Abstract
Introduction
Changing running technique or equipment can alter tibial loads. The efficacy of interventions to modify tibial loads during running is yet to be synthesised and evaluated. This article reviewed the effect of running technique and footwear interventions on tibial loading during running.
Methods
Electronic databases were searched using terms relevant to tibial load and running. Interventions were categorised according to their approach (i.e., footwear; barefoot running; speed; surface; overground versus treadmill; orthotics, insoles and taping; and technique); if necessary, further subgrouping was applied to these categories. Standardised mean differences (SMDs) with 95% confidence intervals (CIs) for changes in tibial loading were calculated and meta-analyses performed where possible.
Results
Database searches yielded 1617 articles, with 36 meeting the inclusion criteria. Tibial loading increased with (1) barefoot running (SMD 1.16; 95% CI 0.50, 1.82); (2) minimalist shoe use by non-habitual users (SMD 0.89; 95% CI 0.40, 1.39); (3) motion control shoe use (SMD 0.46; 95% CI 0.07, 0.84); (4) increased stride length (SMD 0.86; 95% CI 0.18, 1.55); and (5) increased running speed (SMD 1.03; 95% CI 0.74, 1.32). Tibial loading decreased when (1) individuals ran on a treadmill versus overground (SMD − 0.83; 95% CI − 1.53, − 0.12); and (2) targeted biofeedback was used (SMD − 0.93; 95% CI − 1.46, − 0.41).
Conclusions
Running barefoot, in motion control shoes or in unfamiliar minimalist shoes, and with an increased stride length increases tibial loads and may increase the risk of a tibial stress injury during periods of high training load. Adopting interventions such as running on a treadmill versus overground, and using targeted biofeedback during periods of high loads could reduce tibial stress injury.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Running barefoot, in unfamiliar minimalist shoes, in motion control shoes, with increased stride length and increased speed increased tibial loading. Avoiding these conditions during training periods of high volume or when recovering from a tibial stress injury is recommended. |
Running on a treadmill versus overground and the use of targeted biofeedback reduced tibial loading. These strategies could be adopted to reduce tibial loads in training or rehabilitation. |
1 Introduction
Two-thirds of people who meet or exceed physical activity guidelines use running as a mode of exercise [1]. Those who engage in high volumes of running are at an elevated risk of sustaining lower limb bone stress injuries [2, 3]. Stress injuries to the tibia are the fifth (tibial stress syndrome) and ninth (tibial stress fractures) most common running injury; combined, they account for approximately 1 in 10 running injuries [4]. Management and rehabilitation of a tibial stress injury involves a period of rest from high impact exercise and/or full immobilisation, followed by a slow integration back into exercise and sport [5, 6]. The average passive rest period is 8.3 weeks [5], with return to normal activities taking up to 17 weeks depending on severity [7]. These periods away from training and exercise can cause significant reductions in aerobic, anaerobic and muscular fitness in as little as 4 weeks [8]. Prevention of tibial stress injuries is important to avoid the induced inactivity that could impact athlete performance and potential negative health outcomes.
Tibial stress injuries result, in part, from the mechanical fatigue of bone [9]. Cyclic impact loading over time generates minuscule bone cracks (i.e., microdamage) that have the potential to degrade bone material properties [10]. The amount of microdamage can be affected by several variables, including volume, magnitude, and frequency of loads. In healthy individuals, there is a balance between the reabsorption and formation of bone, and strain induced microdamage is removed successfully [10]. However, bone can go into a state of accelerated remodelling if there is inadequate time for successful removal of microdamage. Repeated strain of a high magnitude, without adequate rest or lower magnitude strains coupled with a large volume (i.e., high number of running cycles) or high rate of loading can cause accelerated remodelling to occur [11, 12]. Bone reabsorption will begin to outpace the formation of bone, and microdamage will begin to accumulate. If adequate rest is not introduced in either scenario, a tibial stress fracture can occur [11, 12]. Tibial stress injuries are multifactorial in nature and can be affected by both non-modifiable (i.e., sex, bone density and skeletal alignment) and modifiable risk factors (i.e., training volume and intensity, running biomechanics, equipment and surfaces) [13,14,15]. Changing running technique and biomechanics is one way to alter the load and stress placed on bone [16]. Gait retraining has been used as a tool to modify tibial loads [16,17,18,19,20] through changing speed [19, 20], stride length [21, 22] and cadence [18], step width [16] and foot strike pattern [17, 18]. Foot orthoses and footwear have also been used to modify tibial load [23,24,25,26]. The efficacy of these interventions to change tibial loading is yet to be synthesised and compared, which may help identify the most effective means for reducing tibial loads. The purpose of this review is to synthesise and evaluate the effect of technique and footwear interventions on tibial loads via meta-analyses.
2 Methods
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [27]. The review protocol was registered on the Open Science Framework (21 September 2020; https://osf.io/vm7fk).
2.1 Literature Search Strategy
The Academic Search Complete, SPORTDiscus, Medline compete and CINAHL complete databases were searched, with the last search being run on 12 July 2021. Two searches were run, the first used the key terms and Boolean operators: (tibia*) AND (stress* OR strain*) AND (injur* OR “injury prevention” OR “injury reduction”) AND (intervention OR train* OR program OR strateg*), and the second search aimed at targeting specific intervention types and was run using the terms and Boolean operators: (tibia*) AND (stress* OR strain* OR strain) AND (injur* OR “injury prevention” OR “injury reduction”) AND (step* OR stride* OR shoe* OR orthotic* OR speed OR velocity). No additional filters or search limitations were used. Following these database searches, all titles and abstracts were extracted, and two reviewers (MK, AF) independently reviewed these for relevance. Any conflicts were resolved through consensus. Where consensus could not be reached, a third reviewer (JB) was consulted. Full-text articles were obtained for all articles deemed relevant. The full-text articles were also reviewed independently by the same two reviewers. Articles were assessed for relevance against a set of inclusion and exclusion criteria (see Sect. 2.2, Eligibility). Those articles that met the criteria were included for data extraction. Reference lists of included studies were also reviewed manually to identify any remaining publications. Any titles found to be relevant in the reference lists underwent the same abstract and full-text screening process.
2.2 Eligibility
Studies were included if they (1) examined individuals between the ages of 18 and 45 years; and (2) evaluated the immediate effect of a gait retraining or footwear intervention on a relevant measure of tibial loads during running [28,29,30,31,32]. Gait retraining interventions were considered as those that aimed to modify the participants’ running technique by using targeted technique changes, footwear, surfaces etc. Relevant measures of tibial loading included were (1) stress or strain measures obtained from strain gauges embedded in the tibia, or finite element models; (2) impulse, peak tibial acceleration or impact forces from inertial sensors or accelerometers placed on the tibia; and (3) joint reaction or contact forces at the ankle estimated from musculoskeletal models. Studies that only investigated ground reaction force measures to infer tibial loading were excluded due to ground reaction forces alone not being well correlated with bone strain measures [33]. Recent studies have also implied that wearable sensors such as accelerometers may have the same poor correlations with tibial loading measurements as that of ground reaction forces, particularly at the bone level [33, 34]. While we acknowledge these limitations of tibial acceleration measures, particularly on inclined surfaces, they have been included in this review due to associations with tibial stress injury [35]. Studies were excluded from the review if (1) animal or cadaveric models were used; (2) data from a baseline or control condition to compare the intervention effect were not available; and (3) the full text was not accessible.
2.3 Risk of Bias
Risk of bias of the included studies was assessed using a modified version of the Cochrane Collaboration’s tool for assessing risk of bias in randomised trials [36]. The modified version removed the performance bias criterion as, in most cases, it was considered impossible to blind participants to the interventions used. The authors deemed it important that participants had familiarisation with the interventions, and therefore a familiarisation bias was added. Lastly a statistical bias criterion was added; studies needed to use an appropriate statistical method for paired data and provide an estimate of variability (i.e., standard deviation, standard error) to be evaluated as low risk of bias.
2.4 Data Extraction
Data were extracted from the included articles by a single reviewer (MK). The following data were extracted from the included studies: (1) participant characteristics (i.e. number of participants, male/female ratio, age, height, weight, running kilometres/mileage per week and running experience where available); (2) details of the gait retraining technique used; (3) how running technique was assessed (i.e. overground vs. treadmill, running distance and speed); and (4) the tibial load assessment method (i.e. strain gauge, finite element analysis, accelerometer, musculoskeletal modelling) and measure (i.e. stress, strain, tibial acceleration or impact forces, joint reaction or contact forces) used. Corresponding authors were contacted where data could not be extracted from the full text alone. If no response was provided and data could not be extracted, the study was excluded from further analysis.
2.5 Data and Statistical Analysis
Studies were categorised based on the gait retraining intervention. Based on the set of included studies, each intervention and corresponding data were separated into seven categories: (1) barefoot (i.e. a change from shod to barefoot running); (2) footwear (i.e. modification or change in footwear); (3) speed (i.e. modification to running speed); (4) surface (i.e. modification in running surface); (5) overground versus treadmill (i.e. comparison of overground to treadmill running); (6) orthotics, insoles and taping (i.e., interventions that included the use of orthotics, cushioned insoles or taping/bracing methods); and (7) technique (i.e., biomechanical modifications to running technique). These were separated into further subcategories where appropriate. Footwear interventions were separated into five subcategories: (1) high-cut shoes; (2) shoes with increased cushioning; (3) minimalist shoes (in habitual wearers); (4) minimalist shoes (in non-habitual wearers); and (5) motion control shoes. Orthotics, insoles, and taping interventions were separated into a further five subcategories: (1) cushioned insoles; (2) rigid orthotics; (3) semi-rigid orthotics; (4) soft orthotics; and (5) taping and bracing. Surface interventions were separated into four subcategories: (1) grass; (2) normal versus a padded treadmill; (3) synthetic surfaces; and (4) woodchips. Finally, technique interventions were separated into nine subcategories, (1) anterior trunk lean; (2) increased cadence; (3) forefoot strike; (4) real-time biofeedback; (5) grounded running; (6) increased stride length; (7) decreased stride length; (8) increased step width; and (9) decreased step width. Standardised mean differences (SMDs) with 95% confidence intervals (CIs) were calculated between the baseline and intervention condition(s) for all studies, and meta-analyses were performed where possible to identify pooled effects.
Random-effects meta-analyses were conducted (where possible) using STATA version 16 (StataCorp LLC, College Station, TX, USA). Categories that contained a single intervention were unable to be pooled for meta-analyses. The meta-analyses used mean and standard deviation to calculate SMDs (Hedges’ g) with 95% CIs. Due to several studies having small sample sizes (i.e., < 20), Hedges’ g [37] was opted for over Cohen’s d [38]. Effect sizes were interpreted as trivial (< 0.20), small (0.20–0.59), moderate (0.60–1.19), large (1.20–1.99), and very large (≥ 2.00) [39]. Heterogeneity of studies was assessed using the Higgins I2 statistic. I2 percentages were interpreted as small (> 25%), medium (> 50%) or high (> 75%) levels of heterogeneity between studies [40].
The certainty of pooled evidence was evaluated using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system [41]. Randomised controlled trials were rated as high-grade evidence and observational studies started as low-grade evidence. The overall quality was then downgraded one level to moderate, low and/or very low dependent on the following criteria: imprecision (total sample size < 400 participants), inconsistency (high statistical heterogeneity > 50%), and risk of bias (more than 50% of studies having one or more risk-of-bias item criterion considered high risk).
3 Results
3.1 Literature Search Results
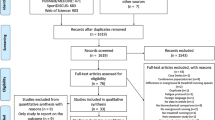
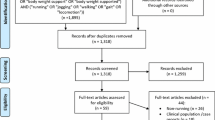
The electronic database and reference list searches yielded a total of 1617 articles for screening, of which 362 were found to be duplicates and were removed prior to title and abstract screening (Fig. 1). Title and abstract screening found 79 potentially relevant articles, of which 43 were removed during full-text screening. The final number of studies included in the review was 36.
3.2 Study Characteristics
All studies were a crossover design, with 19 randomising the order of conditions. The included articles were published between 1996 and 2021 and included 677 participants (of those, 420 were known to be male, 186 were female, and 71 were not specified). Eighty-two interventions were extracted from the 36 included articles (i.e., certain studies included multiple interventions). Of these interventions, four were categorised as barefoot; 11 as footwear; 11 as orthotic, insole and taping; three as overground versus treadmill; 21 as speed; 10 as surface; and 20 as technique-based interventions. Two surface and one speed intervention extracted from the same study [42], and one speed intervention from a separate study [43], were not included in the meta-analysis due to an insufficient number of participants (n = 1). The methods used to evaluate tibial loads included accelerometers placed on the tibia (n = 27, 75%), musculoskeletal models (n = 3, 8.33%), finite element models (n = 2, 5.56%) and tibia-embedded strain/stress gauges (n = 4, 11.11%). Detailed study characteristics for each intervention category are reported in Tables 1, 2, 3, 4, 5, 6 and 7.
3.3 Risk of Bias
Risk-of-bias assessments are reported in Fig. 2. Six studies reported a high risk of random sequence bias, five studies reported high risk of allocation concealment bias, four studies reported high risk of familiarisation bias and three studies reported high risk of statistical bias. None of the included studies were found to have a high risk of bias for the selective reporting or incomplete data criterion. No studies were removed due to risk-of-bias outcomes.
3.4 Quality Assessment
All studies were of crossover design, with all categories except speed having fewer than 100 participants; hence, GRADE identified all pooled variables as having a very low certainty of evidence (see Table 8).
3.5 General Findings
3.5.1 Barefoot
Four studies [23, 44,45,46] examined the effect of barefoot running compared with shod on tibial loading. Barefoot running ‘moderately’ increased tibial load measurements with medium heterogeneity (I2 = 65.88%, SMD 1.16; 95% CI 0.50, 1.82) (Fig. 3; Table 1).
3.5.2 Footwear
One study [47] examined the effect of high-cut shoes on tibial loading and found a ‘moderate’ decrease in tibial loading (I2 = not applicable, SMD − 0.94; 95% CI − 1.76, − 0.13) (Fig. 4a; Table 2). One study [48] examined the effects of two different levels of shoe cushioning on tibial loading compared with a conventional running shoe, with no clear directional effect (I2 = 0%, SMD − 0.30; 95% CI − 0.75, 0.16) (Fig. 4b, Table 2). Two studies [49, 50] comparing minimalist shoe use in habitual wearers with the use of a conventional running shoe found no clear directional effect on tibial loading (I2 = 88.77%, SMD 0.34; 95% CI − 1.33, 2.00) (Fig. 4c, Table 2). Three studies [23, 46, 49] comparing minimalist shoe use in non-habitual wearers with use of a conventional running shoe found a ‘moderate’ increase in tibial loading (I2 = 0%, SMD 0.89; 95% CI 0.40, 1.39) (Fig. 4d, Table 2). Two studies [24, 51] with three different cohorts examined the effect of a motion control shoe, compared with a conventional running shoe, on tibial loading. Motion control shoes resulted in a ‘small’ increase in tibial loading (I2 = 0%, SMD 0.46; 95% CI 0.07, 0.84) (Fig. 4e, Table 2).
Individual and pooled effects of tibial loads when running in a I. high-cut shoe; II. shoe with increased cushioning; III. minimalist shoe (habitual wearers); IV. minimalist shoe (non-habitual wearers); and V. motion control shoe compared with a conventional running shoe. Where two or more interventions from the same study were included in a subcategory, symbols were used to distinguish the difference. aMedium cushioning; bhigh cushioning; chigh-arched participant pool; dlow-arched participant pool. SMD standardised mean difference, CI confidence interval, T2 Tau2, Q Chi2, I2 Higgins I2 statistic
3.5.3 Orthotics, Insoles and Taping
Two studies [52, 53] found that three different cushioned insoles did not have a clear directional effect on tibial loading (I2 = 0%, SMD − 0.03; 95% CI − 0.32, 0.25) (Fig. 5a, Table 3). Two studies [25, 26] found rigid orthotics did not have a clear directional effect on tibial loading (I2 = 0%, SMD − 0.10; 95% CI − 0.59, 0.40) (Fig. 5b, Table 3). One study [54] found semi-rigid orthotics did not have a clear directional effect on tibial loading (I2 = not applicable, SMD − 0.01; 95% CI − 0.89, 0.87) (Fig. 5c, Table 3). Two studies [25, 54] found soft orthotics did not have a clear directional effect on tibial loading (I2 = 0%, SMD − 0.03; 95% CI − 0.51, 0.58) (Fig. 5d, Table 3). One study [55] reported that three different taping and bracing techniques did not have a clear directional effect on tibial loading (I2 = 0%, SMD − 0.05; 95% CI − 0.54, 0.43) (Fig. 5e, Table 3).
Individual and pooled effects of tibial load when running in a conventional shoe with I. cushioned insoles; II. rigid orthotics; III. semi-rigid orthotics; IV. soft orthotics; and V. taping and bracing compared with a conventional running shoe without intervention. Where two or more interventions from the same study were included in a subcategory, symbols were used to distinguish the difference. aPrefabricated insoles; bcustom insoles; ctape; dbrace/cast; etape with brace/cast. SMD standardised mean difference, CI confidence interval, T2 Tau2, Q Chi2, I2 Higgins I2 statistic
3.5.4 Overground Versus Treadmill
Three studies [56,57,58] reported that running on a treadmill, compared with running overground, resulted in a ‘moderate’ decrease in tibial loading (SMD − 0.83; 95% CI − 1.53, − 0.12) (Fig. 6, Table 4), with a ‘small’ amount of heterogeneity detected between interventions (I2 = 48.22%) (Fig. 6).
3.5.5 Speed
Ten studies [19, 20, 31, 46, 48, 59,60,61,62,63] reported 21 changes in speed. Increased speed resulted in a ‘moderate’ increase in tibial loading (SMD 0.87; 95% CI 0.61, 1.13) (Fig. 7, Table 5). A statistically significantly moderate to high level of heterogeneity was detected between studies (I2 = 75.15%) (Fig. 7).
Pooled effects of tibial load when running with increased speed. Where two or more interventions from the same study were included in the speed category, symbols were used to distinguish the difference. a2.5 m/s vs. 3.5 m/s; b2.5 m/s vs. 4.5 m/s; c2.7 m/s vs. 3.27 m/s; d2.7 m/s vs. 4.08 m/s; e3.2 m/s vs. 3.8 m/s; f3.2 m/s vs. 4.5 m/s; g3.2 m/s vs. 5.1 m/s; h3.2 m/s vs. 5.7 m/s; i3.2 m/s vs. 6.4 m/s; j2.7 m/s vs. 3.0 m/s; k2.7 m/s vs. 3.3 m/s; l2.7 m/s vs. 3.7 m/s; m90% preferred vs. preferred speed (female population); n90% preferred vs. preferred speed (female population); o90% preferred vs. preferred speed (male population); p90% preferred vs. 110% preferred speed (male population). SMD standardised mean difference, CI confidence interval, T2 Tau2, Q Chi2, I2 Higgins I2 statistic
3.5.6 Surface
Two studies [56, 58] found that changing from concrete to grass did not have a clear directional effect on tibial loading (I2 = 0%, SMD − 0.21; 95% CI − 0.69, 0.27) (Fig. 8a, Table 6). One study [56] found that changing from a normal to a padded treadmill had no clear directional effect on tibial loading (I2 = not applicable, SMD − 0.41; 95% CI − 1.17, 0.34) (Fig. 8b, Table 6). Four studies [56, 58, 59, 61] found that changing from concrete to a synthetic surface did not have a clear directional effect on tibial loading (SMD − 0.45; 95% CI − 0.98, 0.09) (Fig. 8c, Table 6), with a ‘moderate’ level of heterogeneity detected between these studies (I2 = 60.64%) (Fig. 8c). One study [59] found that changing from a concrete to a woodchip track did not have a clear directional effect on tibial loading (I2 = not applicable, SMD − 0.31; 95% CI − 0.78, 0.16) (Fig. 8d, Table 6).
3.5.7 Technique
One study [17] found that increasing anterior trunk lean by 10° had no clear directional effect on tibial loading (I2 = not applicable, SMD 0.45; 95% CI − 0.18, 1.08) (Fig. 9a, Table 7). Two studies [17, 18] found that increasing cadence by 10% had no clear directional effect on tibial loading (I2 = 46.11%, SMD − 0.25; 95% CI − 0.88, 0.37) (Fig. 9b, Table 7). Five studies [17, 18, 26, 44, 45] found that changing from a rearfoot to a forefoot strike pattern did not have a clear directional effect on tibial loading (SMD − 0.84; 95% CI − 2.41, 0.72) (Fig. 9c, Table 7). There was also an ‘extremely high’ level of heterogeneity between studies on foot strike pattern (I2 = 95.96%). Two studies [64, 65] reporting four biofeedback variables found that real-time biofeedback decreased tibial loading ‘moderately’ (I2 = 0%, SMD − 0.93; 95% CI − 1.46, − 0.41) (Fig. 9d, Table 7). One study [60] found that transitioning to grounded running resulted in a ‘very large’ decrease in tibial loading (I2 = not applicable, SMD − 2.45; 95% CI –3.11, − 1.79) (Fig. 9e, Table 7). One study [66] found that increasing stride length causes a ‘moderate’ increase in tibial loading (I2 = 15.80%, SMD 0.86; 95% CI 0.18, 1.55) (Fig. 9f, Table 7), while two studies [21, 66] found that decreasing stride length had no clear directional effect on tibial loading (I2 = 0%, SMD − 0.30; 95% CI − 0.79, 0.19) (Fig. 9g, Table 7). One study [16] found that increasing step width had no clear effect on tibial loading (I2 = not applicable, SMD 0.32; 95% CI − 0.36, 1.00) (Fig. 9h, Table 7). Similarly, decreasing step width also had no clear directional effect (I2 = not applicable, SMD − 0.34; 95% CI − 1.02, 0.34) (Fig. 9i, Table 7).
Individual and pooled effects of tibial load of interventions that modified running technique by I. increasing anterior trunk lean; II. increasing cadence; III. changing runners to a forefoot strike; IV. using real-time biofeedback; V. using grounded running; VI. increasing stride length; VIII. decreasing stride length; IX. increasing step width; and i decreasing step width. Where two or more interventions from the same study were included in a subcategory, symbols were used to distinguish the difference. a− 20% of preferred stride length; b− 10% of preferred stride length; c+ 10% of preferred stride length; d+ 20% of preferred stride length; evisual feedback period; fpost-visual feedback period. SMD standardised mean difference, CI confidence interval, T2 Tau2, Q Chi2, I2 Higgins I2 statistic
4 Discussion
The magnitude of tibial loading during running is an important risk factor for tibial stress injuries [35, 67]. A wide array of interventions have been adopted to modify tibial loads during running [16, 20, 21, 65]. This review found the greatest increase in tibial loading when individuals ran barefoot compared with shod (SMD 3.43). Increased tibial load was also found for non-habitual users running in a minimalist shoe (SMD 0.89), increased running speed (SMD 0.87), increased stride length (SMD 0.86) and motion control shoe use (SMD 0.46). Tibial loading decreased with targeted biofeedback (SMD − 0.93) and when running on a treadmill versus overground (SMD − 0.83). These findings can be useful to prescribe interventions to potentially reduce tibial loading during training or when returning from tibial stress injury.
This review found that increases in running speed moderately increase tibial loading. A significantly moderate to high level of heterogeneity was detected between studies (I2 = 74.80%), likely explained by the variability in speeds used. While all studies increased speed, the magnitude of increase and baseline speeds varied among studies, which created considerable variability in individual effect sizes. Irrespective of the variability, there was a consistent effect for elevated tibial loading with an increase in running speed. Due to high heterogeneity, the magnitude of the overall effect size should be interpreted with caution. Furthermore, tibial stress injuries are multifactorial and high-speed running speeds combined with other factors such as high training volume or limited rest could increase the risk of injury.
This review also found that as individuals increase above preferred stride length, a moderate increase in tibial loads occurred. An increase in stride length has been noted to increase ground reaction forces during running [68], and these increases may result in larger braking forces being absorbed by the tibia [62, 66]. However, this review found a decrease in preferred stride length had no clear directional effect on tibial loading. This outcome is both supported and contradicted by the kinematics and kinetics of altered stride length. One study found that hip, knee, and ankle joint moments did not change when stride length was decreased in a shod condition, yet vertical ground reaction forces decreased [68]. Another study found decreased stride length (resulting in increased stride frequency) can reduce joint loads, vertical impact peaks, vertical instantaneous and average loading rate [69]. As previously noted, reaction forces alone are not well correlated with bone strain measures and may only have a small contribution to tibial loads [33]. More research may be warranted to gain better insight into the effects decreasing stride length has on tibial loading, and the associated mechanisms driving any potential change.
No clear directional effect was found on tibial loading when runners increased cadence and changed to a forefoot strike. There were limited studies examining altered cadence, with the two studies [17, 18] included having opposite directional effects (SMD − 0.57 vs. SMD 0.07). A few differences that could have potentially affected the outcomes were the difference in participant sex (male only vs. males and females), average age (21.7 ± 2.6 vs. 32.1 ± 9.8), the difference in the weekly running mileage of participants (17.1 ± 10.1 vs. 33.5 ± 17.5) and the total number of outcome variables tested in a session (11 vs. 3) [17, 18]. Other aspects of the studies were very similar, including the placement of tibial accelerometers. The mixed evidence presented suggests further investigation into the effects of cadence on tibial loading is warranted. Variable outcomes of included studies [17, 18, 26, 44, 45] also played a role in the inconclusive results of foot strike interventions. This was reflected in the high heterogeneity reported between the included studies (I2 = 95.96%). A potential reason for this high variance was that these studies included a mix of normal shod [17, 18, 26] and barefoot running [44, 45]. It is possible that shifting to a forefoot strike under these different footwear conditions has a variable effect on any changes in tibial loads.
Our results indicated a moderate decrease in tibial loading when audio or visual biofeedback was used. The studies in this analysis used a sound or visual cue to keep tibial acceleration peaks under a set threshold. It is unclear if these reductions were caused by kinetics; one included study found participants with the greatest reduction in accelerations also showed reductions in ground reaction forces, and instantaneous and average loading rates [64]. Although these changes were observed, ground reaction forces are often only a small contributor to loads experienced by the bone [19, 70]. It is also unclear if the observed reductions occurred due to changes in running kinematics, as these were not reported in either study [64, 65]. The relatively simplistic nature of these feedback processes suggests biofeedback could be a useful approach for reducing tibial loading in the field (e.g., including sound cues in a runner’s headphones). There is also the potential that biofeedback has long-term effects on tibial load measures. Decreases in peak tibial accelerations have been observed 1 month after completing gait retraining using visual biofeedback [71]. However, further studies with longer-term follow-ups are required to better understand the retention of gait retraining interventions in real-world scenarios.
Barefoot compared with shod running moderately increased tibial load measures. The studies [23, 44,45,46] within this analysis all used novice barefoot runners and may therefore not reflect tibial load changes in habitual barefoot runners. During early exposure to barefoot running, habitually shod runners may not alter landing patterns from a heel strike to a mid/forefoot pattern [72]. Individuals who naturally run with a rear foot strike pattern may be prone to increased lower limb loading during the impact phase of running when transitioning to barefoot [73]. These results suggest that the introduction of barefoot running to those inexperienced with the concept could elevate tibial loads. Although no studies in this review included habitual barefoot runners, a similar notion was observed when considering the use of minimalist shoes. Our review found that minimalist shoes worn by those unaccustomed to this footwear moderately increased tibial loading. The same increase in tibial loading was not observed in habitual minimalist shoe users; however these results are likely influenced by survivor bias, where included subjects were unlikely to experience any negative effects of minimalist shoes, allowing them to become habitual users. Hence, these results may not broadly represent the entire running population. Where a runner plans to shift to barefoot running or running in a minimalist shoe, a gradual introduction may be required to not increase the risk of tibial stress injury. Furthermore, introducing barefoot running or minimalist shoes in those who are rehabilitating or at risk of a tibial stress injury should likely be avoided.
This review also found increases in tibial loads when a motion control shoe was adopted during running. The aim of a motion control shoe is to reduce rear foot eversion during running and walking [51]. A consequence of increased rearfoot eversion may be an altered load distribution within the lower extremity, predisposing individuals to a tibial stress injury [74]. Elevated rear foot eversion while running has been cited as a potential risk factor for tibial stress injuries [74, 75]. Although the included studies indicated that rearfoot eversion was reduced with motion control shoes, the meta-analysis still found small increases in tibial loading. Despite the motion control shoes targeting a mechanism thought to be linked to tibial stress injury risk (i.e., rearfoot eversion), this did not translate to a reduction in measured tibial loads. The evidence from this review suggests that motion control shoes may not reduce tibial loads during running.
Only one study was identified that examined high- versus low-cut shoes [47]. The study indicated that there was a ‘moderate’ decrease in tibial loading when wearing a high-compared with low-cut football cleat (SMD − 0.94) [47]. High-cut shoes may be beneficial in reducing tibial loads when worn during running activities; however these assumptions are currently only applicable to football cleats. This may be beneficial to those who regularly wear football cleats, but the application of this research is limited and cannot be applied to the majority who are at risk of tibial stress injuries (i.e., distance runners). Further research is warranted in other types of high-cut footwear and is necessary before any conclusions can be made regarding the effect these may have on tibial loading in other running populations.
Cushioned shoes and insoles were found to not have a clear directional effect on tibial loading during running. Individuals adapt their running kinematics to match the stiffness of shoes and surfaces [76, 77]. A runner increases leg stiffness, particularly at the ankle joint, when running in softer shoes to maintain large enough ground reaction forces for running [76]. Increases in leg stiffness may increase the loads that are transmitted through the musculoskeletal system [77]. The above-mentioned adaptations may be why minimal changes were observed when comparing normal shoes with softer, more compliant shoes and insoles. This review also found that rigid and soft orthotics had no clear directional effect on tibial loading. Semi-rigid orthotics were also included in the study; however meta-analysis was not possible due to only one study examining this intervention. Our review also found no clear directional effect of ankle taping or bracing on tibial loading when used during running. Minimal effects on tibial load were likely observed due to a lack of change in foot and ankle biomechanics with these interventions [25, 26].
No clear directional effect was found when individuals changed from a concrete surface to a grass surface, and no clear directional effect was found when running on a synthetic surface compared with a concrete surface. Lastly, only one study looked at running on a padded treadmill versus a conventional treadmill, and only one study compared running on concrete and running on a woodchip surface; therefore, a meta-analysis was not performed for either of these interventions. The overarching aim of all these interventions is to produce a more cushioned, compliant running surface. As discussed earlier, individuals adapt their running kinematics to match the stiffness of shoes [76, 77], with these adaptations also extending to when running on softer surfaces; this could again explain why no changes in tibial stress measures were observed when running on softer surfaces. However, this review found a moderate decrease in tibial loading when individuals ran on a treadmill versus overground surface (e.g., concrete). The difference in stiffness between overground and treadmill surfaces is a theory for the changes in tibial load [78]; however, our results comparing surfaces suggest that this may not be the predominant reason for a reduction in tibial load during treadmill running. Treadmill running has been shown to reduce vertical displacement [78]. The reduced vertical displacement decreases the amount of vertical acceleration, which in turn decreases the vertical forces during running [78]. Individuals running on a treadmill may also reduce braking and propulsion forces compared with when running overground [78]. It is proposed that the altered loading conditions experienced when running on a treadmill versus overground are the more likely mechanisms responsible for the decreased tibial loading observed. There is evidence to suggest treadmill running may elevate tibial loading due to greater ankle joint moments and planter flexor muscle forces [78]. However, strain gauges attached directly to the tibia have shown decreases in both compressive and tensile loads of the tibia when running on a treadmill compared with overground [57]. Individuals in training periods of higher load or recovering from previous tibial stress injury could use treadmills to supplement overground training. This could potentially reduce overall or cumulative loads on the tibia and reduce the risk of injury/re-injury.
Several technique-based interventions could not be pooled for meta-analyses. These included increased and decreased step width, increased anterior trunk lean and grounded running. Modifications to step width and trunk lean had no directional effect on tibial loading, and therefore neither could be supported for modifying tibial load based on current evidence. Adopting a grounded running technique generated a ‘very large’ decrease in tibial accelerations (SMD − 2.45). This result is not particularly surprising, as the typically slower speeds and biomechanical alterations associated with grounded running reduce the biomechanical loading of the lower extremities [79]. Grounded running may therefore be a promising technique adaptation for reducing tibial loads, yet there is little understanding of the feasibility of gait-retraining techniques for promoting grounded running long-term [79].
4.1 Limitations
This review included a vast number of tibial load variables; however the majority (75%) of the measures used to assume tibial load were accelerometry metrics from wearable sensors. Recent studies have indicated that wearable sensors may have the same poor correlations as ground reaction forces for the measurement of tibial loading, particularly at the bone level [33, 34]. However, the vertical average loading rate recent studies used to assume this poor correlation were found to be well correlated to estimates of bone force when running on level ground [33]. All studies included in this paper were run on a level ground. Reductions in accelerometry-based loads are also likely indicative of a ‘softer’ foot strike. There is recent evidence to suggest that ‘softer’ foot strike or running in a more flexed posture could increase muscle forces during running, causing an increase in bone loading that may be missed by accelerometry measures [80]. It has been identified that for every 1 g increase in peak positive acceleration, the likelihood of having a history of TSF increases by a factor of 1.361 [35]. The magnitude of peak tibial acceleration could also predict group membership (injured vs. uninjured) in 70% of cases [35]. Preliminary prospective findings have also indicated that individuals with tibial stress injury show 15% greater tibial acceleration than their uninjured counterparts [81]. This evidence suggests that acceleration measures may be a good predictor of tibial stress injury, and altering these measures could therefore be an important component of injury prevention. While the relationship between accelerometry and tibial stress needs further investigation, we acknowledge the limitations of tibial acceleration measures, particularly on inclined surfaces. As work in this area progresses, researchers should persist with higher-quality measures (e.g., bone-level stress estimates) to improve the understanding these interventions have on tibial loading. Further insights on the effect of muscle forces, tibial moments, tibial stress and strain are likely necessary to yield the greatest understanding of what interventions are the most effective.
The included studies contain a large amount of variance in the methodological processes used to understand the effects of the chosen interventions. These differences occur in the experimental procedures (running speed, placement of accelerometer, run time, etc.), measurement type used (accelerometry, estimation from musculoskeletal model, strain gauges, etc.) and the equipment used during these methods (variation in shoe brands etc.). A subjective decision to group interventions was made, despite certain variations in methodology, to provide an understanding of the broader overall effects of interventions. Due to this, some intervention categories examined have shown high heterogeneity, even when results are conclusive (e.g., speed).
There was also variation in the populations used across included studies, and all studies investigated healthy individuals. The physical activity/sport profiles of participants varied, with studies including team sport athletes, recreational runners, elite runners, and even sedentary populations. Due to the populations only being healthy individuals, it is unclear if these interventions would have the same effect on those who are presenting with pain, and current or existing tibial stress injuries. Caution should be exercised when applying the interventions to these populations.
This review only examined the immediate (i.e., within session) effects of interventions. Training variables such as weekly running volume, training frequency, and frequency of rest days are important to consider in the context of tibial stress injuries. Interpretation of results from this review should be considered in light of these other training variables that may affect tibial stress injuries.
Finally, the GRADE system assessed all pooled evidence as very low certainty. This was predominantly driven by the type of research (crossover trials) and the lack of participants. Considering this, caution may be necessary with the interpretation of these findings. There is a need for more robust, randomised trials with larger participant samples in the area of gait retraining interventions for modifying tibial loads during running.
5 Conclusion
This review found that tibial loading increased when running barefoot, in motion control shoes, and when minimalist shoes were adopted by non-habitual users. Increased stride length and running speed were also found to increase tibial loading. These conditions may need to be avoided during training periods of high running volume or be avoided by runners recovering from a tibial stress injury. Running on a treadmill versus overground, as well as the use of biofeedback, can reduce tibial loading. These interventions could be adopted to reduce tibial load in healthy populations during training. These interventions may also be beneficial to individuals in rehabilitation from tibial stress injuries, but it is unknown if this population will respond the same or differently to the intervention. Caution should be exercised when interpreting for injured runners as the interventions included in this review may have not been evaluated in injured populations.
References
Hootman JM, Macera CA, Ainsworth BE, Addy CL, Martin M, Blair SN. Epidemiology of musculoskeletal injuries among sedentary and physically active adults. Med Sci Sports Exerc. 2002;34:838–44.
Rizzone KH, Ackerman KE, Roos KG, Dompier TP, Kerr ZY. The epidemiology of stress fractures in collegiate student-athletes, 2004–2005 through 2013–2014 academic years. J Athl Train. 2017;52:966–75.
Korpelainen R, Orava S, Karpakka J, Siira P, Hulkko A. Risk factors for recurrent stress fractures in athletes. Am J Sports Med. 2001;29:304–10.
Taunton JE. A retrospective case-control analysis of 2002 running injuries. Br J Sports Med. 2002;36:95–101.
Beck BR. Tibial stress injuries. An aetiological review for the purposes of guiding management. Sports Med. 1998;26:265–79.
Harris JD, Varner KE. Stress fractures of the tibia. In: Miller TL, Kaeding CC, editors. Stress fractures in Athletes. Cham: Springer International Publishing; 2015. p. 137–47.
Jamieson M, Everson S, Siegel C, Miller TL. Expected time to return to athletic participation following stress fracture in division I collegiate athletes. Orthop J Sports Med. 2016;4(7).
Mujika I, Padilla S. Detraining: Loss of training-induced physiological and performance adaptations. Part I. Sports Med. 2000;30:79–87.
Burr DB, Milgrom C, Boyd R, Higgins RGC, Radin EL. Experimental stress fractures of the tibia. Clin J Sport Med. 1991;1:70.
Pattin CA, Caler WE, Carter DR. Cyclic mechanical property degradation during fatigue loading of cortical bone. J Biomech. 1996;29:69–79.
Brewer RB, Gregory AJM. Chronic lower leg pain in athletes: A guide for the differential diagnosis, evaluation, and treatment. Sports Health. 2012;4:121–7.
Fredericson M, Bergman AG, Hoffman KL, Frcp C, Dillingham MS. Tibial stress reaction in runners correlation of clinical symptoms and scintigraphy with a new magnetic resonance imaging grading system. Am J Sports Med. 1995;23(4):472–81.
Bennell K, Matheson G, Meeuwisse W, Brukner P. Risk factors for stress fractures. Sports Med. 1999;28(2):91–122.
Beck BR, Rudolph K, Matheson GO, Bergman AG, Norling TL. Risk factors for tibial stress injuries. Clin J Sport Med. 2014;25:230–6.
Warden SJ, Burr DB, Brukner PD. Stress fractures: pathophysiology, epidemiology, and risk factors. Curr Osteoporos Rep. 2006;43(3):103–9.
Meardon SA, Derrick TR. Effect of step width manipulation on tibial stress during running. J Biomech. 2014;47:2738–44.
Huang Y, Xia H, Chen G, Cheng S, Cheung RTHH, Shull PB. Foot strike pattern, step rate, and trunk posture combined gait modifications to reduce impact loading during running. J Biomech. 2019;86:102–9.
Yong JR, Silder A, Montgomery KL, Fredericson M, Delp SL. Acute changes in foot strike pattern and cadence affect running parameters associated with tibial stress fractures. J Biomech. 2018;76:1–7.
Brent Edwards W, Taylor D, Rudolphi TJ, Gillette JC, Derrick TR, Taylor D, et al. Effects of running speed on a probabilistic stress fracture model. Clin Biomech. 2010;25:372–7.
Hunter JG, Garcia GL, Shim JK, Miller RH. Fast running does not contribute more to cumulative load than slow running. Med Sci Sports Exerc. 2019;51:1178–85.
Edwards WB, Taylor D, Rudolphi TJ, Gillette JC, Derrick TR. Effects of stride length and running mileage on a probabilistic stress fracture model. Med Sci Sports Exerc. 2009;41:2177–84.
Pérez-Soriano P, Sanchis-Sanchis R, Lison JF, Sánchez-Zuriaga D, Llana-Belloch S, et al. Impact attenuation during gait wearing unstable vs traditional shoes. Eur J Hum Mov. 2019;42:30–41.
Sinclair J, Greenhalgh A, Brooks D, Edmundson CJ, Hobbs SJ. The influence of barefoot and barefoot-inspired footwear on the kinetics and kinematics of running in comparison to conventional running shoes. Footwear Sci. 2013;5:45–53.
Butler RJ, Davis IS, Hamill J. Interaction of arch type and footwear on running mechanics. Am J Sports Med. 2006;34:1998–2005.
Butler RJ, Davis IM, Laughton CM, Hughes M. Dual-function foot orthosis: effect on shock and control of rearfoot motion. Foot Ankle Int. 2003;24:410–4.
Laughton CACA, Davis IM, Hamill J, McClay Davis I, Hamill J. Effect of strike pattern and orthotic intervention on tibial shock during running. J Appl Biomech. 2003;19:153–68.
Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D, Antes G, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;339: b2535.
Hreljac A, Imamura RT, Escamilla RF, Edwards WB, MacLeod T. The relationship between joint kinetic factors and the walk-run gait transition speed during human locomotion. J Appl Biomech. 2008;24:149–57.
Hreljac A. Effects of physical characteristics on the gait transition speed during human locomotion. Hum Mov Sci. 1995;14:205–16.
Brisswalter J, Mottet D. Energy cost and stride duration variability at preferred transition gait speed between walking and running. Can J Appl Physiol. 1996;21:471–80.
Sheerin KR, Besier TF, Reid D. The influence of running velocity on resultant tibial acceleration in runners. Sports Biomech. 2020;19(6):750–60.
Rotstein A, Inbar O, Berginsky T, Meckel Y. Preferred transition speed between walking and running: effects of training status. Med Sci Sports Exerc. 2005;37:1864–70.
Matijevich ES, Branscombe LM, Scott LR, Zelik KE. Ground reaction force metrics are not strongly correlated with tibial bone load when running across speeds and slopes: implications for science, sport and wearable tech. PLoS One. 2019;14:1–19.
Matijevich ES, Scott LR, Volgyesi P, Derry KH, Zelik KE. Combining wearable sensor signals, machine learning and biomechanics to estimate tibial bone force and damage during running. Hum Mov Sci. 2020;74: 102690.
Milner CE, Ferber R, Pollard CD, Hamill J, Davis IS. Biomechanical factors associated with tibial stress fracture in female runners. Med Sci Sports Exerc. 2006;38:323–8.
Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343: d5928.
Hedges LV, Olkin I. Statistical methods for meta-analysis. Cambridge: Academic Press; 2014.
Cohen J. Statistical power analysis for the behavioral sciences. Power Anal Behav Sci. 1977;567:645.
Hopkins WG. A new view of statistics. Internet Society for Sport Science; 2000. https://www.sportsci.org/resource/stats/. Accessed 14 Nov 2020.
Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490.
Burr DBDB, Milgrom C, Fyhrie D, Forwood M, Nyska M, Finestone A, et al. In vivo measurement of human tibial strains during vigorous activity. Bone. 1996;18:405–10.
Milgrom C, Finestone AS, Voloshin A. Differences in the principal strain angles during activities performed on natural hilly terrain versus engineered surfaces. Clin Biomech. 2020;80: 105146.
Lucas-Cuevas AG, Priego Quesada JI, Giménez JV, Aparicio I, Jimenez-Perez I, Pérez-Soriano P. Initiating running barefoot: Effects on muscle activation and impact accelerations in habitually rearfoot shod runners. Eur J Sport Sci. 2016;16:1145–52.
Olin ED, Gutierrez GM. EMG and tibial shock upon the first attempt at barefoot running. Hum Mov Sci. 2013;32:343–52.
Sinclair J, Taylor PJ, Andrews S. Influence of barefoot, barefoot inspired and conventional shoes on tibial accelerations and loading kinetics during running in natural rearfoot strikers. Comp Exerc Physiol. 2013;9:161–7.
Sinclair J, Rooney E, Naemi R, Atkins S, Chockalingam N. Effects of footwear variations on three-dimensional kinematics and tibial accelerations of specific movements in American football. J Mech Med Biol. 2017;17:1750026.
Lam W-KK, Liebenberg J, Woo J, Park S-KK, Yoon S-HH, Cheung RT-HH, et al. Do running speed and shoe cushioning influence impact loading and tibial shock in basketball players? Peer J. 2018;6:e4753.
Sinclair J, Taylor PJ, Liles NB. Effects of running with minimal and conventional footwear in habitual and non-habitual users: a musculoskeletal simulation and statistical parametric mapping based approach. Footwear Sci. 2020;12:25–38.
Izquierdo-Renau M, Queralt A, Encarnación-Martínez A, Perez-Soriano P. Impact acceleration during prolonged running while wearing conventional versus minimalist shoes. Res Q Exerc Sport. 2021;92:182–8.
Butler RJ, Hamill J, Davis I. Effect of footwear on high and low arched runners’ mechanics during a prolonged run. Gait Posture. 2007;26:219–25.
Lucas-Cuevas AG, Camacho-García A, Llinares R, Priego Quesada JI, Llana-Belloch S, Pérez-Soriano P, et al. Influence of custom-made and prefabricated insoles before and after an intense run. PLoS One. 2017;12:1–14.
O’Leary K, Vorpahl KA, Heiderscheit B. Effect of cushioned insoles on impact forces during running. J Am Podiatr Med. 2008;98:36–41.
Ekenman I, Milgrom C, Finestone A, Begin M, Olin C, Arndt T, et al. The role of biomechanical shoe orthoses in tibial stress fracture prevention. Am J Sports Med. 2002;30:866–70.
Kersting UG, Kriwet A, Brüggemann GP. The role of footwear-independent variations in rearfoot movement on impact attenuation in heel-toe running. Res Sports Med. 2006;14:117–34.
Fu W, Fang Y, Liu DMS, Wang L, Ren S, Liu Y. Surface effects on in-shoe plantar pressure and tibial impact during running. J Sport Health Sci. 2015;4:384–90.
Milgrom C, Finestone A, Segev S, Olin C, Arndt T, Ekenman I. Are overground or treadmill runners more likely to sustain tibial stress fracture? Br J Sports Med. 2003;37:160–3.
Milner CE, Hawkins JL, Aubol KG. Tibial acceleration during running is higher in field testing than indoor testing. Med Sci Sports Exerc. 2020;52:1361–6.
Boey H, Aeles J, Schütte K, Vanwanseele B. The effect of three surface conditions, speed and running experience on vertical acceleration of the tibia during running. Sports Biomech. 2017;16:166–76.
Bonnaerens S, Fiers P, Galle S, Aerts P, Frederick EC, Kaneko Y, et al. Grounded running reduces musculoskeletal loading. Med Sci Sports Exerc. 2019;51:708–15.
Greenhalgh A, Sinclair J, Leat A, Chockalingam N. Influence of footwear choice, velocity and surfaces on tibial accelerations experienced by field hockey participants during running. Footwear Sci. 2012;4:213–9.
Mercer J, Vance J, Hreljac A, Hamill J. Relationship between shock attenuation and stride length during running at different velocities. Eur J Appl Physiol. 2002;87:403–8.
Meardon SA, Derrick TR, Willson JD, Baggaley M, Steinbaker CR, Marshall M, et al. Peak and per-step tibial bone stress during walking and running in female and male recreational runners. Am J Sports Med. 2021;49:2227–37.
Crowell HP, Milner CE, Hamill J, Davis IS. Reducing impact loading during running with the use of real-time visual feedback. J Orthop Sports Phys Ther. 2010;40:206–13.
Wood CM, Kipp K. Use of audio biofeedback to reduce tibial impact accelerations during running. J Biomech. 2014;47:1739–41.
Derrick TR, Hamill J, Caldwell GE. Energy absorption of impacts during running at various stride lengths. Med Sci Sports Exerc. 1998;30:128–35.
Davis I, Milner CE, Hamill J. Does increased loading during running lead to tibial stress fractures? a prospective study. Med Sci Sports Exerc. 2004;36:S58.
Thompson MA, Gutmann A, Seegmiller J, McGowan CP. The effect of stride length on the dynamics of barefoot and shod running. J Biomech. 2014;47:2745–50.
Sakaguchi M, Nakazawa K, Hobara H. Step frequency and lower extremity loading during running. Int J Sports Med. 2012;33:310–3.
Edwards WB. Modeling overuse injuries in sport as a mechanical fatigue phenomenon. Exerc Sport Sci Rev. 2018;46:224–31.
Crowell HP, Davis IS. Gait retraining to reduce lower extremity loading in runners. Clin Biomech. 2011;26:78–83.
Cheung RTH, Rainbow MJ. Landing pattern and vertical loading rates during first attempt of barefoot running in habitual shod runners. Hum Mov Sci. 2014;34:120–7.
Paquette MR, Zhang S, Dahl BL. Acute effects of barefoot, minimal shoes and running shoes on lower limb mechanics in rear and forefoot strike runners. Footwear Sci. 2013;5:9–18.
Milner CE, Hamill J, Davis IS. Distinct hip and rearfoot kinematics in female runners with a history of tibial stress fracture. J Orthop Sports Phys Ther. 2010;40:59–66.
Pohl MB, Mullineaux DR, Milner CE, Hamill J, Davis IS. Biomechanical predictors of retrospective tibial stress fractures in runners. J Biomech. 2008;41:1160–5.
Ferris DP, Louie M, Farley CT. Running in the real world: adjusting leg stiffness for different surfaces. Proc Biol Sci. 1998;265:989–94.
Smith G, Watanatada P. Adjustment to vertical displacement and stiffness with changes to running footwear stiffness. Med Sci Sports Exerc. 2002;34:S178.
van Hooren B, Fuller JT, Buckley JD, Miller JR, Sewell K, Rao G, et al. Is motorized treadmill running biomechanically comparable to overground running? A systematic review and meta-analysis of cross-over studies. Sports Med. 2020;50(4):785–813.
Davis S, Fox A, Bonacci J, Davis F. Mechanics, energetics and implementation of grounded running technique: a narrative review. BMJ Open Sport Exerc Med. 2020;6:1–8.
Baggaley M, Derrick TR, Vernillo G, Millet GY, Brent EW. Internal tibial forces and moments during graded running. J Biomech Eng. 2022;144(1): 011009.
Pohl MB, Davis IS, Hamill J. Prospective study of kinetic factors associated with tibial stress fractures in runners. American Society of Biomechanics Annual Meeting; 2007. p. 22–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. No funding was used for the conception of this review.
Conflict of interest
Meghan Keast, Jason Bonacci and Aaron Fox declare they have no conflicts of interest in relation to the contents of this article.
Availability of data and material
The data analysed in this review can be found in the referenced published articles or from the corresponding author upon reasonable request.
Code availability
Not applicable.
Authors’ contributions
Meghan Keast performed database searches, identified relevant articles, extracted and analysed the data, and is the primary author of this manuscript. Aaron Fox identified relevant articles, assisted with data analysis, and gave guidance and contributed to the written manuscript. Jason Bonacci contributed to the manuscript through editing and feedback. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Keast, M., Bonacci, J. & Fox, A. Acute Effects of Gait Interventions on Tibial Loads During Running: A Systematic Review and Meta-analysis. Sports Med 52, 2483–2509 (2022). https://doi.org/10.1007/s40279-022-01703-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-022-01703-1