Abstract
The feasibility to unravel genetic and genomic signatures for disorders affecting the auditory system has accelerated since arriving in the post-genomics era roughly 20 years ago. Newly emerging studies have provided initial landmarks signaling heritability and thus, a genetic link, to severe tinnitus. Tinnitus, the phantom perception of ringing in the ears, is experienced by at least 15% of the adult population and can be extremely disabling. Despite its ubiquity, there is no cure for tinnitus and modalities offering relief are often of limited success. Because tinnitus is frequently reported in patients with acquired conductive or sensorineural hearing impairment, it has been widely accepted that tinnitus is secondary to and a symptom arising from hearing impairment. However, tinnitus has also been identified in the absence of auditory dysfunction and in young individuals, resulting in a debate about its origins. Genetics studies have identified severe tinnitus as a complex disorder arising from gene and environment interactions, refining its classification as a neurological disorder and, in at least a subset of patients, it appears not as a symptom of another health issue. This current opinion summarizes several recent studies that have challenged a long-accepted dogma and postulates how this information could eventually be used in the future to help patients. It is with great hope that this knowledge opens translational paths to provide relief for the many who suffer from the burden of tinnitus on a daily basis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Several recent lines of evidence show a heritable and genetic background for severe tinnitus. |
Specific clinical aspects that characterize tinnitus, also termed clinical heterogeneity, are especially extensive for tinnitus. This expansive heterogeneity has clouded previous studies that have dissected tinnitus heritability and genetics. Newly emerging studies have begun to overcome previous limitations and deliver candidate genes for tinnitus. |
The emergence of severe tinnitus having a genetic link and several studies now uncovering genetic targets is a transformational concept. Severe tinnitus is now recognized as a complex disorder with contributing genetic factors and environmental components. This concept differs compared with the long-standing consensus definition. |
Newly emerging genes provide the first insights into the molecular players in severe tinnitus. This also serves as critical information for effective therapy development to alleviate severe tinnitus. |
1 “Standing on the Shoulders of Giants”: Traction in the Post-Genomics Era
The completion of the Human Genome Project rendered the nucleic acid sequence of humans. The human genome is one of many possible approaches from which to understand basic human biology and disease. Historically, the genomics community has long debated about the total number of human genes thought to comprise the human genome, with dynamic estimates from 120,000 genes in 2000 [1] to just under 20,000 in 2021 [2]. This historical debate has been echoed in the genetics community interested in learning the genes encoding critical proteins in the auditory system. These estimates have ranged from 1% out of the then estimated 30,000 genes in the human genome, or roughly 300 genes, in 2003 [3] to roughly 1000 genes nearly two decades later [4]. Given the shear complexity of the inner ear, it remains challenging to gain traction into the genetic architecture of various auditory disorders. However, the genomics field has never been at such an advantageous position to finally dissect heritability and understand disease-associated variants and genes, for both ultra-rare “single gene” Mendelian disorders and complex genetic conditions, and is an exquisite example of advancement by “standing on the shoulders of giants” [5]. The cost of sequencing has significantly dropped in the past decades, making studies that include large sequencing cohorts a reality. This current opinion explores major advances from the perspective of the heritability and genetics of tinnitus, which has gained promising momentum in recent years. Mounting convincing evidence has re-defined our understanding of tinnitus as a symptom of a disease or syndrome and carved a new definition that classifies severe tinnitus as a neurological disorder and termed “tinnitus disorder” arising via complex gene–environment interactions [6].
2 Prevalence and Characterization of Tinnitus
The inner ear is a strikingly intricate and delicate organ, comprising abundantly diverse cell types, rich with unique and distinct protein signatures. However, a fully functional auditory system is not solely reliant on the health of the inner ear and involves higher centers of the auditory system in the brain. This sensitive balance defined by homeostasis and maintenance of cells, while simultaneously being subjected to various cellular stressors and aging processes throughout the lifetime, is essential for a healthy and functional auditory system. With disruption, several consequences may result such as hearing impairment, vestibular dysfunction, and/or tinnitus. The causes of these are extremely heterogeneous, which is exceptionally true for tinnitus, a major quality-of-life disruptor for many around the world.
Tinnitus is a phantom perception of ringing in the ears and can be subjective or objective and caused by auditory or non-auditory conditions. It can be continuous, pulsatile, episodic, or affect one or both ears. Tinnitus can affect all possible frequencies and last only a few moments, as with acute tinnitus, or manifest for many months or years, as in the case of chronic tinnitus [7]. Mechanisms triggering the transition from acute to chronic tinnitus are largely unknown, although depression, anxiety, and tinnitus-related distress seem to be emotional comorbidities that are key to its general manifestation [7, 8]. Widely employed tinnitus assessments are self-reporting questionnaires and psychoacoustic measures. Although these have greatly improved in recent years, the extreme clinical heterogeneity of tinnitus (both with and without a hearing impairment) and the lack of objective measures make assigning patients to a single clinical sub-category an exceptionally challenging task [9].
While severity can greatly vary, it is reported to affect roughly 15% of the world’s population and is a debilitating condition for between 1 and 3% of the population [10], although published figures range from 5.1 to 42.7% depending on its definition [11, 12]. For comparison, the World Health Organization estimates that more than 1.5 billion people, or roughly 20% of the global population, live with some degree of hearing loss [13]. Accurate global estimates of tinnitus mirroring the World Health Organization’s efforts for hearing loss are lacking. These efforts may have been hampered for many reasons; among these are attributions to its complex definition, clinical heterogeneity, and multiple subtypes [14].
Despite its pervasiveness, there is no cure for tinnitus. Many of the currently available interventions such as hearing aids and cochlear implants, wide-band sound therapy, counseling, and cognitive behavioral therapy do indeed improve quality of life by reducing psychological distress and possibly tinnitus loudness through use of devices. However, most do not treat the primary cause [14, 15]. Several pathophysiological mechanisms have been studied: neuroplastic response to sensory deprivation (in the context of hearing loss) [16]; increased spontaneous firing rates in neurons of the central auditory system (central gain) [17]; failure to generate central gain [18]; increased neural synchrony [17, 19]; and map reorganization. Map reorganization follows an analogy equating loss of sensory input (hearing loss) to limb amputation. Map reorganization suggests tinnitus reaches conscious awareness following compensation due to aberrant neuronal activity in the primary sensory cortex that is connected to a broader network including the frontal, parietal, and limbic brain [20]. More research is urgently needed to understand the molecular basis of tinnitus.
3 Dissecting Genetic vs Environmental Factors: Insights into Heritability
For decades, environmental factors have been thought of as the main drivers of tinnitus. This is supported by several studies, especially the earliest familial aggregation studies, observing no obvious correlation in siblings and/or parent-offspring groups [21, 22]. Furthermore, numerous explored candidate genes in patients with tinnitus lacked significant associations and/or replication (summary detailed in [23]), and more recently, a genome-wide association study (GWAS) with a small sample size rendered no significant associations [24]. The latter study was an important first step, as a GWAS is a preliminary step in establishing specific genes and chromosomal regions by investigating genetic variation in cases (patients) and healthy controls.
A series of recent, self-reported, questionnaire-based studies delivered important insights into heritability calculations. A longitudinal twin-based epidemiological study that relied on self-reported tinnitus calculated a heritability of 0.40 [25] and considered a complex interplay of genetics and environment contributing to tinnitus. Until this point in the hunt for genetic clues for tinnitus, one of the most logical explanations for the lack of significant genetic factors was extensive heterogeneity, which should be viewed as a broad spectrum of multiple subtypes instead of a single entity [26]. By considering sex-specific factors, another study identified a greater heritability in male individuals (0.68) compared with female individuals (0.41) with bilateral tinnitus [27]. As twin studies introduce bias owing to shared-environment effects, an adoption study was performed using medical registry data to determine whether a shared environment is associated with tinnitus [28]. The authors uncovered a familial transmission of clinically significant tinnitus with respect to adoptees relative to their biological parents but not adoptive parents and calculated a heritability of 0.32, suggesting a limited association of shared environmental effects with tinnitus heritability. Familial clustering analysis dissected whether four types (bilateral, unilateral, constant, and severe tinnitus) of self-reported tinnitus segregate. All were found to be significant, most importantly for severe tinnitus in women, and strongly supported that genetic factors may impact the development of severe and bilateral tinnitus [29]. In aggregate, these studies alluded to the importance of expanding this work with genetic studies.
4 Genetics Studies Begin to Unravel a Genetic Architecture for Tinnitus
As described above, the growing body of literature has pointed to a heritability in tinnitus. However, to reach this stage, several issues had to be addressed and will need to be continuously refined and improved in the future, to carefully avoid pitfalls that may lead researchers astray. For example, the study design with respect to patient and control inclusion, with no possibility of objective diagnostic tools, is based on self-reporting on questionnaires. By standardizing tinnitus questionnaires, an area that has made tremendous headway and includes translation into multiple languages [30], heterogeneity can be reduced and comparisons between different studies can be made with a greater level of clinical detail [26]. Furthermore, control individuals should match the study population with respect to age, sex, population background, and stress/anxiety traits [23] and significant findings need to be replicated in other patient cohorts. Early candidate gene studies suffered because of underpowered designs and a lack of replication that included fewer subjects using objective measures [23] and early heritability studies seem to have underestimated the level of clinical heterogeneity.
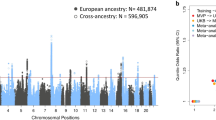
The widespread availability of sequencing data from various cohorts has opened a new research possibility providing masses of data and the number of subjects required for statistical significance, although usually at the risk of insufficiently detailed phenotyping. This balance of potential limitations vs benefits has been an essential approach to uncover genes, variants, and pathways. These molecular signatures serve as signposts from which the field can use to orient itself for further targeted experimental approaches. The identification of these specific targets is a prerequisite before the development of genetic intervention and treatment, making the efforts embarking on these studies extremely important [31]. GWASs are based off large datasets from self-reported tinnitus and have been instrumental for identifying significant variants and have taken many forms throughout the brief history of tinnitus genetics research. Thus, they identify significant common variants with small effect sizes that confer susceptibility to any type of tinnitus, i.e., tinnitus occurring as a symptom or tinnitus disorder. The most important GWAS findings are summarized in Table 1.
The first GWAS surfaced in 2020 and used questionnaire data from 172,995 UK Biobank and 260,832 Million Veteran Program participant discovery and replication cohorts, respectively, and uncovered a polygenic profile with multiple significant risk loci and genes [32]. In aggregate, six genome-wide significant loci and 27 genes in gene-based analyses were mapped, with many of these replicating in the replication cohort [32]. Significant correlations with hearing loss, neuroticism, insomnia, and major depressive disorder were uncovered. Notably, the association between tinnitus and hearing loss was bidirectional; this means hearing loss genes were found to lead to tinnitus as were tinnitus genes found to lead to hearing loss. This study defined tinnitus as a complex disorder and indicated the importance of genes and environmental factors for a full manifestation of tinnitus. Following this milestone, in 2021, a case-control GWAS on 172,608 UK Biobank participants identified the gene RCOR1 with the highest significance in the most severe tinnitus sub-group and identified an additional 11 other independent genetic loci. This study did not include a replication cohort but described candidate genes for further investigation. Although both GWAS approaches used UK Biobank participants, there was little apparent overlap in the strongest associations possibly because of the phenotype definition of tinnitus. However, some degree of suggestive associations (Table 1, bold) was uncovered, signaling some consistency between the two GWAS reports that used slightly different approaches.
5 Extreme Phenotype Approach
Another strategy that has uncovered novel insights into the genetics of tinnitus has been by applying an extreme phenotype approach, where tinnitus occurs as a disorder. Here, under a complex disease model hypothesis and by only selecting a small number of patients with severe tinnitus (extreme phenotype), the large phenotypic heterogeneity can be greatly narrowed. A single variant and gene burden analyses can be performed to identify an enrichment of rare variants with large effect sizes that associate with an extreme phenotype [33].
Exome sequencing of Spanish patients with Ménière’s disease and an extreme tinnitus phenotype uncovered 24 synaptic genes with an enrichment of rare missense variants [34]. A burden of rare variants in three candidate genes, ANK2, TSC2, and AKAP9, was replicated in an independent Swedish cohort with severe tinnitus and was specific to the tinnitus phenotype through inclusion of data of patients with epilepsy who did not have tinnitus. Gene-set enrichment analyses uncovered membrane trafficking and cytoskeletal binding in neurons [34]. This was the first study to link rare variants or large effect sizes to severe tinnitus and suggested genes associated with axonal branching and neuron connectivity in the brain. These genes are expressed in the spiral ganglion neurons of the auditory system, and, interestingly, ANK2 and TSC2 are specifically expressed in the limbic regions of the brain [34].
6 Conclusions and Future Directions
Genetics research exploring tinnitus is still at an early stage but there is a growing body of epidemiological and genetic data supporting that severe tinnitus has a genetic contribution [27, 29, 34]. This shifts the definition of at least a subset of tinnitus from being a symptom of an underlying disorder to being recognized as a neurological disorder, termed tinnitus disorder, that may be due to hyper-connectivity or synaptic reorganization and neuronal excitability in the brain. However, well-defined deep phenotyping of patients with tinnitus will continue to be a challenge but an important hurdle for selecting homogeneous patient groups and reducing the possibilities of false-negative results, which will be essential, as large sequencing cohorts are increasingly available. Well-defined clinical phenotypes must consider aspects such as early age of onset, sex, co-morbidities such as hearing loss and stress/anxiety, audiometry, tinnitus questionnaires, and psychoacoustic evaluations [33].
While it remains a challenge to assess the effect of a single variant for a complex disorder such as tinnitus, one can hypothesize whether the assessment of genetic risk in the shape of polygenic risk scores can provide a relative risk to help adjust “modifiable” environmental exposures and factors that may contribute to tinnitus in the future. These risk scores consider the additive effects of common variants [35] but still need considerable refinement before they can enter the clinical setting [36]. However, being able to have a level of genetic certainty to genetically diagnose patients and recommend therapeutic approaches to truly alleviate burdensome and distressing tinnitus remains a goal that many hope will not be too far in the future.
References
Liang F, Holt I, Pertea G, Karamycheva S, Salzberg SL, Quackenbush J. Gene index analysis of the human genome estimates approximately 120,000 genes. Nat Genet. 2000;25:239–40.
Nurk S, Koren S, Rhie A, Rautiainen M, Bzikadze AV, Mikheenko A, et al. The complete sequence of a human genome. Genomics; 2021 May. http://biorxiv.org/lookup/doi/https://doi.org/10.1101/2021.05.26.445798. Accessed 31 Jan 2021.
Friedman TB, Griffith AJ. Human nonsyndromic sensorineural deafness. Annu Rev Genomics Hum Genet. 2003;4:341–402.
Ingham NJ, Pearson SA, Vancollie VE, Rook V, Lewis MA, Chen J, et al. Mouse screen reveals multiple new genes underlying mouse and human hearing loss. PLoS Biol. 2019;17:e3000194.
Isaac Newton. Letter to Robert Hooke, 1675.
De Ridder D, Schlee W, Vanneste S, Londero A, Weisz N, Kleinjung T, et al. Chapter 1. Tinnitus and tinnitus disorder: theoretical and operational definitions (an international multidisciplinary proposal). In: Schlee W, Langguth B, Kleinjung T, Vanneste S, De Ridder D, editors. Progress in brain research. Elsevier; 2021: p. 1–25.
Wallhäusser-Franke E, D’Amelio R, Glauner A, Delb W, Servais JJ, Hörmann K, et al. Transition from acute to chronic tinnitus: predictors for the development of chronic distressing tinnitus. Front Neurol. 2017;8:605.
Bhatt JM, Bhattacharyya N, Lin HW. Relationships between tinnitus and the prevalence of anxiety and depression. Laryngoscope. 2017;127:466–9.
Langguth B, Kreuzer PM, Kleinjung T, De Ridder D. Tinnitus: causes and clinical management. Lancet Neurol. 2013;12:920–30.
Dobie RA. Depression and tinnitus. Otolaryngol Clin North Am. 2003;36:383–8.
McCormack A, Edmondson-Jones M, Somerset S, Hall D. A systematic review of the reporting of tinnitus prevalence and severity. Hear Res. 2016;337:70–9.
Shargorodsky J, Curhan GC, Farwell WR. Prevalence and characteristics of tinnitus among US adults. Am J Med. 2010;123:711–8.
World Health Organization. World report on hearing. Geneva: World Health Organization; 2021. https://apps.who.int/iris/handle/10665/339913. Accessed 31 Jan 2021.
Baguley D, McFerran D, Hall D. Tinnitus. Lancet. 2013;382:1600–7.
McFerran DJ, Stockdale D, Holme R, Large CH, Baguley DM. Why is there no cure for tinnitus? Front Neurosci. 2019;13:802.
Eggermont JJ, Roberts LE. The neuroscience of tinnitus. Trends Neurosci. 2004;27:676–82.
Noreña AJ, Eggermont JJ. Changes in spontaneous neural activity immediately after an acoustic trauma: implications for neural correlates of tinnitus. Hear Res. 2003;183:137–53.
Knipper M, van Dijk P, Schulze H, Mazurek B, Krauss P, Scheper V, et al. The neural bases of tinnitus: lessons from deafness and cochlear implants. J Neurosci. 2020;40:7190–202.
Seki S, Eggermont JJ. Changes in spontaneous firing rate and neural synchrony in cat primary auditory cortex after localized tone-induced hearing loss. Hear Res. 2003;180:28–38.
Ridder DD, Elgoyhen AB, Romo R, Langguth B. Phantom percepts: tinnitus and pain as persisting aversive memory networks. Proc Natl Acad Sci USA. 2011;108:8075–80.
Hendrickx JJ, Huyghe JR, Demeester K, Topsakal V, Van Eyken E, Fransen E, et al. Familial aggregation of tinnitus: a European multicentre study. B-ENT. 2007;3(Suppl. 7):51–60.
Kvestad E, Czajkowski N, Engdahl B, Hoffman HJ, Tambs K. Low heritability of tinnitus: results from the Second Nord-Trøndelag Health Study. Arch Otolaryngol Head Neck Surg. 2010;136:178–82.
Vona B, Nanda I, Shehata-Dieler W, Haaf T. Genetics of tinnitus: still in its infancy. Front Neurosci. 2017;11:236.
Gilles A, Van Camp G, Van de Heyning P, Fransen E. A pilot genome-wide association study identifies potential metabolic pathways involved in tinnitus. Front Neurosci. 2017;11:71.
Bogo R, Farah A, Karlsson KK, Pedersen NL, Svartengren M, Skjönsberg Å. Prevalence, incidence proportion, and heritability for tinnitus: a longitudinal twin study. Ear Hear. 2017;38:292–300.
Lopez-Escamez JA, Bibas T, Cima RFF, Van de Heyning P, Knipper M, Mazurek B, et al. Genetics of tinnitus: an emerging area for molecular diagnosis and drug development. Front Neurosci. 2016;10:377.
Maas IL, Brüggemann P, Requena T, Bulla J, Edvall NK, Hjelmborg JVB, et al. Genetic susceptibility to bilateral tinnitus in a Swedish twin cohort. Genet Med. 2017;19:1007–12.
Cederroth CR, PirouziFard M, Trpchevska N, Idrizbegovic E, Canlon B, Sundquist J, et al. Association of genetic vs environmental factors in Swedish adoptees with clinically significant tinnitus. JAMA Otolaryngol Head Neck Surg. 2019;145:222–9.
Trpchevska N, Bulla J, Prada Hellberg M, Edvall NK, Lazar A, Mehraei G, et al. Sex-dependent aggregation of tinnitus in Swedish families. J Clin Med. 2020;9:E3812.
Genitsaridi E, Partyka M, Gallus S, Lopez-Escamez JA, Schecklmann M, Mielczarek M, et al. Standardised profiling for tinnitus research: the European School for Interdisciplinary Tinnitus Research Screening Questionnaire (ESIT-SQ). Hear Res. 2019;377:353–9.
Boussaty EC, Friedman RA, Million Veteran Program, Clifford RE. Hearing loss and tinnitus: association studies for complex-hearing disorders in mouse and man. Hum Genet. 2021. https://doi.org/10.1007/s00439-021-02317-9.
Clifford RE, Maihofer AX, Stein MB, Ryan AF, Nievergelt CM. Novel risk loci in tinnitus and causal inference with neuropsychiatric disorders among adults of European ancestry. JAMA Otolaryngol Head Neck Surg. 2020;146:1015–25.
Amanat S, Gallego-Martinez A, Lopez-Escamez JA. Genetic inheritance and its contribution to tinnitus. Curr Top Behav Neurosci. 2021;51:29–47.
Amanat S, Gallego-Martinez A, Sollini J, Perez-Carpena P, Espinosa-Sanchez JM, Aran I, et al. Burden of rare variants in synaptic genes in patients with severe tinnitus: an exome based extreme phenotype study. EBioMedicine. 2021;66:103309.
Li R, Chen Y, Ritchie MD, Moore JH. Electronic health records and polygenic risk scores for predicting disease risk. Nat Rev Genet. 2020;21:493–502.
Adeyemo A, Balaconis MK, Darnes DR, Fatumo S, Granados Moreno P, Hodonsky CJ, et al. Responsible use of polygenic risk scores in the clinic: potential benefits, risks and gaps. Nat Med. 2021;27:1876–84.
Wells HRR, Abidin FNZ, Freidin MB, Williams FMK, Dawson SJ. Genome-wide association study suggests that variation at the RCOR1 locus is associated with tinnitus in UK Biobank. Sci Rep. 2021;11:6470.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by funding from the German Research Foundation through the Collaborative Research Center SFB889.
Conflict of interest
Barbara Vona has no relevant affiliations or financial involvement with any organization or entity with a financial interest in, or financial conflict with, the subject matter or materials discussed in the article, including employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
Author contributions
Barbara Vona was the sole author of this article.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Vona, B. The Road Traveled and Journey Ahead for the Genetics and Genomics of Tinnitus. Mol Diagn Ther 26, 129–136 (2022). https://doi.org/10.1007/s40291-022-00578-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40291-022-00578-2