Abstract
Purpose
To assess the available evidence to support a genetic contribution and define the role of common and rare variants in tinnitus.
Methods
After a systematic search and quality assessment, 31 records including 383,063 patients were selected (14 epidemiological studies and 17 genetic association studies). General information on the sample size, age, sex, tinnitus prevalence, severe tinnitus distribution, and sensorineural hearing loss was retrieved. Studies that did not include data on hearing assessment were excluded. Relative frequencies were used for qualitative variables to compare different studies and to obtain average values. Genetic variants and genes were listed and clustered according to their potential role in tinnitus development.
Results
The average prevalence of tinnitus estimated from population-based studies was 26.3% for any tinnitus, and 20% of patients with tinnitus reported it as an annoying symptom. One study has reported population-specific differences in the prevalence of tinnitus, the white ancestry being the population with a higher prevalence. Genome-wide association studies have identified and replicated two common variants in the Chinese population (rs2846071; rs4149577) in the intron of TNFRSF1A, associated with noise-induced tinnitus. Moreover, gene burden analyses in sequencing data from Spanish and Swede patients with severe tinnitus have identified and replicated ANK2, AKAP9, and TSC2 genes.
Conclusions
The genetic contribution to tinnitus is starting to be revealed and it shows population-specific effects in European and Asian populations. The common allelic variants associated with tinnitus that showed replication are associated with noise-induced tinnitus. Although severe tinnitus has been associated with rare variants with large effect, their role on hearing or hyperacusis has not been established.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tinnitus has been considered an annoying symptom usually associated with sensorineural hearing loss (SNHL) or anxiety commonly found in the aging population [1]; its origin is multifactorial, but it is attributed mostly to environmental factors, noise exposure being the best-known risk factor [2]. Although tinnitus prevalence studies across different populations are scarce, multiple epidemiological studies in large cohorts of individuals with tinnitus, including twins [3, 4], adoptees [5], and familial aggregation studies [6] have reported evidence to support a significant tinnitus heritability, particularly for severe bilateral tinnitus [3, 7]. This hidden inheritance is explained by genetic variation in the DNA sequence and different genetic studies have reported common and rare variants associated with different tinnitus phenotypes [7,8,9,10,11].
A precise phenotype definition is an essential requisite for genetic association studies in complex traits, since the effect size of common and rare variants on the phenotype is small or large according to their allelic frequency [12]. For rare variants, the effect can be large, small, or negligible, but for common variants, the effect only can be small or moderate. As a result, the frequency of the tinnitus phenotype is related to the expected additive effect of common and rare variants, determining the effect size associated with the phenotype [13, 14].
The term “tinnitus disorder” has been proposed for a rare form of tinnitus reported in around 1–2% of the population that is associated with emotional distress, cognitive dysfunction, and/or autonomic arousal, leading to behavioral changes and functional disability [15]. These patients also report SNHL and hyperacusis [16] and should be considered a severe form of tinnitus requiring a multidisciplinary intervention for its management [17,18,19].
The aim of this systematic review is to assess the available evidence to support a genetic contribution and define the role of common and rare variants in the human genome to tinnitus.
Methods
This review has followed the guidelines from “Preferred Reported Items for Systematic Reviews and Meta-Analyses” [20] (Annex 1). The protocol was registered on PROSPERO (registration number: CRD42023440491).
Study Design
According to the methodology for systematic reviews, the items related to the PICO question are listed as follows, so the studies have been selected according to the following characteristics:
-
Participants: patients with a diagnosis for tinnitus, or referring it as a relevant symptom.
-
Intervention: determination of the prevalence of tinnitus, estimation of familial aggregation of this symptom, and measurement of concordance rate between twins. Description of genetic variants potentially linked to the development of chronic tinnitus
-
Controls: controlled and uncontrolled studies
-
Main outcomes: tinnitus prevalence across different populations, according to the ethnicity and genetic variants associated with tinnitus.
-
Study designs: case–control studies, population-based studies, family aggregation studies, and twin studies.
Search Strategy
The bibliographic search was conducted on 15 May 2023. PubMed and Scopus databases were used with the following combination of MesH terms: (“tinnitus”) AND (“prevalence” OR “inheritance” OR “heritage” OR “heritability” OR “genes” OR “genetics” OR “families” OR “familial” OR “twins”), and it was limited to original articles published in the last 10 years.
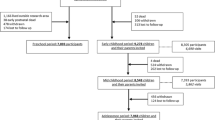
Once the search was performed in both databases, duplicates were eliminated and articles whose title or abstract did not adjust to the objectives of the review were discarded. The selected records were read and those that did not meet the inclusion criteria were excluded. The flowchart with the steps followed in this search is shown in Fig. 1.
Exclusion Criteria
-
Studies without any audiological information (pure tone audiometry, auditory brain responses, self-report hearing loss…)
-
Studies with self-reported tinnitus without additional phenotyping by psychometric questionnaires
-
Animal studies
-
Studies published in other languages than English or Spanish
-
Single case reports
Data Extraction
Two reviewers (PP-C, AGM) independently extracted study characteristics and outcomes from all the included studies, and data were compared. A third reviewer (JALE) was consulted when a consensus could not be reached.
Each article was reviewed to extract the most relevant information according to the objective of this review. For population-based studies, information was collected on authors, year of publication, country and continent, target population, years of registry, sample size, and tinnitus prevalence for each study. Information on severe/bothersome tinnitus was retrieved for each record, according to open questions to patients, or quality-of-life questionnaires. In the familial aggregation studies, the information retrieved was as follows: authors and year of publication, sample size, number of relatives with chronic tinnitus, and the total number of siblings in each family. To sum up, in the genetic studies, we include the reference, country, study design, objective, sample size, sex, mean age, and main results of each study.
Data Synthesis
General information on the sample size, age, sex, tinnitus prevalence, severe tinnitus distribution, and SNHL was recorded. Qualitative variables are presented as relative frequencies to compare them among studies and to obtain average values. Quantitative variables are expressed as mean ± standard deviations (SD). All statistical analyses were performed using SPSS Statistics package v22 (IBM, Armonk, NY).
Quality Assessment
The quality of each study was analyzed according to the type of study. The Risk Of Bias In Non-randomized Studies—of Exposure (ROBINS-E) tool [21] was used for observational epidemiology studies (prevalence studies) and candidate gene studies. GWAS studies were not assessed through the risk of bias, as no proper tool is available for such aim. The seven domains in this tool include the following: (1) bias due to confounding, (2) bias in the measurement of the exposure, (3) bias in the selection of participants into the study, (4) bias due to post-exposure interventions, (5) bias due to missing data, (6) bias in the measurement of outcomes, and (7) bias in the selection of the reported results. However, domain 4 was not relevant in this review and was excluded. The risk of bias ranged from “Low” to “Moderate,” “High,” or “Very High,” and the overall risk of bias was established based on the judgement for all the domains. A color scale was used to summarize it (green, low risk; yellow, moderate risk; red, high risk; black, very high risk). This has been summarized in Table 3.
Results
According to the eligibility criteria, 31 studies (383,063 patients) were included (14 prevalence studies and 17 genetic studies). The following flowchart details the selection process and filtering of the included articles (Fig. 1).
Prevalence Studies for Tinnitus
Fourteen studies were selected to estimate the prevalence of tinnitus, including 88,920 subjects. Three were performed in North America, two of them in the USA [22, 23] and one in Canada [24], and eight were performed in Europe, including studies from Poland [25, 26], UK [27], Netherlands [28], Sweden [4], Germany [29, 30], and Denmark [31], and three in Asia (South Korea) [32,33,34]. The age ranged from 11 to 99 years, with 46.3 ± 15.2 years old.
The mean prevalence for hearing loss was 25% for all the population-based studies, and 34.7% in the subgroup of patients with self-reported tinnitus, based on pure tone audiograms. The hearing thresholds were 15.6 ± 10 dB HL (pure tone average, PTA) in subjects without tinnitus and 24.4 ± 19.3 dB HL in patients with tinnitus, respectively.
In terms of self-reported tinnitus, the mean prevalence from all the population-based studies was 26.9%, and 20.7% of patients with tinnitus described it as an annoying symptom, based on different phenotyping tools, such as THI, TFI, or a direct question about it burden level.
Next, we compared the prevalence of tinnitus in European, North American, and Asian studies, according to the ethnic background. European studies registered a mean prevalence of tinnitus of 37.3%, with a mean prevalence of 44.2% for SNHL and a mean age of 35.4 ± 26.4 years old. Studies based on the North American population recorded a mean prevalence of tinnitus of 19.1%, with a mean prevalence of 29.2% for SNHL and a mean age of 42.8 ± 1.2 years, and studies in the Asian population a mean prevalence of 20%, with a mean prevalence of 12.3% for SNHL and a mean age of 53.5 ± 3.7 years (Table 1). Two studies analyzed African American populations with a prevalence of 21.6% (mean age 53.7 ± 11.5 years old.
Genetic Studies
Seventeen genetic studies were included [8, 9, 35,36,37,38,39,40,41,42,43,44,45,46,47,48,49], with a global sample size of 294,143 subjects (45.4% males), and 55,267 patients with tinnitus (18.8% males).
All the studies included reported no significant differences in terms of age between subgroups. Three studies excluded patients with SNHL [36, 42, 47], two studies reported no significant differences in audiograms between tinnitus and control subjects [39, 40], six studies included subjects with some degree of hearing impairment, including SNHL [8, 37, 45, 49], presbycusis [35], or noise-induced hearing loss [41], and the rest of them failed to report information on hearing stage [9, 38, 43, 44, 46, 48].
These genetic studies included four genome-wide association studies (GWAS) using genotyping arrays [9, 41, 46, 47], nine candidate gene studies [35, 36, 38,39,40, 42, 43, 45, 48], two sequencing studies using exome sequencing [8] and genome-sequencing data [49], and one mitochondrial DNA sequencing study [37]. Besides, there was a methylation study in the BDNF and GDNF genes [44].
All of them sought to find genetic variants associated with the development of tinnitus (Table 2 and Supplementary information). Figure 2 summarizes the main genes reported in GWAS and gene burden analyses. None of the candidate gene studies was replicated.
The genetic landscape of tinnitus. The effect of the variants associated with tinnitus as a function of their allelic frequency was observed in tinnitus genomic studies. A Blue dots represent OR for common variants associated with self-reported tinnitus in genome-wide association studies (GWAS). Odds ratios from GWAS studies were calculated from beta values from their respective studies. Some studies have not been included since information on neither beta nor odds ratio was published. B Orange dots represent OR for genes enriched in rare missense variants in exome/genome sequencing studies selecting an individual with tinnitus extreme phenotype or specific population cohorts through burden test analysis. Odds ratios represent the entire gene enrichment, not a single variant. However, the MAF value represents the MAF of the most common variant reported in the analysis. If variants were not reported, the higher MAF values were used
The allelic variants and genes reported were associated with the following mechanisms:
-
Regulation of the neural activity, including the auditory pathway, such as the BDNF (brain-derived neurotrophic factor) gene and the GDNF (glial cell line-derived neurotrophic factor) gene, both involved in the early development of central auditory pathway and the inner ear; the ANK2 (Ankyrin 2) gene, which encodes two different polypeptide including giant Ankyrin-2, a neuro-specific isoform variant expressed broadly in the central nervous system that keeps connectivity and neural activity in the CNS; the GPM6A (glycoprotein M6A) gene, which encodes neural glycoprotein M6a and plays an essential role in neural growth; the NAV2 (neuron navigator 2) gene, which is involved in neuronal and different sensory organs development; the TMEM132D (transmembrane protein 132D) gene, which encodes a transmembrane protein known for its capacity to act as a cell-surface marker for oligodendrocyte differentiation and neuronal morphogenesis; BCR (breakpoint cluster region) gene, which encodes the Rho family low molecular weight G-protein, abundantly expressed in the central nervous system and crucial for neurogenesis; and RCOR1 (REST corepressor 1) gene which encodes a component of a transcriptional repressor complex which represses neuronal gene expression in non-neuronal cells.
-
Receptors or transporters of neurotransmitters, including the polymorphic region (5-HTTLPR) of the serotonin transporter gene SLC6A4; the GRM7 gene, which encodes the metabotropic glutamate receptor subtype 7 (mGluR7); and the GABRB3 (GABA(A) beta-3 subunit) gene, which encodes a receptor for neuromediators involved in the descending part of the auditory pathways.
-
Metabolism and enzymatic pathways, such as the AKAP9 (A-kinase anchoring protein 9) gene, whose known function is binding to the protein kinase A (PKA) regulatory subunit to enclose it to different parts of the cell where phosphorylation is needed; the COMT (catechol-O-methyltransferase) gene, which inactivates dopamine, norepinephrine, and epinephrine neurotransmitters in the mammalian brain; and the ACE (angiotensin-converting enzyme) gene, which encodes a crucial enzyme in the renin-angiotensin system and is related to the cardiovascular and body water regulation.
-
Voltage-gated channels and cellular homeostasis mediators, such as the TSC2 (tuberous sclerosis complex 2) gene, which encodes a tumor suppressor protein part of the TSC involved in the negative regulation of mTORC1 (mechanistic target of rapamycin complex 1) activity; the CACNA1E (calcium voltage-gated channel subunit alpha1 subunit E) gene, which encodes a part of the “high-voltage activated” channel involved in the firing patterns modulation of neurons important for information processing, the ADD1 (α-adducin) gene, which is related to the volume and sodium homeostasis by interacting with the epithelial sodium channel; the KCNQ1 (potassium voltage-gated channel subfamily Q member 1); and KCNE1 (potassium voltage-gated channel subfamily E regulatory subunit 1), which form a voltage-gated potassium channel expressed in the marginal cell membrane of the stria vascularis.
-
Inflammation, such as the TNFRSF1A (tumor necrosis factor receptor superfamily member 1A) gene, which encodes a member of the TNF receptor superfamily of proteins involved in the TNF pathway. Two non-coding variants showed an association and were replicated in the Chinese population associated with noise-induced tinnitus (rs2846071; rs4149577).
-
Structural genes, such as WDPCP (WD repeat containing planar cell polarity) gene, which is related to the PCP effector proteins to regulate ciliogenesis during development and regulation of the actin cytoskeleton.
-
Mitochondrial DNA variants related to hearing loss.
Although four studies replicated their findings in another cohort, with similar characteristics to the discovery cohort [8, 9, 41, 49], none of the other studies replicated their findings in a second independent cohort.
Quality Assessment of Studies
The detailed analysis based on the seven domains of ROBINS-E is summarized in Table 3. According to this, fifteen of the studies had a moderate risk of bias [8, 22, 24,25,26,27, 29, 30, 35,36,37, 39,40,41,42], and twelve studies were evaluated to have a high risk of bias [4, 23, 28, 31,32,33,34, 38, 43,44,45, 48].
Discussion
Tinnitus is a multifactorial condition and a growing health problem associated with hearing loss. It is related to a wide variety of comorbidities, such as anxiety, hyperacusis, SNHL, headache and some otologic and neurologic conditions [50]. Moreover, tinnitus has a significant impact on the lives of patients who suffer from it and represents an economic burden for the health system [51].
Tinnitus has a significant heritability according to twins [3, 4], adoptees [5], and familial aggregation studies [6]; however, tinnitus heterogeneity and its association with several comorbidities make it difficult to decipher the genetic variation contributing to tinnitus. This challenge is higher with common variants located in non-coding regions that may show small effect by regulating multiple genes in different pathways. In this work, we have reviewed the records published in the last 10 years, on the genetic basis of tinnitus, after establishing a search strategy focused on prevalence studies to compare differences across different populations, genetic association studies, and familial aggregation studies.
One of the novel approaches of this systematic review is to consider the evaluation of the hearing as an essential part of the tinnitus phenotyping, as both conditions have a strong association [52]. For this reason, we have limited the selection to genetic studies that included the hearing thresholds of participants to control the confounding effect of genetic variants associated with hearing loss in tinnitus [53]. This has led to the exclusion of some GWAS reporting genetic associations with tinnitus; however, these genetic findings should be considered with caution, since these studies did not include hearing loss data and/or included military veterans with a history of noise exposure as replication cohort [8, 11, 49].
Several GWAS using biobank datasets have reported few common variants with significant associations with tinnitus [9, 11, 46, 47]; however, these studies were based on the participant responses to general health questionaries where tinnitus was self-reported, without a confirmed diagnosis in digital health records. Furthermore, these studies did not include the hearing thresholds, and it is difficult to determine if the reported associations are mediated by the underlying hearing loss, which is associated with tinnitus in the general population, particularly those over 50 years old. Finally, most of these studies did not include an independent replication cohort, and these associations have not been confirmed in later studies using other UK Biobank datasets [46, 47].
Prevalence and Tinnitus Phenotype
Tinnitus can receive different definitions, but there are some major characteristics, like its duration, that help to define this symptom. According to the latest clinical practice guidelines [54], chronic tinnitus is defined by a duration of at least 6 months. Most of the genetic studies present a homogeneous distribution of tinnitus patients, based on the duration criteria for chronic tinnitus. However, this information could be missed in some prevalence studies based on self-reported questionnaires [55].
Tinnitus disorder is a condition with a lower frequency in the general population. A more precise definition of tinnitus is needed for clinical and genomic research studies.
A standard approach to investigate the combined effect of environmental and genetic contributions in complex disorders is to compare prevalence across different populations with different ancestry living in the same geographical area or a specific population migrating to another continent. Here, we have compared the prevalence of tinnitus to determine the effect of ethnicity and population structure on tinnitus. The genetic uniformity, based on the reproduction between similar individuals or subjects with common ancestors, results in a decrease in the genetic diversity with a lower allele enrichment and less responsiveness to environmental changes [7].
Most studies report conservative tinnitus prevalence rates to be between 10 and 19% of adults [52, 56]. In addition, annoying tinnitus usually affects a low percentage of these patients [57]. Our results show slightly higher rates for both tinnitus prevalence (26.3%) and bothersome tinnitus (20%), which could be partly explained by the approach of the questions regarding tinnitus, in a self-reported questionnaire. Of note, the study of Choi et al. [22] performed in the USA reported a prevalence three times higher in white European compared to the Asian population and intermediate values for Hispanic and African-American individuals. Further prevalence studies in ethnically diverse countries are needed to compare tinnitus incidence in the same geographical areas according to ancestry.
Genetic Signature of Tinnitus
Some recent studies have integrated genetic knowledge into the tinnitus background, using different techniques. The main approaches focus on single variation and association analysis. GWAS have identified and replicated common variants in patients self-reporting tinnitus and noise-induced hearing loss, as well as tinnitus related to misophonia in population-based cohorts [9, 11, 41, 46, 47, 58]. GWAS studies have been useful in targeting potential regulators in tinnitus development in large population cohorts. UK Biobank has been demonstrated to be a valuable resource for association analysis using both self-reported questionnaires for tinnitus and genetic data. Different studies using this cohort have successfully pinpointed potential biomarkers for tinnitus; however, most of these studies lack a replication cohort to conclusively identify the causal genes, and these markers are common variants with a small effect [59, 60]. Of note, Trpchevska et al. [61] performed a large tinnitus GWAS including 723,166 participants from different cohorts, but no signal reached GWAS significance.
An alternative approach consists of selecting individuals with extreme phenotype (individuals with severe or mild symptoms at the ends of the phenotype distribution) and using omics data to identify rare and ultrarare variants by gene burden analysis [62]. This approach leads to more accurate identification of candidate genes. An enrichment of rare variants in patients with severe tinnitus has allowed the identification of target genes, which were replicated in an independent cohort [8]. Burden tests for rare biomarkers have been used successfully to identify potential genes with different rare variants enriched in tinnitus cohorts, using both exome and genome data [8, 49]. However, this overload of rare variation in certain genes may be a population-specific effect, and functional analyses of these rare variants are needed using cellular and animal models. Population stratification can be reasonably ruled out by segregation analysis of rare variants in multiple unrelated individuals.
A third approach is the combination of multiple bioinformatic tools analyzing different types of rare variants (i.e., single nucleotide variants, short indels, large structural variants, or copy number variants), as it has been described in other brain disorders [63, 64]. The identification of genes such as CACNA1E or NAV2, showing enrichment of missense and large structural variants in patients with tinnitus may lead to defining new druggable targets for tinnitus. However, this approach is limited by the clinical information of the cohort, in order to control the effect of other associated comorbidities.
A better understanding of the genetic structure of tinnitus may lead to explaining the difference in the phenotype. Though tinnitus may be a common symptom, tinnitus disorder, in its current definition as a condition associated with emotional, cognitive, or behavioral changes, may be considered a rare disease (less than 1–2% of the general population compared with tinnitus as a symptom). The differences between both may be the result of the combined effect of multiple common and rare variants, with an additive or epistatic effect leading to a complete or severe phenotype.
Limitations
This systematic review has some limitations. As most of the prevalence studies were performed by retrieving data from population-based registries, the information available for tinnitus phenotyping, including its time of evolution, laterality, or psychoacoustic characteristics, is incomplete. Tinnitus is a heterogeneous symptom, so it is essential to perform a deep phenotyping of this condition, including all the reported comorbidities to control association biases.
A second limitation is the low sample size associated with the tinnitus extreme phenotype approach that limits statistical power and cannot avoid population-specific effects. However, the main concern for most of the genetic studies is the lack of a second independent cohort to replicate genetic associations.
Since most of the reviewed studies exhibited a moderate to high risk of bias, the conclusions must be considered with caution, and future genetic studies should include a more precise selection of tinnitus individuals and a validation cohort.
Conclusions
-
1.
The genetic contribution to tinnitus is mediated by common and rare variations, and it is likely to have population-specific effects.
-
2.
Common allelic variants associated with tinnitus with a small effect have been associated with noise-induced tinnitus.
-
3.
Rare missense variants with a large effect have been associated with severe tinnitus, although their effect on other comorbidities such as hearing or hyperacusis has not been established.
References
Jafari Z, Kolb BE, Mohajerani MH (2019) Age-related hearing loss and tinnitus, dementia risk, and auditory amplification outcomes. Ageing Res Rev 56:100963. https://doi.org/10.1016/j.arr.2019.100963
Le TN, Straatman LV, Lea J, Westerberg B (2017) Current insights in noise-induced hearing loss: a literature review of the underlying mechanism, pathophysiology, asymmetry, and management options. J Otolaryngol Head Neck Surg 46(1):41. https://doi.org/10.1186/s40463-017-0219-x
Maas IL, Brüggemann P, Requena T, Bulla J, Edvall NK, Hjelmborg JVB, Szczepek AJ, Canlon B, Mazurek B, Lopez-Escamez JA, Cederroth CR (2017) Genetic susceptibility to bilateral tinnitus in a Swedish twin cohort. Genet Med 19(9):1007–1012. https://doi.org/10.1038/gim.2017.4
Bogo R, Farah A, Karlsson KK, Pedersen NL, Svartengren M, Skjönsberg A (2017) Prevalence, incidence proportion, and heritability for tinnitus: a longitudinal twin study. Ear Hear 38(3):292–300. https://doi.org/10.1097/AUD.0000000000000397
Cederroth CR, PirouziFard M, Trpchevska N, Idrizbegovic E, Canlon B, Sundquist J, Sundquist K, Zöller B (2019) Association of genetic vs environmental factors in Swedish adoptees with clinically significant tinnitus. JAMA Otolaryngol Head Neck Surg 145(3):222–229. https://doi.org/10.1001/jamaoto.2018.3852
Trpchevska N, Bulla J, Prada Hellberg M, Edvall NK, Lazar A, Mehraei G, Uhlen I, Schlee W, Canlon B, Gallus S, Lopez-Escamez JA, Cederroth CR (2020) Sex-dependent aggregation of tinnitus in Swedish families. J Clin Med 9(12):3812. https://doi.org/10.3390/jcm9123812
Amanat S, Gallego-Martinez A, Lopez-Escamez JA (2021) Genetic inheritance and its contribution to tinnitus. Curr Top Behav Neurosci 51:29–47. https://doi.org/10.1007/7854_2020_155
Amanat S, Gallego-Martinez A, Sollini J, Perez-Carpena P, Espinosa-Sanchez JM, Aran I, Soto-Varela A, Batuecas-Caletrio A, Canlon B, May P, Caderroth CR, Lopez-Escamez JA (2021) Burden of rare variants in synaptic genes in patients with severe tinnitus: an exome based extreme phenotype study. EBioMedicine 66:103309. https://doi.org/10.1016/j.ebiom.2021.103309
Bhatt IS, Wilson N, Dias R, Torkamani A (2022) A genome-wide association study of tinnitus reveals shared genetic links to neuropsychiatric disorders. Sci Rep 12(1):22511. https://doi.org/10.1038/s41598-022-26413-6
Boussaty EC, Friedman RA, Clifford RE (2022) Hearing loss and tinnitus: association studies for complex-hearing disorders in mouse and man. Hum Genet 141(3–4):981–990. https://doi.org/10.1007/s00439-021-02317-9
Clifford RE, Maihofer AX, Stein MB, Ryan AF, Nievergelt CM (2020) Novel risk loci in tinnitus and causal inference with neuropsychiatric disorders among adults of European ancestry. JAMA Otolaryngol Head Neck Surg 146(11):1015–1025. https://doi.org/10.1001/jamaoto.2020.2920
Lopez-Escamez JA, Bibas T, Cima RF, Van de Heyning P, Knipper M, Mazurek B, Szczepek AJ, Cederroth CR (2016) Genetics of tinnitus: an emerging area for molecular diagnosis and drug development. Front Neurosci 10:377. https://doi.org/10.3389/fnins.2016.00377
Sullivan PF, Geschwind DH (2019) Defining the genetic, genomic, cellular, and diagnostic architectures of psychiatric disorders. Cell 177(1):162–183. https://doi.org/10.1016/j.cell.2019.01.015
Visscher PM, Yengo L, Cox NJ, Wray NR (2021) Discovery and implications of polygenicity of common diseases. Science 373(6562):1468–1473. https://doi.org/10.1126/science.abi8206
De Ridder D, Schlee W, Vanneste S, Londero A, Weisz N, Kleinjung T et al (2021) Tinnitus and tinnitus disorder: theoretical and operational definitions (an international multidisciplinary proposal). Prog Brain Res 260:1–25. https://doi.org/10.1016/bs.pbr.2020.12.002
Cederroth CR, Lugo A, Edvall NK, Lazar A, Lopez-Escamez JA, Bulla J et al (2020) Association between hyperacusis and tinnitus. J Clin Med 9(8):2412. https://doi.org/10.3390/jcm9082412
Henton A, Tzounopoulos T (2021) What’s the buzz? The neuroscience and the treatment of tinnitus. Physiol Rev 101(4):1609–1632. https://doi.org/10.1152/physrev.00029.2020
Langguth B, Elgoyhen AB, Cederroth CR (2019) Therapeutic approaches to the treatment of tinnitus. Annu Rev Pharmacol Toxicol 59:291–313. https://doi.org/10.1146/annurev-pharmtox-010818-021556
Langguth B, Kreuzer PM, Kleinjung T, De Ridder D (2013) Tinnitus: causes and clinical management. Lancet Neurol 12(9):920–930. https://doi.org/10.1016/s1474-4422(13)70160-1
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71
Higgins J, Morgan R, Rooney A, Taylor K, Thayer K, Silva R et al (2022) Risk of bias in non-randomized studies of -exposure (ROBINS-E). Retrieved from https://www.riskofbias.info/welcome/robins-e-tool
Choi JS, Yu AJ, Voelker CCJ, Doherty JK, Oghalai JS, Fisher LM (2020) Prevalence of tinnitus and associated factors among Asian Americans: results from a national sample. Laryngoscope 130(12):E933-e940. https://doi.org/10.1002/lary.28535
House L, Bishop CE, Spankovich C, Su D, Valle K, Schweinfurth J (2018) Tinnitus and its risk factors in African Americans: the Jackson Heart Study. Laryngoscope 128(7):1668–1675. https://doi.org/10.1002/lary.26964
Ramage-Morin PL, Banks R, Pineault D, Atrach M (2019) Tinnitus in Canada. Health Rep 30(3):3–11. https://doi.org/10.25318/82-003-x201900300001-eng
Dziendziel B, Skarżyński PH, Rajchel JJ, Gos E, Skarżyński H (2019) Prevalence and severity of tinnitus in Polish otosclerosis patients qualified for stapes surgery. Eur Arch Otorhinolaryngol 276(6):1585–1590. https://doi.org/10.1007/s00405-019-05317-8
Lima AF, Moreira FC, Costa IE, Azevedo C, Mar F, Dias L (2022) Tinnitus and otosclerosis: an exploratory study about the prevalence, features and impact in daily life. Int Arch Otorhinolaryngol 26(3):e390–e395. https://doi.org/10.1055/s-0041-1739967
Humphriss R, Hall AJ, Baguley DM (2016) Prevalence and characteristics of spontaneous tinnitus in 11-year-old children. Int J Audiol 55(3):142–148. https://doi.org/10.3109/14992027.2015.1120890
Oosterloo BC, Croll PH, Baatenburg de Jong RJ, Ikram MK, Goedegebure A (2021) Prevalence of tinnitus in an aging population and its relation to age and hearing loss. Otolaryngol Head Neck Surg 164(4):859–868. https://doi.org/10.1177/0194599820957296
Hackenberg B, Döge J, O’Brien K, Bohnert A, Lackner KJ, Beutel ME et al (2023) Tinnitus and its relation to depression, anxiety, and stress—a population-based cohort study. J Clin Med 12(3):1169. https://doi.org/10.3390/jcm12031169
Hackenberg B, O’Brien K, Döge J, Lackner KJ, Beutel ME, Münzel T et al (2023) Tinnitus prevalence in the adult population—results from the Gutenberg Health Study. Medicina (Lithuania) 59(3):620. https://doi.org/10.3390/medicina59030620
Nemholt S, Schmidt JH, Wedderkopp N, Baguley DM (2019) A cross-sectional study of the prevalence and factors associated with tinnitus and/or hyperacusis in children. Ear Hear 41(2):344–355. https://doi.org/10.1097/AUD.0000000000000759
Choi JE, Ahn J, Park HW, Baek SY, Kim S, Moon IJ (2017) Prevalence of minimal hearing loss in South Korea. PLoS ONE 12(2):e0171635. https://doi.org/10.1371/journal.pone.0171635
Park KH, Lee SH, Koo JW, Park HY, Lee KY, Choi YS et al (2014) Prevalence and associated factors of tinnitus: data from the Korean National Health and Nutrition Examination Survey 2009–2011. J Epidemiol 24(5):417–426. https://doi.org/10.2188/jea.je20140024
Park RJ, Moon JD (2014) Prevalence and risk factors of tinnitus: the Korean National Health and Nutrition Examination Survey 2010–2011, a cross-sectional study. Clin Otolaryngol 39(2):89–94. https://doi.org/10.1111/coa.12232
Haider HF, Flook M, Aparicio M, Ribeiro D, Antunes M, Szczepek AJ et al (2017) Biomarkers of presbycusis and tinnitus in a Portuguese older population. Front Aging Neurosci 9:346. https://doi.org/10.3389/fnagi.2017.00346
Jeong JE, Jeon S, Han JS, Cho EY, Hong KS, Park SN, Kim JJ (2021) The mediating effect of psychological distress on the association between BDNF, 5-HTTLPR, and tinnitus severity. Psychiatry Investig 18(3):187–195. https://doi.org/10.30773/pi.2020.0295
Lechowicz U, Pollak A, Raj-Koziak D, Dziendziel B, Skarżyński PH, Skarżyński H, Ołdak M (2018) Tinnitus in patients with hearing loss due to mitochondrial DNA pathogenic variants. Eur Arch Otorhinolaryngol 275(8):1979–1985. https://doi.org/10.1007/s00405-018-5028-y
Orenay-Boyacioglu S, Coskunoglu A, Caki Z, Cam FS (2016) Relationship between chronic tinnitus and glial cell line-derived neurotrophic factor gene rs3812047, rs1110149, and rs884344 polymorphisms in a Turkish population. Biochem Genet 54(4):552–563. https://doi.org/10.1007/s10528-016-9741-1
Vanneste S, Alsalman O, De Ridder D (2018) COMT and the neurogenetic architecture of hearing loss induced tinnitus. Hear Res 365:1–15. https://doi.org/10.1016/j.heares.2018.05.020
Vanneste S, Mohan A, De Ridder D, To WT (2021) The BDNF Val(66)Met polymorphism regulates vulnerability to chronic stress and phantom perception. Prog Brain Res 260:301–326. https://doi.org/10.1016/bs.pbr.2020.08.005
Xie C, Niu Y, Ping J, Wang Y, Yang C, Li Y, Zhou G (2021) Genome-wide association study identifies new loci associated with noise-induced tinnitus in Chinese populations. BMC Genom Data 22(1):31. https://doi.org/10.1186/s12863-021-00987-y
Yüce S, Sancakdar E, Bağci G, Koç S, Kurtulgan HK, Bağcı B, Doğan M, Uysal IÖ (2016) Angiotensin-converting enzyme (ACE) I/D and alpha-adducin (ADD1) G460W gene polymorphisms in Turkish patients with severe chronic tinnitus. J Int Adv Otol 12(1):77–81. https://doi.org/10.5152/iao.2016.1732
Watabe T, Kanzaki S, Sato N, Matsunaga T, Muramatsu M, Ogawa K (2020) Single nucleotide polymorphisms in tinnitus patients exhibiting severe distress. Sci Rep 10(1):13023. https://doi.org/10.1038/s41598-020-69467-0
Orenay-Boyacioglu S, Caliskan M, Boyacioglu O, Coskunoglu A, Bozkurt G, Cam FS (2019) Chronic tinnitus and BDNF/GDNF CpG promoter methylations: a case–control study. Mol Biol Rep 46(4):3929–3936. https://doi.org/10.1007/s11033-019-04837-0
Rottenberg J, Zallmann M, Kostrica R, Jurajda M, Talach T (2014) The significance of (CA)n tandem repeat in GABA(A) beta-3 subunit gene in tinnitus manifestation. Ceska a Slovenska Neurologie a Neurochirurgie 77(4):473–478
Wells HRR, Abidin FNZ, Freidin MB, Williams FMK, Dawson SJ (2021) Genome-wide association study suggests that variation at the RCOR1 locus is associated with tinnitus in UK Biobank. Sci Rep 11(1):6470. https://doi.org/10.1038/s41598-021-85871-6
Urbanek ME, Zuo J (2021) Genetic predisposition to tinnitus in the UK Biobank population. Sci Rep 11(1):18150. https://doi.org/10.1038/s41598-021-97350-z
Bhatt IS, Dias R, Torkamani A (2021) Association analysis of candidate gene polymorphisms and tinnitus in young musicians. Otol Neurotol 42(9):e1203–e1212. https://doi.org/10.1097/mao.0000000000003279
Gallego-Martinez A, Escalera-Balsera A, Trpchevska N, Robles-Bolivar P, Roman-Naranjo P, Frejo L, Perez-Carpena P, Bulla J, Gallus S, Canlon B, Cederroth CR, Lopez-Escamez JA (2022) Using coding and non-coding rare variants to target candidate genes in patients with severe tinnitus. NPJ Genom Med 7(1):70. https://doi.org/10.1038/s41525-022-00341-w
Yew KS (2014) Diagnostic approach to patients with tinnitus. Am Fam Physician 89(2):106–113
Tziridis K, Friedrich J, Brüeggemann P, Mazurek B, Schulze H (2022) Estimation of tinnitus-related socioeconomic costs in Germany. Int J Environ Res Public Health 19(16):10455. https://doi.org/10.3390/ijerph191610455
Axelsson A, Ringdahl A (1989) Tinnitus–a study of its prevalence and characteristics. Br J Audiol 23(1):53–62. https://doi.org/10.3109/03005368909077819
Elmer S, Schmitt R, Giroud N, Meyer M (2023) The neuroanatomical hallmarks of chronic tinnitus in comorbidity with pure-tone hearing loss. Brain Struct Funct. https://doi.org/10.1007/s00429-023-02669-0
Cima RFF, Mazurek B, Haider H, Kikidis D, Lapira A, Noreña A, Hoare DJ (2019) A multidisciplinary European guideline for tinnitus: diagnostics, assessment, and treatment. HNO 67:10–42. https://doi.org/10.1007/s00106-019-0633-7
Monacelli G, Zhang L, Schlee W, Langguth B, Ward TE, Murphy TB (2023) Adaptive data collection for intraindividual studies affected by adherence. Biom J 65:e2200203. https://doi.org/10.1002/bimj.202200203
Jarach CM, Lugo A, Scala M, van den Brandt PA, Cederroth CR, Odone A et al (2022) Global prevalence and incidence of tinnitus: a systematic review and meta-analysis. JAMA Neurol 79(9):888–900. https://doi.org/10.1001/jamaneurol.2022.2189
Hallberg LR, Erlandsson SI (1993) Tinnitus characteristics in tinnitus complainers and noncomplainers. Br J Audiol 27(1):19–27. https://doi.org/10.3109/03005369309077885
Smit DJA, Bakker M, Abdellaoui A, Hoetink AE, Vulink N, Denys D (2022) A genome-wide association study of a rage-related misophonia symptom and the genetic link with audiological traits, psychiatric disorders, and personality. Front Neurosci 16:971752. https://doi.org/10.3389/fnins.2022.971752
Cresswell M, Casanova F, Beaumont RN, Wood AR, Ronan N, Hilton MP, Tyrrell J (2022) Understanding factors that cause tinnitus: a Mendelian randomization study in the UK Biobank. Ear Hear 43(1):70–80. https://doi.org/10.1097/aud.0000000000001074
Dawes P, Fortnum H, Moore DR, Emsley R, Norman P, Cruickshanks K et al (2014) Hearing in middle age: a population snapshot of 40- to 69-year olds in the United Kingdom. Ear Hear 35(3):e44–e51. https://doi.org/10.1097/aud.0000000000000010
Trpchevska N, Freidin MB, Broer L, Oosterloo BC, Yao S, Zhou Y, Nagtegaal AP et al (2022) Genome-wide association meta-analysis identifies 48 risk variants and highlights the role of the stria vascularis in hearing loss. Am J Hum Genet 109(6):1077–1091. https://doi.org/10.1016/j.ajhg.2022.04.010
Amanat S, Requena T, Lopez-Escamez JA (2020) A systematic review of extreme phenotype strategies to search for rare variants in genetic studies of complex disorders. Genes (Basel) 11(9):987. https://doi.org/10.3390/genes11090987
Halvorsen M, Huh R, Oskolkov N, Wen J, Netotea S, Giusti-Rodriguez P et al (2020) Increased burden of ultra-rare structural variants localizing to boundaries of topologically associated domains in schizophrenia. Nat Commun 11(1):1842. https://doi.org/10.1038/s41467-020-15707-w
Monlong J, Girard SL, Meloche C, Cadieux-Dion M, Andrade DM, Lafreniere RG et al (2018) Global characterization of copy number variants in epilepsy patients from whole genome sequencing. PLoS Genet 14(4):e1007285. https://doi.org/10.1371/journal.pgen.1007285
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions PP-C is funded by the Andalusian Health Government (Grant RH-0150–2020). JAL-E has received funding from GNP-182 GENDER-Net Co-Plus Fund including support from “La Caixa” Foundation (ID 100010434), under agreement LCF/PR/DE18/52010002, the European Union’s Horizon 2020 Research and Innovation Programme, Grant Agreement No. 848261, and The University of Sydney to support research on genetics of Meniere disease (K7013_B3413 Grant). AG-M is funded by the Andalusian Health Government (Grant DOC_01677).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Perez-Carpena, P., Lopez-Escamez, J.A. & Gallego-Martinez, Á. A Systematic Review on the Genetic Contribution to Tinnitus. JARO 25, 13–33 (2024). https://doi.org/10.1007/s10162-024-00925-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10162-024-00925-6