Abstract
Introduction
Erythrocytosis is attributed to various clinical and molecular factors. Many cases of JAK2-unmutated erythrocytosis remain undiagnosed. We investigated the characteristics and causes of JAK2-unmutated erythrocytosis.
Methods
We assessed the clinical and laboratory results of patients with erythrocytosis without JAK2 mutations and performed targeted next-generation sequencing (NGS) panels for somatic and germline mutations.
Results
In total, 117 patients with JAK2-unmutated erythrocytosis were included. The median hemoglobin and hematocrit levels were 17.9 g/dL and 53.4%, respectively. Erythropoietin levels were not below the reference range. Thrombotic events were reported in 17 patients (14.5%). Among JAK2-unmutated patients, 44 had undergone targeted panel sequencing consisting of myeloid neoplasm-related genes, and 16 had one or more reportable variants in ASXL1 (5/44), TET2, CALR, FLT3, and SH2B3 (2/44). Additional testing for germline causes revealed eight variants in seven genes in eight patients, including NF1, BPGM, EPAS1, PIEZO1, RHAG, SH2B3, and VHL genes. One NF1 pathogenic, one BPGM likely pathogenic, and six variants of undetermined significance were detected.
Conclusion
Somatic and germline mutations were identified in 36.4% and 33.3 % of the JAK2-unmutated group; most variants had unknown clinical significance. Not all genetic causes have been identified; comprehensive diagnostic approaches are crucial for identifying the cause of erythrocytosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
We evaluated potential factors contributing to JAK2-unmutated erythrocytosis and highlighted the importance of genetic mutation analysis. |
Our findings revealed genetic mutations in approximately one-third of patients with erythrocytosis. |
Integrative approaches and additional investigations are necessary to identify the cause of erythrocytosis and functional and clinical impact of variants of uncertain significance. |
1 Introduction
Erythrocytosis is defined as elevated hemoglobin (Hb), hematocrit (Hct), or red cell mass (RCM) and is affected by sex, race, and habitat altitude [1, 2]. Erythrocytosis can be classified as primary or secondary depending on the presence of mutations or due to increased RCM by elevated serum erythropoietin (EPO). Primary erythrocytosis can be further classified as inherited (due to germline mutations) or acquired (polycythemia vera (PV) due to somatic JAK2 mutations) [3]. Secondary erythrocytosis is attributed to various causes, hypoxia, high altitude, smoking, obstructive sleep apnea, and medications such as androgen and sodium-glucose cotransporter 2 (SGLT2) inhibitors [4], and requires thorough evaluation to identify the causes. Nonetheless, many patients remain undiagnosed and do not undergo further investigations following the exclusion of PV [5], and symptoms are managed through phlebotomy. Recent advances in genetic testing have increasingly identified patients with germline mutations in genes, including EPAS1, EGLN1, VHL, EPO, EPOR, PIEZO1, and BPGM. Various heterozygous mutations have been found, with frequencies ranging from 4% to 18% [6,7,8,9], indicating that inherited erythrocytosis is more common than previously expected. Studies have shown that some patients with secondary erythrocytosis have thrombosis events as in patients with PV [10]. In another study, secondary erythrocytosis patients experienced fewer thrombotic events than low-risk PV patients, showing comparable outcomes even when a substantial number of secondary erythrocytosis patients received low-dose aspirin or regular phlebotomies [11]. Compared to the general population, individuals with erythrocytosis had a higher tendency of thrombotic events [12]. Depending on the cause, phlebotomy can interfere with the natural compensation process [13]. Thus, the need to establish appropriate diagnostic and treatment approaches for patients with secondary erythrocytosis is increasing.
This study aimed to investigate the clinical and molecular features of JAK2-unmutated erythrocytosis through a retrospective analysis of laboratory test results and medical records. Additionally, we conducted a targeted next-generation sequencing (NGS) panel to detect somatic or germline variants associated with erythrocytosis, focusing on the genetic causes of JAK2-unmutated erythrocytosis.
2 Methods
2.1 Study Population and Data Review
The study population included patients tested with JAK2 mutation for erythrocytosis between 2016 and 2022. We retrospectively analyzed laboratory test results, including complete blood count, serum EPO, molecular studies such as JAK2 mutation analysis or targeted NGS panels, cytogenetics, and bone marrow (BM) studies [1, 14]. Clinical characteristics, including smoking habits, thrombotic events, medications, and obstructive sleep apnea, were assessed. The study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (B-2301-805-103), and the requirement for patient consent was waived because the study was based on a retrospective review of electronic medical records.
2.2 JAK2 Mutation Analysis and Next-Generation Sequencing
Each patient underwent either JAK2 mutation analysis or a targeted myeloid NGS panel, as ordered by the clinicians. BM aspirates or peripheral blood were used for genetic testing. Genomic DNA was purified from patient samples using the QIAamp DNA Blood Mini Kit (QIAGEN, Hilden, Germany) following the manufacturer’s instructions. SNaPshot assays were performed for JAK2 V617F mutation analysis. The PCR (polymerase chain reaction) mixture contained primer, PCR buffer, dNTP, and recombinant Taq DNA polymerase (Takara Bio, Shiga, Japan). PCR conditions were as follows: 95 ℃ for 5 min, 39 cycles of 95 ℃ for 30 s, 58 ℃ for 30 s, and 72 ℃ for 30 s, and 72 ℃ for 5 min. Excess primers and unincorporated nucleotides from the PCR reaction were cleaned by ExoSAP-IT (USB corp., Cleveland, OH, USA). SNaPshot Multiplex Kit (Applied Biosystems, Foster City, CA, USA) was used, and SNaPshot PCR reactions were performed for 25 cycles of 96 ℃ for 10 s, 50 ℃ for 5 s, and 60 ℃ for 30 s. The final data were analyzed on GeneMapper v5.0 software (Applied Biosystems). Mutation analysis of JAK2 exon 12 was performed by PCR followed by sequencing. PCR was performed with a PCR mixture consisting of the buffer, dNTP, forward primer (5’-TCAAAGTTCAATGAGTTGACCC-3’), reverse primer (5’-ATCTAACACAAGGTTGGCA-3’), and recombinant Taq DNA polymerase (Takara Bio, Shiga, Japan). PCR conditions were as follows: 95 ℃ for 5 min, 35 cycles of 95 ℃ for 30 s, 58 ℃ for 30 s, and 72 ℃ for 30 s, and 72 ℃ for 5 min. Excess primers and unincorporated nucleotides from the PCR reaction were cleaned by ExoSAP-IT (USB corp.). BigDye Ready Reaction Mix (Applied Biosystems) was used and sequencing PCR reactions were performed for 25 cycles of 96 ℃ for 10 s, 50 ℃ for 5 s, and 60 ℃ for 4 min. The final analysis was performed by Mutation Surveyor software (SoftGenetics, State College, PA, USA).
Oncomine Myeloid Research Assay (Thermo Fisher Scientific, Waltham, MA, USA) was used for targeted NGS analysis of somatic mutations. This targeted panel assay for detection of somatic sequence mutations encompassed 38 target genes, including exon 12-14 of the JAK2 gene. A total of 44 patients underwent the Oncomine Myeloid Research Assay, with library preparation performed using the Ion Chef system (Thermo Fisher Scientific) according to the manufacturer’s instructions. Libraries were prepared with 10 ng of DNA using an Ion AmpliSeq Kit for Chef DL8 (Thermo Fisher Scientific). Template preparations were performed using Ion 510TM, 520TM, and 530TM Kit Chef (Thermo Fisher Scientific). Chip loading was done using Ion 530 chip and followed by sequencing on the Ion S5 XL sequencer. Base calling, alignments, and annotation were performed using the Ion Reporter 5.20 software (Thermo Fisher Scientific). The variants were classified and interpreted according to the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists joint consensus recommendations [15]. Tier I, II, and III variants were included in this study. Patients lacking somatic variants in the myeloid panel were additionally tested for germline variants, if requested by the clinician, with TruSight One Expanded panel (Illumina Inc., San Diego, CA, USA) to analyze variants of EGLN1, HIF1A, EPAS1, VHL, EPO, EPOR, JAK2, SH2B3, CBL, RHAG, and BPGM genes. This panel assay was sequenced on the NextSeq 550Dx (Illumina Inc.) platform with Local Run Manager software version 2.4.0.2385 (Illumina Inc.). Raw NGS reads were aligned to the reference sequence hg19 (GRCh37) using the Burrows-Wheeler Aligner algorithm, and variant calling was performed by Genome Analysis Tool Kit workflows. The Human Gene Mutation Database (HGMD), ClinVar, and gnomAD were used to investigate clinical implications and pathogenicity of mutations. The variants were classified and interpreted according to the American College of Medical Genetics and Genomics guideline [16].
2.3 Statistical Analysis
The R statistical software (https://www.r-project.org) was used for all statistical analyses. Continuous variables are represented as median (range), whereas categorical variables are presented as the number of patients with available results in each category. The Wilcoxon rank-sum test was used to calculate the statistical significance of continuous variables, and Fisher’s exact test was used for categorical variables. A p-value less than 0.05 was considered statistically.
3 Results
3.1 Clinical and Hematologic Characteristics
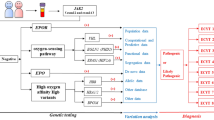
A total of 117 patients with JAK2-unmutated erythrocytosis were included; 82 patients underwent JAK2 V617F mutation analysis, 42 patients underwent JAK2 exon 12 mutation analysis, and 44 patients underwent targeted NGS panel tests (Fig. 1). The clinical characteristics are summarized in Table 1. The median age at diagnosis of patients was 51 years. The male-to-female ratio showed a prominent male predominance. The median Hb and Hct level was 17.9 g/dL and 53.4%, respectively. The median serum EPO level was 10.8 mIU/mL. No patients exhibited EPO levels below the reference range, and the EPO level increased in 6.9% of the patients. Regarding clinical features, thrombotic events were reported in 17 patients (17/117, 14.5%). Patients with a smoking history, including current smokers and ex-smokers, accounted for 70 patients (70/97, 72.1%). Seventeen patients were administered SGLT2 inhibitors (SGLT2i; 17/106, 16.0%). Of the 59 patients in the JAK2-unmutated group who underwent BM examination, two were diagnosed with PV based on panmyelosis from the BM study, cytogenetics, and genetic abnormalities despite JAK2 negativity. The percentage of patients with hypo- to normocellular BM was 95.5% (42/44). Karyotype analysis mainly revealed a normal karyotype (57/59, 96.6%) while two (2/59, 3.4%) JAK2-unmutated patients had cytogenetic abnormalities: one with Y chromosome loss and one with t(4;10;18)(q25;q21;q21.1).
3.2 Gene Mutations Detected in Somatic Mutation Targeted Panel Sequencing
Among the 44 JAK2-unmutated patients who underwent targeted somatic NGS panel assay, 16 (36.4%) had one or more reportable variants. The median number of detected variants was one per patient (range, 1–4). Variants were identified most commonly in ASXL1 (5/44, 11.4%), followed by TET2, CALR, FLT3, and SH2B3 (2/44, 4.5%) (Fig. 2). ASXL1 and SRSF2 tier 1 variants were identified in two patients, who were subsequently diagnosed with PV, based on panmyelosis and clonal abnormalities. Patients with reportable somatic variants showed lower mean corpuscular hemoglobin concentration (MCHC) levels than patients without tier III or higher variants. Other clinical and hematologic factors, including red cell distribution width, neutrophil/lymphocyte ratio, and platelet/lymphocyte ratio, did not reveal significant differences.
3.3 Gene Mutations Identified in Germline Mutation Panel Tests
We performed an additional germline NGS panel to identify possible genetic causes of inherited erythrocytosis in 21 JAK2-unmutated patients (Fig. 1). Among these patients, eight germline variants in seven genes, including NF1, BPGM, EPAS1, PIEZO1, RHAG, SH2B3, and VHL, were identified in eight patients (38.1%) (Table 2). One patient exhibited a BPGM missense variant classified as likely pathogenic. This patient presented with symptoms such as headache, chest discomfort, and respiratory distress, despite having no family history and being a non-smoker. The laboratory results revealed a normal EPO level and persistent erythrocytosis of Hb >19.0g/dL and Hct >50.0%, even after periodic phlebotomy. A frameshift pathogenic variant in the NF1 gene was detected in a patient who had a cafe-au-lait spot and pilocytic astrocytoma and a family history of neurofibromatosis type 1. Six germline variants identified in other genes were classified as variants of uncertain significance (VUS). Among these six patients with VUS, one patient with a VHL variant had a family history of erythrocytosis and coronary artery disease in his father. Four of the six patients did not exhibit any specific symptoms, while the remaining two reported dizziness. All patients had WBC and platelet counts within the reference range.
4 Discussion
JAK2-unmutated erythrocytosis remains a diagnostic challenge [1, 14] since diverse clinical and genetic factors can cause erythrocytosis. In the clinical algorithm for diagnosing erythrocytosis, as the first step, it is recommended to evaluate heart, lung, and renal disease, including duration and presence of typical symptoms, serum EPO level, and JAK2 mutation analysis [17, 18]. After excluding PV, reactive erythrocytosis, and secondary acquired erythrocytosis, somatic and germline mutation analysis of erythrocytosis-related genes should be performed to distinguish various genetic causes. In this study, among 117 JAK2-unmutated patients, 16 revealed variants in the somatic myeloid panel and 7 were identified with variants in the germline panel.
Of the JAK2-unmutated patients, even if serum EPO was in the reference range, increased WBC or PLT counts could be an indication to differentiate MPN using BM biopsy [3] since two cases were diagnosed as PV based on panmyelosis and additional karyotypic abnormalities. Serum EPO levels are included in the diagnostic criteria of PV [1] and help differentiate PV and other secondary causes. However, EPO level can be within reference range in PV patients who are obese or smoke daily. In this case, PV may be underdiagnosed, which shows that the EPO level should be evaluated differently depending on the patient’s condition and make it less reliable marker [19]. We identified one or more somatic variants in 36.4% of JAK2-unmutated patients. Clonal hematopoiesis (CH) is detected in the aging population, and the percentage of CH identified in our study is similar to a previous study on patients with erythrocytosis. Since erythrocytosis with CH is associated with cardiovascular morbidity and all-cause mortality [12], somatic testing to detect CH may imply clinical implications for the patients [20,21,22].
This study identified eight reportable variants in patients who underwent germline testing. Two pathogenic variants were detected, and the remainder were VUS, for which additional functional studies are needed to define their clinical significance. One BPGM likely pathogenic variant was detected from a 24-year-old male patient: NM_001724.5(BPGM):c.535C>T (p.Arg179Cys). This variant was initially classified as VUS owing to lack of pathogenicity; however, a recently published case series reported that patients with this heterozygous variant were associated with inherited erythrocytosis and showed low-normal BPGM deficiency and slightly reduced 2,3-bisphosphoglycerate levels, which alters Hb-oxygen affinity [23]. Reports on novel germline mutations and functional assays are continuously updated, and clinical laboratories should consequently update their classification of VUS detected in patients with unexplained persistent erythrocytosis. Another pathogenic variant was detected in the NF1 gene, which has been reported from patients with multiple unrelated neurofibromatosis. Patients with neurofibromatosis are reported to show secondary erythrocytosis [24]. Despite the increase in genetic testing for inherited erythrocytosis, the detection rate of pathogenic variants is not high in other studies [7, 17], suggesting that unknown genetic causes or unidentified etiologies of erythrocytosis are yet to be investigated. Since rare hereditary erythrocytosis can be due to Hb variants with high oxygen affinity and 2,3 BPG deficiency, standard pulse oximetry and venous P50 measurements are recommended in some erythrocytosis diagnostic algorithms [13, 25, 26]. However, these tests were not conducted in this study, which is a limitation. The low detection rate may also be due to incomplete analysis of certain genes with clinical significance, such as HFE [27], HBA1/HBA2, and HBB [28], not included in our targeted panel.
Clinical factors, including medications, tobacco use, and obstructive sleep apnea, should also be considered to identify secondary erythrocytosis. In particular, JAK2 wild-type erythrocytosis has been reported with SGLT2i, showing self-limiting characteristics and resolution of erythrocytosis after medication discontinuation [4]. Since SGLT2i is used in patients other than those with diabetes mellitus, with increased clinical use, erythrocytosis associated with SGLT2i should be considered in cases of secondary erythrocytosis. In this study, 17 patients (16.0%) were taking SGLT2i, and most of them continued taking the medication, except for one who discontinued SGLT2i and showed resolution of erythrocytosis. Since this patient had been taking SGLT2i before referral to our hospital, we could not identify the baseline Hb levels, but the median Hb and Hct levels were 18.7 g/dL and 54.0%, respectively, while taking SGLT2i; after discontinuation, the median Hb and Hct decreased to 16.9 g/dL and 49.3%, respectively.
Recently, there has been growing interest in the diagnostic utility of blood count parameters in patients with erythrocytosis. Some studies have reported that potential of indices such as neutrophil/lymphocyte ratio and platelet/lymphocyte ratio, demonstrating significantly higher values in PV patients compared to those with secondary erythrocytosis [29, 30]. We analyzed the differences of these indices between patients with and without reportable mutations. Notably, only MCHC levels showed a statistically significant difference between these groups. It is important to mention that the limited number of patients undergoing NGS panel testing may have influenced this outcome.
The study has some limitations. First, this study is based on single-center experience with a patient cohort of JAK2-unmutated erythrocytosis. This could have caused a selection bias to exclude patients with relatively mild erythrocytosis and result in a relatively high detection rate of germline mutation. However, these germline mutations were mostly VUS; thus, further studies are necessary to elucidate the idiopathic etiology and adequately discriminate germline erythrocytosis from other secondary erythrocytosis. Additionally, the study population includes two PV and one essential thrombocythemia patients without JAK2 mutation. While this inclusion might be a limitation when characterizing the secondary erythrocytosis patients, it underscores that the absence of JAK2 mutation does not completely exclude the possibility of MPN. Since this study primarily focused on the diagnostic approach, we included these cases to broaden our study population. A minority of patients in our study underwent only JAK2 V617F mutation analysis. It is worth noting that certain studies indicate a lower frequency of JAK2 exon 12 mutations among Korean patients, with reported frequencies varying across studies [31, 32].
In conclusion, integrative approaches are required to identify the cause of erythrocytosis. Considering the high frequency of somatic and germline mutations, genetic etiology in JAK2-unmutated patients should be investigated. Further research is also crucial to investigate the functional and clinical effects of VUS.
References
Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia. 2022;36(7):1703–19.
Fairbanks VF, Tefferi A. Normal ranges for packed cell volume and hemoglobin concentration in adults: relevance to ‘apparent polycythemia.’ Eur J Haematol. 2000;65(5):285–96.
Barbui T, Thiele J, Gisslinger H, Finazzi G, Carobbio A, Rumi E, et al. Masked polycythemia vera (mPV): results of an international study. Am J Hematol. 2014;89(1):52–4.
Gangat N, Szuber N, Alkhateeb H, Al-Kali A, Pardanani A, Tefferi A. JAK2 wild-type erythrocytosis associated with sodium-glucose cotransporter 2 inhibitor therapy. Blood. 2021;138(26):2886–9.
Ernest V, Abbou N, Tichadou A, Arcani R, Venton G. Characteristics of JAK2 unmutated erythrocytosis: distinctive traits between polycythemia vera and non-polycythemia vera patients. Eur J Intern Med. 2023;107:113–5.
Jalowiec KA, Vrotniakaite-Bajerciene K, Capraru A, Wojtovicova T, Joncourt R, Rovó A, et al. NGS evaluation of a Bernese cohort of unexplained erythrocytosis patients. Genes (Basel). 2021;12(12):1951.
Kristan A, Pajič T, Maver A, Režen T, Kunej T, Količ R, et al. Identification of variants associated with rare hematological disorder erythrocytosis using targeted next-generation sequencing analysis. Front Genet. 2021;12: 689868.
Gangat N, Oliveira JL, Porter TR, Hoyer JD, Al-Kali A, Patnaik MM, et al. Erythrocytosis associated with EPAS1(HIF2A), EGLN1(PHD2), VHL, EPOR or BPGM mutations: the Mayo Clinic experience. Haematologica. 2022;107(5):1201–4.
Filser M, Giansily-Blaizot M, Grenier M, Monedero Alonso D, Bouyer G, Pérès L, et al. Increased incidence of germline PIEZO1 mutations in individuals with idiopathic erythrocytosis. Blood. 2021;137(13):1828–32.
Nguyen E, Harnois M, Busque L, Sirhan S, Assouline S, Chamaki I, et al. Phenotypical differences and thrombosis rates in secondary erythrocytosis versus polycythemia vera. Blood Cancer J. 2021;11(4):75.
Krečak I, Holik H, Zekanović I, Morić Perić M, Marketin T, Coha B, et al. Thrombotic risk in secondary polycythemia resembles low-risk polycythemia vera and increases in specific subsets of patients. Thromb Res. 2022;209:47–50.
Wouters HJ, Mulder R, van Zeventer IA, Schuringa JJ, van der Klauw MM, van der Harst P, et al. Erythrocytosis in the general population: clinical characteristics and association with clonal hematopoiesis. Blood Adv. 2020;4(24):6353–63.
Gangat N, Szuber N, Tefferi A. JAK2 unmutated erythrocytosis: 2023 update on diagnosis and management. Am J Hematol. 2023;98(6):965–81.
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–405.
Li MM, Datto M, Duncavage EJ, Kulkarni S, Lindeman NI, Roy S, et al. Standards and guidelines for the interpretation and reporting of sequence variants in cancer: a Joint Consensus Recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J Mol Diagn. 2017;19(1):4–23.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–24.
Anžej Doma S, Drnovšek E, Kristan A, Fink M, Sever M, Podgornik H, et al. Diagnosis and management of non-clonal erythrocytosis remains challenging: a single centre clinical experience. Ann Hematol. 2021;100(8):1965–73.
Gangat N, Szuber N, Pardanani A, Tefferi A. JAK2 unmutated erythrocytosis: current diagnostic approach and therapeutic views. Leukemia. 2021;35(8):2166–81.
Lupak O, Han X, Xie P, Mahmood S, Mohammed H, Donthireddy V. The role of a low erythropoietin level for the polycythemia vera diagnosis. Blood Cells Mol Dis. 2020;80: 102355.
Quattrocchi A, Maiorca C, Billi M, Tomassini S, De Marinis E, Cenfra N, et al. Genetic lesions disrupting calreticulin 3’-untranslated region in JAK2 mutation-negative polycythemia vera. Am J Hematol. 2020;95:263–7.
Oh ST, Simonds EF, Jones C, Hale MB, Goltsev Y, Gibbs KD Jr, et al. Novel mutations in the inhibitory adaptor protein LNK drive JAK-STAT signaling in patients with myeloproliferative neoplasms. Blood. 2010;116(6):988–92.
Chauveau A, Nibourel O, Tondeur S, Paz DL, Mansier O, Paul F, et al. Absence of CALR mutations in JAK2-negative polycythemia. Haematologica. 2017;102(1): e15.
van Dijk MJ, van Oirschot BA, Stam-Slob MC, Waanders E, van der Zwaag B, van Beers EJ, et al. Heterozygosity for bisphosphoglycerate mutase deficiency expressing clinically as congenital erythrocytosis: a case series and literature review. Br J Haematol. 2023;200(2):249–55.
Van Baren N, Issa A, Delannoy A. Von Recklinghausen neurofibromatosis and hematologic malignancies: 2 case reports in adulthood. Acta Clin Belg. 1993;48(3):164–70.
Agarwal N, Mojica-Henshaw MP, Simmons ED, Hussey D, Ou CN, Prchal JT. Familial polycythemia caused by a novel mutation in the beta globin gene: essential role of P50 in evaluation of familial polycythemia. Int J Med Sci. 2007;4(4):232–6.
McMullin MF. Diagnostic workflow for hereditary erythrocytosis and thrombocytosis. Hematology. 2019;2019(1):391–6.
Burlet B, Bourgeois V, Buriller C, Aral B, Airaud F, Garrec C, et al. High HFE mutation incidence in idiopathic erythrocytosis. Br J Haematol. 2019;185(4):794–5.
Mangin O. High oxygen affinity hemoglobins. Rev Med Interne. 2017;38(2):106–12.
Krečak I, Holik H, Morić Perić M, Zekanović I, Coha B, Gverić-Krečak V, et al. High platelet-to-lymphocyte ratio may differentiate polycythemia vera from secondary polycythemia. Wien Klin Wochenschr. 2022;134(11):483–6.
Krečak I, Lucijanić M. Can we use platelet-to-lymphocyte ratio (PLR) to differentiate JAK2-unmutated erythrocytosis from polycythemia vera? Eur J Intern Med. 2023;108:120–1.
Kim JT, Cho YG, Choi SI, Lee YJ, Kim HR, Jang SJ, et al. JAK2 V617F and exon 12 genetic variations in Korean patients with BCR/ABL1-negative myeloproliferative neoplasms. Korean J Lab Med. 2010;30(6):567–74.
Park CH, Lee KO, Jang JH, Jung CW, Kim JW, Kim SH, et al. High frequency of JAK2 exon 12 mutations in Korean patients with polycythaemia vera: novel mutations and clinical significance. J Clin Pathol. 2016;69(8):737–41.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open Access funding enabled and organized by Seoul National University.
Conflict of Interest
The authors have no conflicts of interest to declare.
Availability of Data and Material
Data are not publicly available due to ethical reasons as they contain personal information of the patients. Further inquiries can be directed to the corresponding author.
Ethical Approval
This study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (B-2301-805-103).
Informed Consent
The requirement for patient consent was waived because the study was based on a retrospective review of electronic medical records.
Code availability
Not applicable.
Author Contributions
LY, KSA, LJY, LJO, BSM, and HSM collected clinical and laboratory data. SSH, KJ, and PKU interpreted the results and performed data analyses. LY performed statistical analyses and drafted the manuscript. HSM searched the literature, conceived the study concept, and edited the manuscript. All the authors have read and approved the final version of this manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Lee, Y., Seo, S.H., Kim, J. et al. Diagnostic Approaches to Investigate JAK2-Unmutated Erythrocytosis Based on a Single Tertiary Center Experience. Mol Diagn Ther 28, 311–318 (2024). https://doi.org/10.1007/s40291-024-00703-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40291-024-00703-3