Abstract
Immune checkpoint blockade therapies have generated efficacious responses in certain tumor types; however, the responses of breast carcinomas have been largely limited. Moreover, the identity of various parameters that can predict responses to immunotherapies, and at the same time, serve as putative biomarkers that can be therapeutically targeted to enhance the effectiveness of immunotherapies for breast cancers, remains to be comprehensively delineated. Activation of epithelial–mesenchymal plasticity in cancer cells, including those of the breast, increases their tumor-initiating potential and promotes their aggressiveness and resistance to multiple treatment regimens. Moreover, the residence of cancer cells in alternating epithelial or mesenchymal plastic phenotypic states can also influence their immuno-modulatory properties and susceptibilities to immune checkpoint blockade therapies. In this current opinion, we discuss the lessons that can be learnt from epithelial–mesenchymal transition to potentiate the efficacy of immunotherapy for breast cancers. We also discuss strategies to sensitize more-mesenchymal cancer cells to anti-tumor immunity and immune checkpoint blockade therapies, with the hope that these can serve as new translational avenues for the treatment of human breast tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
A vast majority of breast tumors are unresponsive to checkpoint blockade immunotherapies and a comprehensive understanding of the biomarkers that can predict responsiveness is lacking. |
Epithelial–mesenchymal plasticity alters the immunomodulatory properties of cancer cells and drives their resistance to checkpoint blockade immunotherapies. The residence of cancer cells in these phenotypic states can itself be indicative of responsiveness. |
1 Introduction
The use of various immunotherapeutic regimens to harness the immune system against tumors has revolutionized cancer treatment by generating durable responses and improving overall survival. Of these various therapies, immune checkpoint blockade (ICB) therapies in particular have seen unprecedented success in various types of solid tumors [1]. However, while ICB therapies administered individually or in combination have been successful in treating certain non-small cell lung cancers and melanomas, the responses of certain other cancers, such as those of the breast, have been largely limited [2, 3]. Moreover, the underlying reasons for the responsiveness of only a subset of breast tumors and the non-responsiveness of others to ICB therapies requires further investigation.
The expression of cancer cell-intrinsic and cell-extrinsic biomarkers that could be used to predict the responsiveness of breast tumors to ICB could represent one strategy to potentiate the efficacy of immunotherapies. Indeed, the expression of the programmed death ligand 1 (PD-L1), which inhibits the function of T-cells upon engagement with the programmed cell death protein 1 (PD-1) receptor, and the mismatch repair pathway are some of the approved predictive biomarkers for breast tumors [4,5,6]. However, the heterogeneity of PD-L1 expression across primary breast tumors and their subsequent metastases, the unresponsiveness of certain PD-L1-positive tumors to anti-PD-L1 ICB therapies, and the striking ability of certain PD-L1-negative tumors to mount proficient responses to PD-L1 inhibitors brings into question the clinical utility of this biomarker [3, 7,8,9]. It also undermines the need to identify additional parameters that could potentially be used in combination with PD-L1 expression to unequivocally predict the responses of breast tumors to ICB therapies.
The epithelial–mesenchymal transition (EMT) is a cell biological process that facilitates the conversion of epithelial cells to more-mesenchymal derivatives and is typically observed during the physiological processes of embryonic development and wound healing [10, 11]. In the context of cancer progression, activation of the EMT program empowers carcinoma cells to acquire tumor-initiating abilities, invasiveness, motility, and resistance to multiple targeted therapies and chemotherapies [12,13,14,15]. In addition, we and others have demonstrated that the residence of cancer cells in the epithelial or mesenchymal phenotypic states can itself be predictive of responsiveness or non-responsiveness to ICB therapies [16,17,18,19].
Given the limited responses of breast tumors to ICB treatment, intercepting the EMT program which is activated in a subset of breast tumors, could represent an attractive strategy to potentiate the efficacy of ICB therapies. However, the utility of considering the residence of carcinoma cells along various points on the epithelial–mesenchymal spectrum as a predictive criterion for ICB therapies, has been limited. Although the EMT program has been associated with resistance to immunotherapies in multiple cancer types, in this current opinion, we specifically focus on breast tumors [17, 20,21,22,23]. To this end, we comment on the lessons that can be learnt from the EMT program for improving immunotherapy for breast cancers. We specifically discuss (1) the current state of responses of breast tumors to immunotherapy, (2) associations between the EMT program and resistance to anti-tumor immunity, specifically in the context of breast cancers, (3) the probability of considering the phenotypic plasticity of cancer cells themselves, as a predictive biomarker for immunotherapies, and (4) the prospect of intercepting the epithelial–mesenchymal plasticity of breast cancer cells to potentiate the efficacy of ICB therapies.
2 Immune Checkpoint Blockade Therapy for Breast Cancers
Inhibitory receptors such as PD-1 and cytotoxic T lymphocyte-associated protein 4 (CTLA4) function to repress the activities of T cells upon engagement with their cognate ligands. Blocking these regulatory interactions using anti-PD-1/anti-PD-L1 or anti-CTLA4 antibodies largely restores T cell function, enabling them to eliminate carcinomas [1, 24,25,26,27]. Breast carcinomas span various different subtypes, including hormone receptor-positive breast cancers, human epidermal growth factor receptor 2 (HER2)-positive (HER-2+) breast cancers, and triple-negative breast cancers (TNBCs), which lack the expression of HER2 and hormone receptors [28, 29]. Although certain types of solid tumors such as melanomas and non-small cell lung cancers have been particularly responsive to anti-PD-1 or anti-CTLA4 ICB, these therapies have been less efficacious in the treatment of breast cancers [30,31,32,33,34].
In a study that evaluated the effects of anti-CTLA4 ICB together with an aromatase inhibitor in estrogen receptor-positive (ER+) HER2- breast cancer patients, short-term stable disease was observed in approximately half the patients. However, a small subset of patients who received this combination also developed various toxicities [2, 35]. Combining anti-CTLA4 ICB with other therapeutic regimens that target alternative immune checkpoints or other immune-suppressive components within the tumor microenvironment remains an active area of investigation. Most ICB regimens for the treatment of breast cancer have been largely centered on perturbing the PD-1/PD-L1 pathway. This is due to the fact that TNBCs, which are the most aggressive subset of breast cancers, have derived the most clinical benefit from anti-PD-1 or anti-PD-L1 ICB therapies relative to HER2+ or hormone receptor-positive breast cancers, which have remained largely unresponsive [3]. However, even for TNBCs, the objective response rates (ORRs) for anti-PD-1 or PD-L1 ICB monotherapy are still low (ranging from approximately 5–23%) relative to other solid tumors that have higher ORRs (approximately 40–45%) [3, 7, 30,31,32,33, 36,37,38,39].
In contrast to either anti-PD-1 or anti-CTLA4 ICB monotherapy, combinations of ICB and chemotherapy have had significantly greater success in early and advanced TNBC [2,3,4, 34]. The results from a phase 1b trial (NCT01375842) demonstrated that combining atezolizumab with nab-paclitaxel resulted in ORRs of 39.4% in some patients with metastatic disease that had received 0–2 pretreatments [40]. Moreover, the results from the randomized IMpassion 130 phase 3 trial demonstrated a clinically relevant prolonged survival in PD-L1-positive, treatment-naïve, metastatic TNBC patients that had received the same combination [41]. This combination therapy of atezolizumab and nab-paclitaxel has now been approved as the first immunotherapy for PD-L1-positive metastatic TNBC by the Food and Drug Administration (FDA) and European Commission [3, 4]. In addition, combining ICB with multiple forms of targeted therapies has also resulted in favorable responses in pre-clinical models as well as in patients; however, a comprehensive understanding of therapeutic combinations that could be applied to multiple breast cancer subsets, is still lacking [2, 3].
2.1 Predictive Biomarkers
The use of predictive biomarkers has proved beneficial in enhancing the efficacy of ICB in multiple tumor types. In the context of breast cancers, one widely used biomarker for predicting responsiveness to ICB is PD-L1, which can dampen the function of T cells upon engagement with the PD-1 receptor [5, 6]. PD-L1 expression as a predictive biomarker has been studied in patients with early stage TNBC that received combinations of anti-PD-L1 immunotherapy and chemotherapy. The results from the GeparNuevo trial demonstrated that PD-L1 expression did in fact associate positively with higher pathological complete remission rates [42]. However, results from the KEYNOTE-522 trial showed that clinical benefit was observed regardless of PD-L1 status (Table 1) [2, 43]. Similar conflicting findings have also been observed in patients with advanced TNBC who received combinations of ICB (atezolizumab) and chemotherapy (nab-paclitaxel). In the IMpassion 130 trial, a clinically meaningful increase in progression-free survival (PFS) was observed in patients whose tumors expressed PD-L1 relative to patients whose tumors were PD-L1 negative [41]. In sharp contrast, the results from the IMpassion 131 trial demonstrated that PD-L1 expression had little to no impact on PFS [44]. Moreover, a subset of TNBC patients do not derive any benefit from anti-PD-L1 immunotherapy administered individually or in combination with chemotherapy, in spite of their tumors being positive for PD-L1. Thus, the reliability of using PD-L1 as a predictive marker is in question given the conflicting data on PD-L1 expression and clinical benefit in breast cancer patients [3, 4]. Other predictive markers include immune cells and transcriptomic signatures within the tumor microenvironment. As observed in the IMpassion 130 trial, the presence of tumor infiltrating lymphocytes, CD8+ T cells, and the presence of a BRCA mutation was associated with clinical benefit in patients with incurable TNBC that had received combination therapy of atezolizumab and nab-paclitaxel [7]. In the randomized GeparNuevo trial for early stage TNBC, tumor mutational burden provided predictive value when used individually or in combination with tumor infiltrating lymphocytes or immune transcriptomic signatures [42].
While these recent advances will likely open multiple new avenues for the treatment of breast cancers, the vast majority of breast cancer patients are still unresponsive to ICB therapies. Moreover, the underlying reasons for the responsiveness of only a subset of breast tumors and the non-responsiveness of others to ICB therapies requires further investigation. The aforementioned need is complicated by the fact that the identities of breast carcinoma cell-derived factors that could possibly be targeted in combination with ICB are poorly defined. This raises the question of whether additional markers are required for predicting which breast cancer subtypes are most likely to benefit from ICB administered as a monotherapy or in combination with other agents.
3 EMT as a Driver of Resistance to Anti-tumor Immunity
The EMT is a cell biological process that potentiates the aggressive properties of carcinomas and drives the metastatic dissemination of tumor cells including those of breast cancers [11, 45]. During this process, cells typically shed the expression of epithelial markers, such as E-cadherin, and express instead, mesenchymal markers, such as vimentin, fibronectin and certain master EMT-inducing transcription factors (EMT-TFs), notably ZEB1, TWIST1, SNAI1 and SNAI2; once expressed, these EMT-TFs regulate the expression of genes associated with the more-mesenchymal states of carcinoma cells. The EMT is a highly dynamic process that gives rise to a continuum of states arrayed along the epithelial–mesenchymal spectrum. Indeed, some of these states express both epithelial and mesenchymal properties and are often referred to as hybrid, partial, or quasi-mesenchymal states [46,47,48,49]. Among the acquired EMT characteristics are invasiveness and motility, which enable carcinoma cells to metastasize to distant anatomical sites. Equally important, the EMT program can generate carcinoma cells with tumor-initiating properties [12, 13], often termed as cancer stem cells (CSCs) [12, 50]. CSCs have elevated drug resistance and are more refractory to standard chemotherapeutic regimens [14].
Specifically in the context of breast cancers, activation of epithelial–mesenchymal plasticity is typically observed in poorly differentiated breast cancer cell lines and tumors [51,52,53,54]. In a more recent study, Jorgensen and colleagues analyzed the expression of epithelial (E-cadherin-expressing), hybrid (co-expression of E-cadherin and N-cadherin), and mesenchymal (N-cadherin or vimentin-expressing) states in over 500 breast cancer patients and observed the presence of hybrid and mesenchymal states in TNBC patient samples. In contrast, epithelial states were largely dominant in the luminal A subtype [51]. Along similar lines, two other studies revealed that the mesenchymal markers N-cadherin and vimentin positively correlated with a higher histological grade and the absence of hormone receptors in breast tumors [52, 53]. Thus, a loss of E-cadherin and gain of N-cadherin and vimentin has been reported in TNBCs, invasive lobular carcinomas and a subset of invasive ductal carcinomas [52, 53]. Additionally, the expression of the EMT-TFs SNAI1 and TWIST correlate positively with high-grade invasive breast carcinomas [55, 56]. Importantly, multiple studies have demonstrated that patients with hybrid or mesenchymal breast tumors have poorer overall and disease-free survival, relative to patients whose tumors express the epithelial marker, E-cadherin [51, 53, 57].
In addition to the aforementioned well-documented features of the EMT, there are emerging connections between this program and the types of stromal cells that congregate within the tumor microenvironment. We and others have demonstrated that epithelial and mesenchymal breast tumors are differentially susceptible to immune attack [16, 58, 59]. Activation of the EMT program in carcinoma cells induces the expression of multiple cell surface receptors, secreted paracrine factors, and metabolites, many of which drive immune-evasion, regulate the assembly of an immune-suppressive tumor microenvironment, and drive resistance to immunotherapies [11, 60,61,62]. Importantly, many of these immune regulatory factors that are expressed by cancer cells undergoing an EMT are also indicative of poor prognosis of TNBCs. Moreover, a subset of TNBCs themselves, activate components of the EMT program [51, 52]. This striking overlap of immune-regulatory factors that are expressed by cancer cells undergoing an EMT and by TNBC tumors, brings to the forefront the attractive possibility of using the EMT program as a parameter that can be (1) used as a predictive criterion for responses of breast tumors to immunotherapies and (2) intercepted to potentiate the susceptibility of breast tumors to immunotherapies. In this section, we specifically highlight a few EMT-regulated, cancer cell-intrinsic immune-modulatory factors that are also indicative of poor prognosis in TNBCs. We also discuss the functional importance of such factors in altering the tumor microenvironment and driving resistance of breast tumors to ICB therapies.
3.1 EMT and Regulation of Cell-Intrinsic Factors
(1) PD-L1: Over the past few years, several studies have demonstrated that the EMT program can directly regulate the expression of PD-L1 in breast tumors [63, 64]. SnailHi murine quasi-mesenchymal cell lines and their corresponding tumors express elevated levels of PD-L1 relative to their more-epithelial SnailLO counterparts [16]. Importantly, activation of an EMT program in more-epithelial cells by doxycycline-induced expression of the ZEB1 or SNAI1 EMT-TFs resulted in a fivefold increase in PD-L1 expression [16]. Similarly, Noman and colleagues demonstrated that human breast cancer cell lines with more-mesenchymal features expressed elevated levels of PD-L1 in a manner that was highly dependent on the ZEB1 and SNAI1 EMT-TFs [65]. Both SNAI1 and ZEB1 have been documented to regulate PD-L1 by distinct mechanisms. Activation of ZEB1 drives PD-L1 expression by inhibiting the expression of mir200C [65]. SNAI1 is thought to drive the expression of PD-L1 by inducing CKLF-like MARVEL transmembrane domain-containing protein 6 (CMTM6), an important regulator of cell-surface PD-L1 [66]. Finally, PD-L1 expression is significantly associated with the expression of EMT-markers in claudin-low breast tumors, a highly aggressive subset of breast cancers that are known to activate components of the EMT program [67,68,69]. Although the first approved immunotherapy for human breast cancers includes targeting PD-L1 in combination with chemotherapy, a large fraction of PD-L1-expressing tumors remain unresponsive. This undermines the importance of additional biomarkers that can be used in combination with PD-L1 expression to predict responsiveness of breast tumors to ICB. Given the strong causal association between EMT-TFs and PD-L1 expression, using a combination of both these parameters as predictive criteria for TNBCs is particularly appealing.
(2) Antigen processing and presentation: In addition to PD-L1, activation of the EMT program also results in reduced expression of major histocompatibility complex class 1 (MHC-I) molecules that are required for presenting antigen to CD8+ T cells. Murine breast epithelial cell lines established from MMTV-PYMT mice expressed significantly higher levels of MHC-I and β2-microglobulin (B2M) relative to more-mesenchymal cell lines [16]. Strikingly, SNAI1- or ZEB1-induced activation of an EMT program in epithelial cells resulted in a ten- to 100-fold decrease in the expression of surface MHC-I [16]. Importantly, these MHC-I expressing-epithelial tumors had significantly higher numbers of cytotoxic CD8+ T cells relative to mesenchymal tumors that expressed low levels of MHC-I. Thus, EMT-induced downregulation of molecules associated with antigen processing and presentation represents another mechanism by which the EMT program could enable carcinoma cells to actively evade adaptive immunity. The expression of MHC-I is regulated by interferon gamma (IFN-γ) via activation of the JAK/STAT signaling pathway [70, 71]. Interestingly, multiple interferon-stimulating genes are intrinsically downregulated in more mesenchymal, CSC-enriched subpopulations contained within transformed human mammary epithelial cells [72]. This cell-intrinsic loss of IFN signaling in more mesenchymal cells may likely explain why cells that have undergone an EMT express significantly lower levels of cell-surface MHC-I.
(3) CD47: CD47 is a cell-surface glycoprotein that is widely accepted as a checkpoint molecule that regulates the activity of myeloid cells [73]. Engagement of CD47 with its cognate receptors, signal regulatory protein alpha (SIPR1a) and thrombospondin-1 (TSP1) expressed on the surface of macrophages, provides a ‘don’t eat me’ signal that precludes the ability of macrophages to phagocytose their targets [74]. Multiple cancer cells overexpress CD47 on their surface as a strategy to shield themselves from engulfment by innate immune cells [73, 75]. Indeed, CD47 is strongly expressed by TNBC tumors and is indicative of poor prognosis [76]. Most strikingly, the expression of CD47 in these TNBC tumors was found to be strongly associated with reduced expression of E-cadherin and increased expression of more-mesenchymal markers, such as vimentin, once again indicating a correlation with the EMT program. Noman and colleagues have demonstrated that SNAI1 and ZEB1 directly induce the expression of CD47 in more-mesenchymal human breast cancer cell lines by binding to E-boxes within the CD47 promoter. Importantly, such EMT-induced activation of CD47 in more-mesenchymal human breast cancer cells prevented them from being phagocytosed by macrophages in vitro [77]. Along these lines, Chip-Seq analysis of a murine MMTV-PYMT cell line also revealed SNAI1 binding to the CD47 promoter, supporting a causal connection between the EMT program and expression of CD47 [78, 79]. Taken together, these studies indicate that myeloid checkpoints that are indicative of poor prognosis in breast tumors are also tightly regulated by the EMT program.
(4) CD73: Under conditions of hypoxia, extracellular adenosine triphosphate (ATP) within the tumor microenvironment is converted to immunosuppressive adenosine by the actions of various ectoenzymes. ATP is dephosphorylated to generate adenosine monophosphate (AMP) by CD39, following which CD73 dephosphorylates AMP to generate adenosine [80, 81]. Adenosine is known to blunt the anti-tumor potential of multiple innate and adaptive immune cells by binding to various adenosine receptors expressed on their cell surface [82,83,84,85]. In the context of breast cancers, CD73 expression is associated with poor prognosis and drives chemotherapy resistance in TNBCs [86, 87]. However, the underlying factors that drive cell-intrinsic expression of CD73 in cancer cells remains relatively unexplored. We and others have demonstrated that the EMT program can regulate the expression of CD73. CD73 is robustly expressed on more-mesenchymal murine and human breast cancer cell lines relative to those displaying more-epithelial features [77, 79, 88, 89]. Direct induction of the EMT program by transforming growth factor beta (TGFβ), TWIST1, or SNAI1 resulted in elevated expression of CD73 in more-epithelial human breast cancer cells [77, 88, 89]. Moreover, SNAI1 binding was also observed within the CD73 promoter in both human as well as murine cancer cells that had activated the EMT program [77, 79].
3.2 EMT, Regulation of the Tumor Microenvironment, and Responses to ICB Therapies
Upon activation of the EMT program, the multitude of immune-suppressive factors expressed by cancer cells can directly impinge on and regulate the functions of immune cells within the tumor microenvironment. In this section, we specifically discuss the cell-extrinsic effects of the EMT program in shaping the tumor microenvironment and driving refractory responses of breast tumors to ICB therapies.
Using in vitro co-culture assays, Akalay and colleagues observed that activation of an EMT-program in human breast cancer cells impaired their susceptibility to CD8+ T cells by inhibiting the formation of an immunological synapse as well as by the induction of autophagy [59]. Moreover, small interfering RNA (siRNA)-induced silencing of the autophagy protein, BCN1, in breast cancer cell lines that had undergone an EMT, restored their susceptibility to CD8+ T cell lysis [90]. These studies suggest that one mechanism by which mesenchymal breast cancer cells resist elimination by CD8+ T cells is by the induction of autophagy. In addition, activation of an EMT program in human breast cancer cells via priming with a mixture of inflammatory cytokines such as TGFβ, tumor necrosis factor alpha (TNFα), and IFNγ led to loss of E-cadherin and upregulation of vimentin. These mesenchymal breast cancer cells could in turn, dampen the proliferation of NK cells in in vitro co-culture assays. In sharp contrast, the same mesenchymal cancer cells promoted the formation of immune-suppressive, regulatory T cells (Tregs) and B cells instead [91].
To determine whether the EMT program influences the assembly of the tumor microenvironment in vivo, we established novel, pre-clinical models of epithelial and mesenchymal breast tumors by deriving cell lines from late-stage tumors arising in the transgenic MMTV-PYMT autochthonous breast carcinoma mouse model. Some of these cell lines were more epithelial and expressed high levels of E-cadherin, while others were more mesenchymal and expressed vimentin and the ZEB1, TWIST1, and SNAI1 EMT-TFs. These cell lines upon implantation in vivo generated corresponding, phenotypically distinct epithelial or mesenchymal tumors in immunocompetent syngeneic hosts. Most strikingly, the epithelial tumors were infiltrated by CD8+ T cells, expressed MHC-I, and were sensitive to anti-CTLA4. In sharp contrast, mesenchymal tumors expressed PD-L1, recruited immunosuppressive Tregs and M2-like macrophages, and excluded exhausted CD8+ T cells to the tumor periphery. Importantly, mesenchymal tumors were resistant to anti-CTLA4 ICB therapy. Finally, tumors arising from mixtures of epithelial and mesenchymal carcinoma cells also generated an immunosuppressive tumor microenvironment and were resistant to ICB (Fig. 1) [16]. Hence, not only can mesenchymal carcinoma cells recruit immunosuppressive cells to their tumors, but minority populations (< 10%) of these mesenchymal carcinoma cells protect majority populations of their epithelial neighbors within such tumors from an immune attack. This would seem to be of great consequence clinically since the majority of carcinomas under treatment in the oncology clinic contain minority populations of more-mesenchymal cells that may well be responsible for dictating the outcome of the tumor as a whole to immune attack.
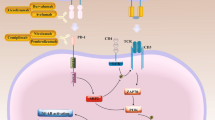
Immunological consequences of the epithelial–mesenchymal transition (EMT) in cancer cells. Activation of an EMT program in epithelial cells results in a progressive loss of epithelial features, resulting in a continuum of partial or mesenchymal phenotypic states. More-epithelial cancer cells express cell-intrinsic immune-stimulatory factors and form tumors that are infiltrated by cytotoxic CD8+ T cells. Epithelial cancer cells also affect the properties of neutrophils and promote the polarization of macrophages towards an M1-like state. These tumors are responsive to immunotherapies. More-mesenchymal cancer cells express cell-intrinsic immune-evasive and suppressive factors and form tumors that exclude CD8+ T-cells to the periphery. They are infiltrated by Tregs and M2-like macrophages and are refractory to immunotherapies. Macs macrophages, MET mesenchymal-to-epithelial transition, Tregs regulatory T cells
To determine the mechanism(s) by which more-mesenchymal tumors mount resistance to ICB, we took a series of transcriptomic approaches and observed that more-mesenchymal carcinoma cells express multiple immunosuppressive factors relative to their epithelial counterparts. Of these various factors, only the tumors arising from quasi-mesenchymal carcinoma cells that lacked the expression of a macrophage chemoattractant (Csf1), osteopontin (Spp1), or CD73 demonstrated a dramatic influx of T cells into the tumor core while also retaining their residence in a more-mesenchymal state. An important consequence of this increased influx of tumor-infiltrating lymphocytes in the knockout tumors was their corresponding responses to anti-CTLA4 ICB. Tumors lacking the expression of either CSF1 or SPP1 were partially sensitized to anti-CTLA4 ICB relative to control quasi-mesenchymal tumors. Most strikingly, quasi-mesenchymal tumors lacking the expression of CD73 were completely sensitized to anti-CTLA4 ICB and remained tumor free upon re-challenge, indicating the activation of immunological memory. Importantly, treatment of quasi-mesenchymal tumor-bearing mice with anti-CD73 or adenosine receptor antagonists in combination with anti-CTLA4 also led to significant reductions in both primary tumors and metastases [79]. These studies demonstrate for the first time that more-mesenchymal cells can be completely sensitized to at least one form of ICB by targeting CD73 in a pre-clinical model of breast cancer. These findings could have lasting clinical implications as more-mesenchymal cells have tumor-initiating abilities and are resistance to therapies. They are also present in certain highly aggressive TNBC subsets. Indeed, clinical trials for breast tumors that combine inhibitors of the adenosinergic signaling axis with ICB are currently ongoing. In light of the aforementioned pre-clinical studies, these treatments could be particularly beneficial for breast tumors that display features of the EMT program.
Along similar lines, Kim and colleagues utilized pre-clinical murine models of breast cancers and observed that neutrophil enriched-breast tumors displayed epithelial features in contrast to macrophage-enriched breast tumors, which are largely mesenchymal and displayed markers associated with the EMT program [92, 93]. These macrophage-enriched, more-mesenchymal tumors resembled the claudin-low subtype of human breast cancers, a subset of which showed tumor regression when macrophage depletion was combined with low-dose chemotherapy [94].
3.3 Reversing the EMT to Enhance Sensitization of Breast Tumors to Immunotherapy
The EMT program is a highly dynamic process in which cells residing in a more-mesenchymal state can revert back into an epithelial state by undergoing the reverse process of the mesenchymal-to-epithelial transition (MET) [95]. As discussed in the preceding sections, the mesenchymal state is associated with the expression of multiple cell-intrinsic, immune-evasive factors that instruct the formation of an immune-suppressive tumor microenvironment and drive resistance of more-mesenchymal tumors to multiple therapies. Thus, altering the phenotypic plasticity of the cancer cells by reversing the EMT program could concomitantly alter immunosuppressive signals within the tumor microenvironment. This would represent an attractive strategy to sensitize breast tumors to anti-tumor immunity.
One well-established factor that is known to regulate epithelial–mesenchymal plasticity is the mir200 family of microRNAs, which can repress the expression of ZEB1 leading to the loss of more-mesenchymal features. Along these lines, transfection of more-mesenchymal human breast cancer cell lines with Pre-mir200a, Pre-mir200b, or Pre-mir200c resulted in a significant reduction in the expression of PD-L1 [65]. Similarly, doxycycline-induced expression of mir200c in multiple human TNBC cell lines led to the acquisition of more-epithelial features, which was also accompanied by a substantial loss in the expression of PD-L1 and other immunosuppressive metabolites tryptophan 2,3-dioxygenase (TDO2), heme oxygenase 1 (HMOX-1), and growth differentiation factor 15 (GDF-15) [96, 97]. Taken together, these studies suggest that reversing the EMT program could enable carcinoma cells to substantially mitigate the expression of cell-intrinsic, immune-evasive, and immune-suppressive markers. Such a reduction in the expression of immune-modulatory factors, especially PD-L1, could have profound consequences on preventing T cell dysfunction that is typically observed in more-mesenchymal tumors. Thus, induction of an MET could possibly represent a strategy to potentiate the susceptibility of primary breast tumors to anti-tumor immunity.
In addition to T cells, reversing the EMT program has also been documented to have secondary consequences on innate immune cells within the tumor microenvironment. Indeed, Williams and colleagues showed that restoration of mir200c in murine and human breast cancer cell lines induced the expression of pro-inflammatory cytokines that promoted the polarization of macrophages towards an M1-like, anti-tumor state relative to control more-mesenchymal cell lines, which polarized macrophages to a M2-like, pro-tumor state instead [98]. Kim and colleagues have observed that over-expressing mir200c in macrophage-enriched mesenchymal tumors not only induced the acquisition of more-epithelial features by the cancer cells, but also increased the frequencies of neutrophils in the tumor microenvironment [92]. In another study, antibody-based neutralization of interleukin-8 (IL-8) resulted in a loss of mesenchymal features and a gain of epithelial features in claudin-low, human TNBCs. This acquisition of more-epithelial features dampened the recruitment of immunosuppressive polymorphonuclear–myeloid-derived suppressor cells (PMN-MDSCs) and enhanced the susceptibility of human breast cancer cell lines to natural killer (NK) and T cell-mediated lysis in vitro [99].
Induction of an MET has also been documented to enhance the susceptibility of breast tumors to ICB therapies. Shen and colleagues identified F-box protein 7 (FBXO7) as a top candidate in an RNA interference (RNAi) screen that was designed to identify genes that regulate the residence of human TNBC cell lines in a more-mesenchymal state [100]. Accordingly, short hairpin RNA (shRNA)-mediated knockdown of FBXO7 in various human TNBC cell lines resulted in reduced expression of more-mesenchymal markers and increased expression of E-cadherin. This acquisition of more-epithelial features was also accompanied by a concomitant increase in immune-stimulatory genes, the T-cell chemo-attractants chemokine (C-X-C motif) ligand 9 (CXCL9) and CXCL10, and molecules associated with antigen presentation. Moreover, pharmacological inhibition of the FBXO7/EYA2SCFFBXW7 signaling axis generated synergistic responses with anti-PD-1 ICB in 4T1 tumor-bearing mice [100].
4 Conclusions and Future Perspectives
Although a subset of TNBCs derive some clinical benefit from immunotherapy administered individually or in combination with chemotherapy, a large proportion of tumors are still unresponsive. Moreover, the underlying mechanisms for heterogeneous responses or a complete lack of response to immunotherapies are elusive. This provokes the question of whether the proportion of occasionally curative responses in breast cancers can be increased by the improvement of currently existing therapeutic protocols and the identification of biomarkers that can predict the probability of responsiveness of breast cancers to ICB therapies. Activation of the EMT program leads to the acquisition of immune-evasive features by carcinoma cells, promotes the assembly of an immune-suppressive tumor microenvironment, and drives resistance to ICB therapies. Given the plasticity of the EMT program, it maybe plausible that hybrid states that co-express both epithelial and mesenchymal features may also express certain immune-evasive features [101]. Of particular interest, is the notion that TNBCs not only activate components of the EMT program, but also seem to express several immune-suppressive factors that can be directly regulated by EMT-TFs. Indeed, recent studies that utilized pre-clinical models of breast cancers have already uncovered the identity of various EMT-associated immune-suppressive factors. Targeting some of these factors could completely sensitize quasi-mesenchymal breast tumors to at least one form of ICB. Moreover, given that the mesenchymal state is associated with the expression of multiple immune-suppressive factors, targeting EMT could not only alter the phenotypic plasticity of cells, but could also concomitantly reverse immunosuppressive signals within the tumor microenvironment incorporating components of the EMT program for informing therapeutic combinatorial strategies can potentiate responses to ICB therapies. Such strategies could be particularly advantageous for the treatment of breast tumors.
References
Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348(6230):56–61. https://doi.org/10.1126/science.aaa8172.
Emens LA. Breast cancer immunotherapy: facts and hopes. Clin Cancer Res. 2018;24(3):511–20. https://doi.org/10.1158/1078-0432.CCR-16-3001.
Keenan TE, Tolaney SM. Role of immunotherapy in triple-negative breast cancer. J Natl Compr Cancer Netw. 2020;18(4):479–89. https://doi.org/10.6004/jnccn.2020.7554.
Emens LA. Immunotherapy in triple-negative breast cancer. Cancer J. 2021;27(1):59–66. https://doi.org/10.1097/PPO.0000000000000497.
Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. https://doi.org/10.1146/annurev.immunol.26.021607.090331.
Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–34. https://doi.org/10.1084/jem.192.7.1027.
Emens LA, Cruz C, Eder JP, Braiteh F, Chung C, Tolaney SM, et al. Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer: a phase 1 study. JAMA Oncol. 2019;5(1):74–82. https://doi.org/10.1001/jamaoncol.2018.4224.
Shen X, Zhao B. Efficacy of PD-1 or PD-L1 inhibitors and PD-L1 expression status in cancer: meta-analysis. BMJ. 2018;362:k3529. https://doi.org/10.1136/bmj.k3529.
Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–7. https://doi.org/10.1038/nature14011.
Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial–mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–96. https://doi.org/10.1038/nrm3758.
Dongre A, Weinberg RA. New insights into the mechanisms of epithelial–mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2019;20(2):69–84. https://doi.org/10.1038/s41580-018-0080-4.
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial–mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–15. https://doi.org/10.1016/j.cell.2008.03.027.
Morel AP, Lievre M, Thomas C, Hinkal G, Ansieau S, Puisieux A. Generation of breast cancer stem cells through epithelial–mesenchymal transition. PLoS One. 2008;3(8):e2888. https://doi.org/10.1371/journal.pone.0002888.
Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29(34):4741–51. https://doi.org/10.1038/onc.2010.215.
Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol. 2017;14(10):611–29. https://doi.org/10.1038/nrclinonc.2017.44.
Dongre A, Rashidian M, Reinhardt F, Bagnato A, Keckesova Z, Ploegh HL, et al. Epithelial-to-mesenchymal transition contributes to immunosuppression in breast carcinomas. Cancer Res. 2017;77(15):3982–9. https://doi.org/10.1158/0008-5472.CAN-16-3292.
Kudo-Saito C, Shirako H, Takeuchi T, Kawakami Y. Cancer metastasis is accelerated through immunosuppression during snail-induced EMT of cancer cells. Cancer Cell. 2009;15(3):195–206. https://doi.org/10.1016/j.ccr.2009.01.023.
Lotsberg ML, Rayford A, Thiery JP, Belleggia G, D’Mello Peters S, Lorens JB, et al. Decoding cancer’s camouflage: epithelial–mesenchymal plasticity in resistance to immune checkpoint blockade. Cancer Drug Resist. 2020;3(4):832–53. https://doi.org/10.20517/cdr.2020.41.
Mullins RDZ, Pal A, Barrett TF, Heft Neal ME, Puram SV. Epithelial–mesenchymal plasticity in tumor immune evasion. Cancer Res. 2022;82(13):2329–43. https://doi.org/10.1158/0008-5472.CAN-21-4370.
Chen L, Gibbons DL, Goswami S, Cortez MA, Ahn YH, Byers LA, et al. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat Commun. 2014;5:5241. https://doi.org/10.1038/ncomms6241.
Lou Y, Diao L, Cuentas ER, Denning WL, Chen L, Fan YH, et al. Epithelial–mesenchymal transition is associated with a distinct tumor microenvironment including elevation of inflammatory signals and multiple immune checkpoints in lung adenocarcinoma. Clin Cancer Res. 2016;22(14):3630–42. https://doi.org/10.1158/1078-0432.CCR-15-1434.
Ortiz-Cuaran S, Swalduz A, Foy JP, Marteau S, Morel AP, Fauvet F, et al. Epithelial-to-mesenchymal transition promotes immune escape by inducing CD70 in non-small cell lung cancer. Eur J Cancer. 2022;169:106–22. https://doi.org/10.1016/j.ejca.2022.03.038.
Plaschka M, Benboubker V, Grimont M, Berthet J, Tonon L, Lopez J, et al. ZEB1 transcription factor promotes immune escape in melanoma. J Immunother Cancer. 2022. https://doi.org/10.1136/jitc-2021-003484.
Pauken KE, Torchia JA, Chaudhri A, Sharpe AH, Freeman GJ. Emerging concepts in PD-1 checkpoint biology. Semin Immunol. 2021;52:101480. https://doi.org/10.1016/j.smim.2021.101480.
Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. 2002;99(19):12293–7. https://doi.org/10.1073/pnas.192461099.
Pentcheva-Hoang T, Egen JG, Wojnoonski K, Allison JP. B7-1 and B7-2 selectively recruit CTLA-4 and CD28 to the immunological synapse. Immunity. 2004;21(3):401–13. https://doi.org/10.1016/j.immuni.2004.06.017.
Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64. https://doi.org/10.1038/nrc3239.
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–52. https://doi.org/10.1038/35021093.
Harbeck N, Penault-Llorca F, Cortes J, Gnant M, Houssami N, Poortmans P, et al. Breast cancer. Nat Rev Dis Prim. 2019;5(1):66. https://doi.org/10.1038/s41572-019-0111-2.
Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med. 2018;50(12):1–11. https://doi.org/10.1038/s12276-018-0191-1.
Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–39. https://doi.org/10.1056/NEJMoa1507643.
Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–28. https://doi.org/10.1056/NEJMoa1501824.
Larkin J, Hodi FS, Wolchok JD. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(13):1270–1. https://doi.org/10.1056/NEJMc1509660.
Kwa MJ, Adams S. Checkpoint inhibitors in triple-negative breast cancer (TNBC): where to go from here. Cancer. 2018;124(10):2086–103. https://doi.org/10.1002/cncr.31272.
Vonderheide RH, LoRusso PM, Khalil M, Gartner EM, Khaira D, Soulieres D, et al. Tremelimumab in combination with exemestane in patients with advanced breast cancer and treatment-associated modulation of inducible costimulator expression on patient T cells. Clin Cancer Res. 2010;16(13):3485–94. https://doi.org/10.1158/1078-0432.CCR-10-0505.
Nanda R, Chow LQ, Dees EC, Berger R, Gupta S, Geva R, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. J Clin Oncol. 2016;34(21):2460–7. https://doi.org/10.1200/JCO.2015.64.8931.
Adams S, Schmid P, Rugo HS, Winer EP, Loirat D, Awada A, et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann Oncol. 2019;30(3):397–404. https://doi.org/10.1093/annonc/mdy517.
Adams S, Loi S, Toppmeyer D, Cescon DW, De Laurentiis M, Nanda R, et al. Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: cohort B of the phase II KEYNOTE-086 study. Ann Oncol. 2019;30(3):405–11. https://doi.org/10.1093/annonc/mdy518.
Dirix LY, Takacs I, Jerusalem G, Nikolinakos P, Arkenau HT, Forero-Torres A, et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN Solid Tumor study. Breast Cancer Res Treat. 2018;167(3):671–86. https://doi.org/10.1007/s10549-017-4537-5.
Adams S, Diamond JR, Hamilton E, Pohlmann PR, Tolaney SM, Chang CW, et al. Atezolizumab plus nab-paclitaxel in the treatment of metastatic triple-negative breast cancer with 2-year survival follow-up: a phase 1b clinical trial. JAMA Oncol. 2019;5(3):334–42. https://doi.org/10.1001/jamaoncol.2018.5152.
Schmid P, Rugo HS, Adams S, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21(1):44–59. https://doi.org/10.1016/S1470-2045(19)30689-8.
Karn T, Denkert C, Weber KE, Holtrich U, Hanusch C, Sinn BV, et al. Tumor mutational burden and immune infiltration as independent predictors of response to neoadjuvant immune checkpoint inhibition in early TNBC in GeparNuevo. Ann Oncol. 2020;31(9):1216–22. https://doi.org/10.1016/j.annonc.2020.05.015.
Schmid P, Cortes J, Pusztai L, McArthur H, Kummel S, Bergh J, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382(9):810–21. https://doi.org/10.1056/NEJMoa1910549.
Miles D, Gligorov J, Andre F, Cameron D, Schneeweiss A, Barrios C, et al. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann Oncol. 2021;32(8):994–1004. https://doi.org/10.1016/j.annonc.2021.05.801.
Nieto MA. Epithelial–mesenchymal transitions in development and disease: old views and new perspectives. Int J Dev Biol. 2009;53(8–10):1541–7. https://doi.org/10.1387/ijdb.072410mn.
Pastushenko I, Brisebarre A, Sifrim A, Fioramonti M, Revenco T, Boumahdi S, et al. Identification of the tumour transition states occurring during EMT. Nature. 2018;556(7702):463–8. https://doi.org/10.1038/s41586-018-0040-3.
Bierie B, Pierce SE, Kroeger C, Stover DG, Pattabiraman DR, Thiru P, et al. Integrin-beta4 identifies cancer stem cell-enriched populations of partially mesenchymal carcinoma cells. Proc Natl Acad Sci USA. 2017;114(12):E2337–46. https://doi.org/10.1073/pnas.1618298114.
Kroger C, Afeyan A, Mraz J, Eaton EN, Reinhardt F, Khodor YL, et al. Acquisition of a hybrid E/M state is essential for tumorigenicity of basal breast cancer cells. Proc Natl Acad Sci USA. 2019;116(15):7353–62. https://doi.org/10.1073/pnas.1812876116.
Zhang Y, Donaher JL, Das S, Li X, Reinhardt F, Krall JA, et al. Genome-wide CRISPR screen identifies PRC2 and KMT2D-COMPASS as regulators of distinct EMT trajectories that contribute differentially to metastasis. Nat Cell Biol. 2022;24(4):554–64. https://doi.org/10.1038/s41556-022-00877-0.
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100(7):3983–8. https://doi.org/10.1073/pnas.0530291100.
Jorgensen CLT, Forsare C, Bendahl PO, Falck AK, Ferno M, Lovgren K, et al. Expression of epithelial–mesenchymal transition-related markers and phenotypes during breast cancer progression. Breast Cancer Res Treat. 2020;181(2):369–81. https://doi.org/10.1007/s10549-020-05627-0.
Khadri FZ, Issac MSM, Gaboury LA. Impact of epithelial–mesenchymal transition on the immune landscape in breast cancer. Cancers (Basel). 2021. https://doi.org/10.3390/cancers13205099.
Bae YK, Choi JE, Kang SH, Lee SJ. Epithelial–mesenchymal transition phenotype is associated with clinicopathological factors that indicate aggressive biological behavior and poor clinical outcomes in invasive breast cancer. J Breast Cancer. 2015;18(3):256–63. https://doi.org/10.4048/jbc.2015.18.3.256.
Tomaskovic-Crook E, Thompson EW, Thiery JP. Epithelial to mesenchymal transition and breast cancer. Breast Cancer Res. 2009;11(6):213. https://doi.org/10.1186/bcr2416.
Blanco MJ, Moreno-Bueno G, Sarrio D, Locascio A, Cano A, Palacios J, et al. Correlation of Snail expression with histological grade and lymph node status in breast carcinomas. Oncogene. 2002;21(20):3241–6. https://doi.org/10.1038/sj.onc.1205416.
Mironchik Y, Winnard PT Jr, Vesuna F, Kato Y, Wildes F, Pathak AP, et al. Twist overexpression induces in vivo angiogenesis and correlates with chromosomal instability in breast cancer. Cancer Res. 2005;65(23):10801–9. https://doi.org/10.1158/0008-5472.CAN-05-0712.
Willipinski-Stapelfeldt B, Riethdorf S, Assmann V, Woelfle U, Rau T, Sauter G, et al. Changes in cytoskeletal protein composition indicative of an epithelial–mesenchymal transition in human micrometastatic and primary breast carcinoma cells. Clin Cancer Res. 2005;11(22):8006–14. https://doi.org/10.1158/1078-0432.CCR-05-0632.
Rashidian M, Ingram JR, Dougan M, Dongre A, Whang KA, LeGall C, et al. Predicting the response to CTLA-4 blockade by longitudinal noninvasive monitoring of CD8 T cells. J Exp Med. 2017;214(8):2243–55. https://doi.org/10.1084/jem.20161950.
Akalay I, Janji B, Hasmim M, Noman MZ, Thiery JP, Mami-Chouaib F, et al. EMT impairs breast carcinoma cell susceptibility to CTL-mediated lysis through autophagy induction. Autophagy. 2013;9(7):1104–6. https://doi.org/10.4161/auto.24728.
Terry S, Savagner P, Ortiz-Cuaran S, Mahjoubi L, Saintigny P, Thiery JP, et al. New insights into the role of EMT in tumor immune escape. Mol Oncol. 2017;11(7):824–46. https://doi.org/10.1002/1878-0261.12093.
Mak MP, Tong P, Diao L, Cardnell RJ, Gibbons DL, William WN, et al. A patient-derived, pan-cancer EMT signature identifies global molecular alterations and immune target enrichment following epithelial-to-mesenchymal transition. Clin Cancer Res. 2016;22(3):609–20. https://doi.org/10.1158/1078-0432.CCR-15-0876.
Gu Y, Zhang Z, Ten Dijke P. Harnessing epithelial–mesenchymal plasticity to boost cancer immunotherapy. Cell Mol Immunol. 2023;20(4):318–40. https://doi.org/10.1038/s41423-023-00980-8.
Segovia-Mendoza M, Romero-Garcia S, Lemini C, Prado-Garcia H. Determining factors in the therapeutic success of checkpoint immunotherapies against PD-L1 in breast cancer: a focus on epithelial–mesenchymal transition activation. J Immunol Res. 2021;2021:6668573. https://doi.org/10.1155/2021/6668573.
Jiang Y, Zhan H. Communication between EMT and PD-L1 signaling: new insights into tumor immune evasion. Cancer Lett. 2020;468:72–81. https://doi.org/10.1016/j.canlet.2019.10.013.
Noman MZ, Janji B, Abdou A, Hasmim M, Terry S, Tan TZ, et al. The immune checkpoint ligand PD-L1 is upregulated in EMT-activated human breast cancer cells by a mechanism involving ZEB-1 and miR-200. Oncoimmunology. 2017;6(1):e1263412. https://doi.org/10.1080/2162402X.2016.1263412.
Xiao M, Hasmim M, Lequeux A, Moer KV, Tan TZ, Gilles C, et al. Epithelial to mesenchymal transition regulates surface PD-L1 via CMTM6 and CMTM7 induction in breast cancer. Cancers (Basel). 2021. https://doi.org/10.3390/cancers13051165.
Alsuliman A, Colak D, Al-Harazi O, Fitwi H, Tulbah A, Al-Tweigeri T, et al. Bidirectional crosstalk between PD-L1 expression and epithelial to mesenchymal transition: significance in claudin-low breast cancer cells. Mol Cancer. 2015;14:149. https://doi.org/10.1186/s12943-015-0421-2.
Prat A, Perou CM. Deconstructing the molecular portraits of breast cancer. Mol Oncol. 2011;5(1):5–23. https://doi.org/10.1016/j.molonc.2010.11.003.
Pommier RM, Sanlaville A, Tonon L, Kielbassa J, Thomas E, Ferrari A, et al. Comprehensive characterization of claudin-low breast tumors reflects the impact of the cell-of-origin on cancer evolution. Nat Commun. 2020;11(1):3431. https://doi.org/10.1038/s41467-020-17249-7.
Fruh K, Yang Y. Antigen presentation by MHC class I and its regulation by interferon gamma. Curr Opin Immunol. 1999;11(1):76–81. https://doi.org/10.1016/s0952-7915(99)80014-4.
Isaacs A, Lindenmann J. Virus interference. I. The interferon. By A. Isaacs and J. Lindenmann, 1957. J Interf Res. 1987;7(5):429–38. https://doi.org/10.1089/jir.1987.7.429.
Doherty MR, Cheon H, Junk DJ, Vinayak S, Varadan V, Telli ML, et al. Interferon-beta represses cancer stem cell properties in triple-negative breast cancer. Proc Natl Acad Sci USA. 2017;114(52):13792–7. https://doi.org/10.1073/pnas.1713728114.
Willingham SB, Volkmer JP, Gentles AJ, Sahoo D, Dalerba P, Mitra SS, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci USA. 2012;109(17):6662–7. https://doi.org/10.1073/pnas.1121623109.
Brown EJ, Frazier WA. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol. 2001;11(3):130–5. https://doi.org/10.1016/s0962-8924(00)01906-1.
Jaiswal S, Chao MP, Majeti R, Weissman IL. Macrophages as mediators of tumor immunosurveillance. Trends Immunol. 2010;31(6):212–9. https://doi.org/10.1016/j.it.2010.04.001.
Yuan J, Shi X, Chen C, He H, Liu L, Wu J, et al. High expression of CD47 in triple negative breast cancer is associated with epithelial–mesenchymal transition and poor prognosis. Oncol Lett. 2019;18(3):3249–55. https://doi.org/10.3892/ol.2019.10618.
Noman MZ, Van Moer K, Marani V, Gemmill RM, Tranchevent LC, Azuaje F, et al. CD47 is a direct target of SNAI1 and ZEB1 and its blockade activates the phagocytosis of breast cancer cells undergoing EMT. Oncoimmunology. 2018;7(4):e1345415. https://doi.org/10.1080/2162402X.2017.1345415.
Ye X, Tam WL, Shibue T, Kaygusuz Y, Reinhardt F, Eaton EN, et al. Distinct EMT programs control normal mammary stem cells and tumour-initiating cells. Nature. 2015;525(7568):256–+. https://doi.org/10.1038/nature14897.
Dongre A, Rashidian M, Eaton EN, Reinhardt F, Thiru P, Zagorulya M, et al. Direct and indirect regulators of epithelial–mesenchymal transition-mediated immunosuppression in breast carcinomas. Cancer Discov. 2021;11(5):1286–305. https://doi.org/10.1158/2159-8290.CD-20-0603.
Antonioli L, Blandizzi C, Pacher P, Hasko G. Immunity, inflammation and cancer: a leading role for adenosine. Nat Rev Cancer. 2013;13(12):842–57. https://doi.org/10.1038/nrc3613.
Vigano S, Alatzoglou D, Irving M, Menetrier-Caux C, Caux C, Romero P, et al. Targeting adenosine in cancer immunotherapy to enhance T-cell function. Front Immunol. 2019;10:925. https://doi.org/10.3389/fimmu.2019.00925.
Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MK, et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci USA. 2006;103(35):13132–7. https://doi.org/10.1073/pnas.0605251103.
Allard B, Turcotte M, Stagg J. Targeting CD73 and downstream adenosine receptor signaling in triple-negative breast cancer. Expert Opin Ther Targets. 2014;18(8):863–81. https://doi.org/10.1517/14728222.2014.915315.
Kjaergaard J, Hatfield S, Jones G, Ohta A, Sitkovsky M. A2A adenosine receptor gene deletion or synthetic A2A antagonist liberate tumor-reactive CD8(+) T cells from tumor-induced immunosuppression. J Immunol. 2018;201(2):782–91. https://doi.org/10.4049/jimmunol.1700850.
Cekic C, Day YJ, Sag D, Linden J. Myeloid expression of adenosine A2A receptor suppresses T and NK cell responses in the solid tumor microenvironment. Cancer Res. 2014;74(24):7250–9. https://doi.org/10.1158/0008-5472.CAN-13-3583.
Fu Z, Chen S, Zhu Y, Zhang D, Xie P, Jiao Q, et al. Proteolytic regulation of CD73 by TRIM21 orchestrates tumor immunogenicity. Sci Adv. 2023;9(1):eadd6626. https://doi.org/10.1126/sciadv.add6626.
Loi S, Pommey S, Haibe-Kains B, Beavis PA, Darcy PK, Smyth MJ, et al. CD73 promotes anthracycline resistance and poor prognosis in triple negative breast cancer. Proc Natl Acad Sci USA. 2013;110(27):11091–6. https://doi.org/10.1073/pnas.1222251110.
Turcotte M, Allard D, Mittal D, Bareche Y, Buisseret L, Jose V, et al. CD73 promotes resistance to HER2/ErbB2 antibody therapy. Cancer Res. 2017;77(20):5652–63. https://doi.org/10.1158/0008-5472.CAN-17-0707.
Hasmim M, Xiao M, Van Moer K, Kumar A, Oniga A, Mittelbronn M, et al. SNAI1-dependent upregulation of CD73 increases extracellular adenosine release to mediate immune suppression in TNBC. Front Immunol. 2022;13:982821. https://doi.org/10.3389/fimmu.2022.982821.
Akalay I, Janji B, Hasmim M, Noman MZ, Andre F, De Cremoux P, et al. Epithelial-to-mesenchymal transition and autophagy induction in breast carcinoma promote escape from T-cell-mediated lysis. Cancer Res. 2013;73(8):2418–27. https://doi.org/10.1158/0008-5472.CAN-12-2432.
Ricciardi M, Zanotto M, Malpeli G, Bassi G, Perbellini O, Chilosi M, et al. Epithelial-to-mesenchymal transition (EMT) induced by inflammatory priming elicits mesenchymal stromal cell-like immune-modulatory properties in cancer cells. Br J Cancer. 2015;112(6):1067–75. https://doi.org/10.1038/bjc.2015.29.
Kim IS, Gao Y, Welte T, Wang H, Liu J, Janghorban M, et al. Immuno-subtyping of breast cancer reveals distinct myeloid cell profiles and immunotherapy resistance mechanisms. Nat Cell Biol. 2019;21(9):1113–26. https://doi.org/10.1038/s41556-019-0373-7.
Soundararajan R, Fradette JJ, Konen JM, Moulder S, Zhang X, Gibbons DL, et al. Targeting the interplay between epithelial-to-mesenchymal-transition and the immune system for effective immunotherapy. Cancers (Basel). 2019. https://doi.org/10.3390/cancers11050714.
Singh S, Lee N, Pedroza DA, Bado IL, Hamor C, Zhang L, et al. Chemotherapy coupled to macrophage inhibition induces T-cell and B-cell infiltration and durable regression in triple-negative breast cancer. Cancer Res. 2022;82(12):2281–97. https://doi.org/10.1158/0008-5472.CAN-21-3714.
Pattabiraman DR, Bierie B, Kober KI, Thiru P, Krall JA, Zill C, et al. Activation of PKA leads to mesenchymal-to-epithelial transition and loss of tumor-initiating ability. Science. 2016;351(6277):aad3680. https://doi.org/10.1126/science.aad3680.
Rogers TJ, Christenson JL, Greene LI, O’Neill KI, Williams MM, Gordon MA, et al. Reversal of triple-negative breast cancer EMT by miR-200c decreases tryptophan catabolism and a program of immunosuppression. Mol Cancer Res. 2019;17(1):30–41. https://doi.org/10.1158/1541-7786.MCR-18-0246.
Williams MM, Hafeez SA, Christenson JL, O’Neill KI, Hammond NG, Richer JK. Reversing an oncogenic epithelial-to-mesenchymal transition program in breast cancer reveals actionable immune suppressive pathways. Pharmaceuticals (Basel). 2021. https://doi.org/10.3390/ph14111122.
Williams MM, Christenson JL, O’Neill KI, Hafeez SA, Ihle CL, Spoelstra NS, et al. MicroRNA-200c restoration reveals a cytokine profile to enhance M1 macrophage polarization in breast cancer. NPJ Breast Cancer. 2021;7(1):64. https://doi.org/10.1038/s41523-021-00273-1.
Dominguez C, McCampbell KK, David JM, Palena C. Neutralization of IL-8 decreases tumor PMN-MDSCs and reduces mesenchymalization of claudin-low triple-negative breast cancer. JCI Insight. 2017. https://doi.org/10.1172/jci.insight.94296.
Shen JZ, Qiu Z, Wu Q, Zhang G, Harris R, Sun D, et al. A FBXO7/EYA2-SCF(FBXW7) axis promotes AXL-mediated maintenance of mesenchymal and immune evasion phenotypes of cancer cells. Mol Cell. 2022;82(6):1123-1139 e8. https://doi.org/10.1016/j.molcel.2022.01.022.
Muralidharan S, Sehgal M, Soundharya R, Mandal S, Majumdar SS, Yeshwanth M, et al. PD-L1 activity is associated with partial EMT and metabolic reprogramming in carcinomas. Curr Oncol. 2022;29(11):8285–301. https://doi.org/10.3390/curroncol29110654.
Acknowledgements
All figures were created using BioRender.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Anushka Dongre has received a research grant (K22 Transition Career Development Award 5K22CA255420) from the National Cancer Institute (NCI), National Institutes of Health (NIH). The open access fee was paid using start-up funds provided to Anushka Dongre by Cornell University.
Conflict of interest
Author Anushka Dongre has received a research grant from the National Cancer Institute (NCI), National Institutes of Health (NIH). Author Isabel O’Connell declares that they have no conflicts of interest that might be relevant to the contents of this article.
Author contributions
IO and AD designed, planned, and wrote the manuscript.
Ethics approval
Not applicable.
Consent to participate and publication
Not applicable.
Data availability
Not applicable.
Code availability
Not applicable.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
O’Connell, I., Dongre, A. Immune Checkpoint Blockade Therapy for Breast Cancer: Lessons from Epithelial–Mesenchymal Transition. Mol Diagn Ther 27, 433–444 (2023). https://doi.org/10.1007/s40291-023-00652-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40291-023-00652-3