene/5654. Accessed 13 Dec 2023.
HTRA2 HtrA serine peptidase 2 [Homo sapiens (human)] - Gene - NCBI. https://www.ncbi.nlm.nih.gov/gene/27429. Accessed 13 Dec 2023.
HTRA3 HtrA serine peptidase 3 [Homo sapiens (human)] - Gene - NCBI. https://www.ncbi.nlm.nih.gov/gene/94031. Accessed 13 Dec 2023.
HTRA4 HtrA serine peptidase 4 [Homo sapiens (human)] - Gene - NCBI. https://www.ncbi.nlm.nih.gov/gene/203100. Accessed 13 Dec 2023
Cilenti L, Kyriazis GA, Soundarapandian MM, et al. Omi/HtrA2 protease mediates cisplatin-induced cell death in renal cells. Am J Physiol Renal Physiol. 2005;288:F371–9. https://doi.org/10.1152/ajprenal.00154.2004.
Wenta T, Rychlowski M, Jurewicz E, et al. The HtrA3 protease promotes drug-induced death of lung cancer cells by cleavage of the X-linked inhibitor of apoptosis protein (XIAP). FEBS J. 2019;286:4579–96. https://doi.org/10.1111/febs.14977.
Wang Y, Lim R, Nie G. Elevated circulating HtrA4 in preeclampsia may alter endothelial expression of senescence genes. Placenta. 2020;90:71–81. https://doi.org/10.1016/j.placenta.2019.12.012.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71. https://doi.org/10.1136/bmj.n71.
Adams AJ, Warram JM, Chaudhuri TR, Zinn KR. Fc receptors (FcR) on the surface of human breast cancer cells may facilitate the cytotoxic effects of hTRA-8, a new humanized apoptosis-inducing antibody. Cancer Res. 2006;66:1291.
Jarząb M, Wenta T, Glaza P, et al. HtrA proteins as possible targets in cancer therapy. Int J Mol Med. 2014;34:S28–S28.
Wysocka M, Wojtysiak A, Okońska M, et al. Design and synthesis of new substrates of HtrA2 protease. Anal Biochem. 2015;475:44–52. https://doi.org/10.1016/j.ab.2015.01.013.
Hartkamp J, Carpenter B, Roberts SGE. The Wilms’ tumor suppressor protein WT1 is processed by the serine protease HtrA2/Omi. Mol Cell. 2010;37:159–71. https://doi.org/10.1016/j.molcel.2009.12.023.
Neddermann M, Backert S. How many protein molecules are secreted by single Helicobacter pylori cells: quantification of serine protease HtrA. Cell Microbiol. 2019;21: e13022. https://doi.org/10.1111/cmi.13022.
Tegtmeyer N, Moodley Y, Yamaoka Y, et al. Characterisation of worldwide Helicobacter pylori strains reveals genetic conservation and essentiality of serine protease HtrA. Mol Microbiol. 2016;99:925–44. https://doi.org/10.1111/mmi.13276.
Liu D, Liu X, Wu Y, et al. Cloning and transcriptional activity of the mouse Omi/HtrA2 gene promoter. Int J Mol Sci. 2016. https://doi.org/10.3390/ijms17010119.
Yeh Y-C, Kuo H-Y, Chang W-L, et al. H. pylori isolates with amino acid sequence polymorphisms as presence of both HtrA-L171 & CagL-Y58/E59 increase the risk of gastric cancer. J Biomed Sci. 2019;26:4. https://doi.org/10.1186/s12929-019-0498-9.
Rai N, Muthukumaran R, Amutha R. Identification of inhibitor against H. pylori HtrA protease using structure-based virtual screening and molecular dynamics simulations approaches. Microb Pathog. 2018;118:365–77. https://doi.org/10.1016/j.micpath.2018.03.027.
Wang Z, Chen M, Fang X, et al. KIF15 is involved in development and progression of Burkitt lymphoma. Cancer Cell Int. 2021;21:261. https://doi.org/10.1186/s12935-021-01967-z.
Kim H, Chaudhuri TR, Buchsbaum DJ, et al. Single-photon emission computed tomography imaging with a humanized, Apoptosis-inducing antibody targeting human death receptor 5 in pancreas and breast tumor xenografts. Cancer Biol Ther. 2007;6:1392–8. https://doi.org/10.4161/cbt.6.9.4540.
Franco R, Collina F, Di Bonito M, et al. HtrA1 loss is related to aggressive behavior parameters in sentinel node positive breast cancer. Histol Histopathol. 2015;30:707–14. https://doi.org/10.14670/HH-30.707.
Yin Y, Wu M, Nie G, et al. HtrA3 is negatively correlated with lymph node metastasis in invasive ductal breast cancer. Tumour Biol J Int Soc Oncodevelopmental Biol Med. 2013;34:3611–7. https://doi.org/10.1007/s13277-013-0942-5.
Wang N, Eckert KA, Zomorrodi AR, et al. Down-regulation of HtrA1 activates the epithelial-mesenchymal transition and ATM DNA damage response pathways. PLoS ONE. 2012;7: e39446. https://doi.org/10.1371/journal.pone.0039446.
Lehner A, Magdolen V, Schuster T, et al. Downregulation of serine protease HTRA1 is associated with poor survival in breast cancer. PLoS ONE. 2013;8: e60359. https://doi.org/10.1371/journal.pone.0060359.
Neuhausen SL, Brummel S, Ding YC, et al. Genetic variation in IGF2 and HTRA1 and breast cancer risk among BRCA1 and BRCA2 carriers. Cancer Epidemiol biomarkers Prev a Publ Am Assoc Cancer Res cosponsored by Am Soc Prev Oncol. 2011;20:1690–702. https://doi.org/10.1158/1055-9965.EPI-10-1336.
Singh H, Li Y, Fuller PJ, et al. HtrA3 is downregulated in cancer cell lines and significantly reduced in primary serous and granulosa cell ovarian tumors. J Cancer. 2013;4:152–64. https://doi.org/10.7150/jca.5702.
Kiflemariam S, Ljungström V, Pontén F, Sjöblom T. Tumor vessel up-regulation of INSR revealed by single-cell expression analysis of the tyrosine kinome and phosphatome in human cancers. Am J Pathol. 2015;185:1600–9. https://doi.org/10.1016/j.ajpath.2015.02.019.
Wenta T, Zurawa-Janicka D, Rychlowski M, et al. HtrA3 is a cellular partner of cytoskeleton proteins and TCP1α chaperonin. J Proteom. 2018;177:88–111. https://doi.org/10.1016/j.jprot.2018.02.022.
Kummari R, Dutta S, Chaganti LK, Bose K. Discerning the mechanism of action of HtrA4: a serine protease implicated in the cell death pathway. Biochem J. 2019;476:1445–63. https://doi.org/10.1042/BCJ20190224.
Wenta T, Rychlowski M, Jarzab M, Lipinska B. HtrA4 protease promotes chemotherapeutic-dependent cancer cell death. Cells. 2019. https://doi.org/10.3390/cells8101112.
Reza A, Tasnim-E-tarik M, Al HR, et al. HTRA1 and HTRA2 expression differentially modulate the clinical prognosis of cancer: a multi-omics analysis using bioinformatics approaches. J Adv Biotechnol Exp Ther. 2022;5:358–80. https://doi.org/10.5455/jabet.2022.d121.
Lv Q, Yang B, Ning C, et al. Hypoxia is involved in the reduction of HtrA3 in patients with endometrial hyperplasia and cancer. Biochem Biophys Res Commun. 2018;503:2918–23. https://doi.org/10.1016/j.bbrc.2018.08.070.
Bowden MA, Di Nezza-Cossens LA, Jobling T, et al. Serine proteases HTRA1 and HTRA3 are down-regulated with increasing grades of human endometrial cancer. Gynecol Oncol. 2006;103:253–60. https://doi.org/10.1016/j.ygyno.2006.03.006.
Narkiewicz J, Lapinska-Szumczyk S, Zurawa-Janicka D, et al. Expression of human HtrA1, HtrA2, HtrA3 and TGF-beta1 genes in primary endometrial cancer. Oncol Rep. 2009;21:1529–37. https://doi.org/10.3892/or_00000385.
Mullany SA, Moslemi-Kebria M, Rattan R, et al. Expression and functional significance of HtrA1 loss in endometrial cancer. Clin Cancer Res. 2011;17:427–36. https://doi.org/10.1158/1078-0432.CCR-09-3069.
Narkiewicz J, Klasa-Mazurkiewicz D, Zurawa-Janicka D, et al. Changes in mRNA and protein levels of human HtrA1, HtrA2 and HtrA3 in ovarian cancer. Clin Biochem. 2008;41:561–9. https://doi.org/10.1016/j.clinbiochem.2008.01.004.
Chien J, Staub J, Hu S-I, et al. A candidate tumor suppressor HtrA1 is downregulated in ovarian cancer. Oncogene. 2004;23:1636–44. https://doi.org/10.1038/sj.onc.1207271.
Chien J, Aletti G, Baldi A, et al. Serine protease HtrA1 modulates chemotherapy-induced cytotoxicity. J Clin Invest. 2006;116:1994–2004. https://doi.org/10.1172/JCI27698.
He X, Ota T, Liu P, et al. Downregulation of HtrA1 promotes resistance to anoikis and peritoneal dissemination of ovarian cancer cells. Cancer Res. 2010;70:3109–18. https://doi.org/10.1158/0008-5472.CAN-09-3557.
He X, Khurana A, Maguire JL, et al. HtrA1 sensitizes ovarian cancer cells to cisplatin-induced cytotoxicity by targeting XIAP for degradation. Int J cancer. 2012;130:1029–35. https://doi.org/10.1002/ijc.26044.
Campioni M, Severino A, Manente L, et al. The serine protease HtrA1 specifically interacts and degrades the tuberous sclerosis complex 2 protein. Mol Cancer Res. 2010;8:1248–60. https://doi.org/10.1158/1541-7786.MCR-09-0473.
Yang X, Xing H, Gao Q, et al. Regulation of HtrA2/Omi by X-linked inhibitor of apoptosis protein in chemoresistance in human ovarian cancer cells. Gynecol Oncol. 2005;97:413–21. https://doi.org/10.1016/j.ygyno.2004.12.055.
Zhao M, Ding JX, Nie GY, et al. HTRA3 is reduced in ovarian cancers regardless of stage. Cancer Invest. 2014;32:464–9. https://doi.org/10.3109/07357907.2014.958496.
Hu X-Y, Xu Y-M, Chen XC, et al. Immunohistochemical analysis of Omi/HtrA2 expression in prostate cancer and benign prostatic hyperplasia. APMIS. 2006;114:893–8. https://doi.org/10.1111/j.1600-0463.2006.apm_271.x.
Moriya Y, Uzawa N, Morita T, et al. The high-temperature requirement factor A3 (HtrA3) is associated with acquisition of the invasive phenotype in oral squamous cell carcinoma cells. Oral Oncol. 2015;51:84–9. https://doi.org/10.1016/j.oraloncology.2014.10.001.
Chen Y, Yang J, Jin H, et al. HtrA3: a promising prognostic biomarker and therapeutic target for head and neck squamous cell carcinoma. PeerJ. 2023;11: e16237. https://doi.org/10.7717/peerj.16237.
Huang X, Ma Z, Qin W. Screening and bioinformatics analyses of key miRNAs Associated with Toll-like Receptor Activation in Gastric Cancer Cells. Medicina (Kaunas). 2023. https://doi.org/10.3390/medicina59030511.
Wu H-X, Tong S-L, Wu C, Wang W-X. HTRA1 gene expression in gastric epithelial cells. Asian Pac J Trop Med. 2014;7:765–71. https://doi.org/10.1016/S1995-7645(14)60133-4.
Catalano V, Mellone P, d’Avino A, et al. HtrA1, a potential predictor of response to cisplatin-based combination chemotherapy in gastric cancer. Histopathology. 2011;58:669–78. https://doi.org/10.1111/j.1365-2559.2011.03818.x.
Lee SH, Lee JW, Kim HS, et al. Immunohistochemical analysis of Omi/HtrA2 expression in stomach cancer. APMIS. 2003;111:586–90. https://doi.org/10.1034/j.1600-0463.2003.1110508.x.
Ji C, Sun L-S, Xing F, et al. HTRA3 is a prognostic biomarker and associated with immune infiltrates in gastric cancer. Front Oncol. 2020;10: 603480. https://doi.org/10.3389/fonc.2020.603480.
Teng W, Zhou C, Li Y. Exploring genes of rectal cancer for new treatments based on protein interaction network. bioRxiv. 2016. https://doi.org/10.1101/037531.
Altobelli E, Latella G, Morroni M, et al. Low HtrA1 expression in patients with long-standing ulcerative colitis and colorectal cancer. Oncol Rep. 2017;38:418–26. https://doi.org/10.3892/or.2017.5700.
Zurawa-Janicka D, Kobiela J, Slebioda T, et al. Expression of HTRA genes and its association with microsatellite instability and survival of patients with colorectal cancer. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21113947.
Schillinger J, Severin K, Kaschani F, et al. HTRA1-dependent cell cycle proteomics. J Proteome Res. 2018;17:2679–94. https://doi.org/10.1021/acs.jproteome.8b00129.
Xiong Z, Fu Z, Shi J, et al. HtrA1 Down-regulation induces cisplatin resistance in colon cancer by increasing XIAP and activating PI3K/Akt pathway. Ann Clin Lab Sci. 2017;47:264–70.
Forse CL, Rahimi M, Diamandis EP, et al. HtrA3 stromal expression is correlated with tumor budding in stage II colorectal cancer. Exp Mol Pathol. 2017;103:94–100. https://doi.org/10.1016/j.yexmp.2017.07.002.
Lokhandwala T, Aly A, Farrelly E, et al. Management of hepatocellular carcinoma from diagnosis in routine clinical practice. Hepatic Oncol. 2022;9:HEP45. https://doi.org/10.2217/hep-2021-0011.
Hepatocellular Carcinoma - Symptoms, Causes, Treatment | NORD. https://rarediseases.org/rare-diseases/hepatocellular-carcinoma/. Accessed 14 May 2023
Zhang X, Zhuge J, Liu J, et al. Prognostic signatures of sphingolipids: Understanding the immune landscape and predictive role in immunotherapy response and outcomes of hepatocellular carcinoma. Front Immunol. 2023;14:1153423. https://doi.org/10.3389/fimmu.2023.1153423.
Li Y, Gong L, Qi R, et al. Paeoniflorin suppresses pancreatic cancer cell growth by upregulating HTRA3 expression. Drug Des Devel Ther. 2017;11:2481–91. https://doi.org/10.2147/DDDT.S134518.
Zhu F, Jin L, Luo T-P, et al. Serine protease HtrA1 expression in human hepatocellular carcinoma. Hepatobil Pancreat Dis Int. 2010;9:508–12.
Bao W, Zhu F, Duan Y, et al. HtrA1 resensitizes multidrug-resistant hepatocellular carcinoma cells by targeting XIAP. Biomed Pharmacother. 2015;70:97–102. https://doi.org/10.1016/j.biopha.2014.12.044.
Zeng X, Li X, Xue X, et al. Activation of apoptosis in hepatocellular carcinoma by the Chinese traditional medicine Hu Qisan. Exp Ther Med. 2013;5:695–700. https://doi.org/10.3892/etm.2012.862.
Xu Z, Chen Y, Xu G, et al. Omi/HtrA2 pro-apoptotic marker differs in various hepatocellular carcinoma cell lines owing to ped/pea-15 expression level. Oncol Rep. 2015;33:905–12. https://doi.org/10.3892/or.2014.3656.
Zurawa-Janicka D, Kobiela J, Stefaniak T, et al. Changes in expression of serine proteases HtrA1 and HtrA2 during estrogen-induced oxidative stress and nephrocarcinogenesis in male Syrian hamster. Acta Biochim Pol. 2008;55:9–19.
Zurawa-Janicka D, Kobiela J, Galczynska N, et al. Changes in expression of human serine protease HtrA1, HtrA2 and HtrA3 genes in benign and malignant thyroid tumors. Oncol Rep. 2012;28:1838–44. https://doi.org/10.3892/or.2012.1988.
Esposito V, Campioni M, De Luca A, et al. Analysis of HtrA1 serine protease expression in human lung cancer. Anticancer Res. 2006;26:3455–9.
Beleford D, Rattan R, Chien J, Shridhar V. High temperature requirement A3 (HtrA3) promotes etoposide- and cisplatin-induced cytotoxicity in lung cancer cell lines. J Biol Chem. 2010;285:12011–27. https://doi.org/10.1074/jbc.M109.097790.
Beleford D, Liu Z, Rattan R, et al. Methylation induced gene silencing of HtrA3 in smoking-related lung cancer. Clin Cancer Res an Off J Am Assoc Cancer Res. 2010;16:398–409. https://doi.org/10.1158/1078-0432.CCR-09-1677.
Baldi A, Mottolese M, Vincenzi B, et al. The serine protease HtrA1 is a novel prognostic factor for human mesothelioma. Pharmacogenomics. 2008;9:1069–77. https://doi.org/10.2217/14622416.9.8.1069.
Xu Y, Jiang Z, Zhang Z, et al. HtrA1 downregulation induces cisplatin resistance in lung adenocarcinoma by promoting cancer stem cell-like properties. J Cell Biochem. 2014;115:1112–21. https://doi.org/10.1002/jcb.24751.
Mao G, Lv L, Liu Y, et al. The expression levels and prognostic value of high temperature required A2 (HtrA2) in NSCLC. Pathol Res Pract. 2014;210:939–43. https://doi.org/10.1016/j.prp.2014.06.030.
Zhao J, Zhang J, Zhang X, et al. High temperature requirement A3 (HTRA3) expression predicts postoperative recurrence and survival in patients with non-small-cell lung cancer. Oncotarget. 2016;7:40725–34. https://doi.org/10.18632/oncotarget.9173.
Zhao J, Feng M, Liu D, et al. Antagonism between HTRA3 and TGFβ1 contributes to metastasis in non-small cell lung cancer. Cancer Res. 2019;79:2853–64. https://doi.org/10.1158/0008-5472.CAN-18-2507.
Xia J, Wang F, Wang L, Fan Q. Elevated serine protease HtrA1 inhibits cell proliferation, reduces invasion, and induces apoptosis in esophageal squamous cell carcinoma by blocking the nuclear factor-κB signaling pathway. Tumour Biol J Int Soc Oncodevelopmental Biol Med. 2013;34:317–28. https://doi.org/10.1007/s13277-012-0553-6.
Renke J, Wasilewska E, Kędzierska-Mieszkowska S, et al. Tumor suppressors-HTRA proteases and interleukin-12-in pediatric asthma and allergic rhinitis Patients. Medicina (Kaunas). 2020. https://doi.org/10.3390/medicina56060298.
Gelsleichter NE, de Souza PO, Teixeira FC, et al. Metastatic melanoma: a preclinical model standardization and development of a chitosan-coated nanoemulsion containing temozolomide to treat brain metastasis. Cell Mol Neurobiol. 2023. https://doi.org/10.1007/s10571-023-01338-4.
Baldi A, De Luca A, Morini M, et al. The HtrA1 serine protease is down-regulated during human melanoma progression and represses growth of metastatic melanoma cells. Oncogene. 2002;21:6684–8. https://doi.org/10.1038/sj.onc.1205911.
de Weille J. On the genesis of neuroblastoma and glioma. Int J Brain Sci. 2014;2014: 217503. https://doi.org/10.1155/2014/217503.
Rosén E, Mangukiya HB, Elfineh L, et al. Inference of glioblastoma migration and proliferation rates using single time-point images. Commun Biol. 2023;6:402. https://doi.org/10.1038/s42003-023-04750-0.
D’Angelo V, Pecoraro G, Indolfi P, et al. Expression and localization of serine protease Htra1 in neuroblastoma: correlation with cellular differentiation grade. J Neurooncol. 2014;117:287–94. https://doi.org/10.1007/s11060-014-1387-4.
Minchenko DO, Kharkova AP, Karbovskyi LL, Minchenko OH. Expression of insulin-like growth factor binding protein genes and its hypoxic regulation in U87 glioma cells depends on ERN1 mediated signaling pathway of endoplasmic reticulum stress. Endocr Regul. 2015;49:73–83. https://doi.org/10.4149/endo_2015_02_73.
Jiang M, Bennani NN, Feldman AL. Lymphoma classification update: T-cell lymphomas, Hodgkin lymphomas, and histiocytic/dendritic cell neoplasms. Expert Rev Hematol. 2017;10:239–49. https://doi.org/10.1080/17474086.2017.1281122.
Wiita AP, Hsu GW, Lu CM, et al. Circulating proteolytic signatures of chemotherapy-induced cell death in humans discovered by N-terminal labeling. Proc Natl Acad Sci U S A. 2014;111:7594–9. https://doi.org/10.1073/pnas.1405987111.
Li S, Wan M, Cao X, Ren Y. Expression of AIF and HtrA2/Omi in small lymphocytic lymphoma and diffuse large B-cell lymphoma. Arch Pathol Lab Med. 2011;135:903–8. https://doi.org/10.5858/2010-0003-OAR1.1.
Cabrera AC, Melo E, Roth D, et al. HtrA1 activation is driven by an allosteric mechanism of inter-monomer communication. Sci Rep. 2017;7:14804. https://doi.org/10.1038/s41598-017-14208-z.
Singh H, Nero TL, Wang Y, et al. Activity-modulating monoclonal antibodies to the human serine protease HtrA3 provide novel insights into regulating HtrA proteolytic activities. PLoS ONE. 2014;9: e108235. https://doi.org/10.1371/journal.pone.0108235.
Glaza P, Osipiuk J, Wenta T, et al. Structural and functional analysis of human HtrA3 protease and its subdomains. PLoS ONE. 2015;10: e0131142. https://doi.org/10.1371/journal.pone.0131142.
de Lima Silva LL, Oliveira AKS, Gama AR, et al. Helicobacter pylori virulence dupA gene: risk factor or protective factor? Brazil J Microbiol. 2021;52:1921–7. https://doi.org/10.1007/s42770-021-00553-9.
MubarakAli D, Akshaya T, Sathya R, Irfan N. Study on the interaction of algal peptides on virulence factors of Helicobacter pylori: In silico approach. Appl Biochem Biotechnol. 2022;194:37–53. https://doi.org/10.1007/s12010-021-03716-4.
Zhou G, Song C, Liu X, et al. Insight into the potential of Meehania fargesii var. radicans against Hp-induced gastric carcinoma based on phytochemical and molecular docking studies. Chem Biodivers. 2022;19:e202200383. https://doi.org/10.1002/cbdv.202200383.
Zawilak-Pawlik A, Zarzecka U, Żyła-Uklejewicz D, et al. Establishment of serine protease htrA mutants in Helicobacter pylori is associated with secA mutations. Sci Rep. 2019;9:11794. https://doi.org/10.1038/s41598-019-48030-6.
Zhang X, Xu Q, Yang L, et al. The genes slyA, STM3120 and htrA are required for the anticancer ability of VNP20009. Oncotarget. 2016;7:81187–96. https://doi.org/10.18632/oncotarget.13217.
Xu W, Zhou T, Zhou J, et al. Attenuated Salmonella VNP20009 mutant (ΔhtrA) is a promising candidate for bacteria-mediated tumour therapy in hosts with TNFR1 deficiency. Lett Appl Microbiol. 2018;67:97–103. https://doi.org/10.1111/lam.12999.
Clanchy FIL, Huang Y-S, Ogbechi J, et al. Induction of IDO1 and kynurenine by serine proteases subtilisin, prostate specific antigen, CD26 and HtrA: A new form of immunosuppression? Front Immunol. 2022;13: 832989. https://doi.org/10.3389/fimmu.2022.832989.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Competing Interests
M.A.R., K.K. and W.M.S. declare that they have no competing interests.
Financial Interests
The authors have no relevant financial or non-financial interests to disclose.
Funding
No funds, grants, or other support was received.
Authors' Contributions
Literature search, article collection and extraction were performed by M.A.R. and K.K.; conceptualisation and writing—original draft was performed by M.A.R.; writing—review and editing were performed by M.A.R., K.K. and W.M.S.; and graphics were created by M.A.R. All authors have read and agreed to the published version of this manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Rosochowicz, M.A., Kulcenty, K. & Suchorska, W.M. Exploring the Role of HtrA Family Genes in Cancer: A Systematic Review. Mol Diagn Ther 28, 347–377 (2024). https://doi.org/10.1007/s40291-024-00712-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40291-024-00712-2