Abstract
Background
It has been proposed that habitual physical activity improves appetite control; however, the evidence has never been systematically reviewed.
Objective
To examine whether appetite control (e.g. subjective appetite, appetite-related peptides, food intake) differs according to levels of physical activity.
Data Sources
Medline, Embase and SPORTDiscus were searched for articles published between 1996 and 2015, using keywords pertaining to physical activity, appetite, food intake and appetite-related peptides.
Study Selection
Articles were included if they involved healthy non-smoking adults (aged 18–64 years) participating in cross-sectional studies examining appetite control in active and inactive individuals; or before and after exercise training in previously inactive individuals.
Study Appraisal and Synthesis
Of 77 full-text articles assessed, 28 studies (14 cross-sectional; 14 exercise training) met the inclusion criteria.
Results
Appetite sensations and absolute energy intake did not differ consistently across studies. Active individuals had a greater ability to compensate for high-energy preloads through reductions in energy intake, in comparison with inactive controls. When physical activity level was graded across cross-sectional studies (low, medium, high, very high), a significant curvilinear effect on energy intake (z-scores) was observed.
Limitations
Methodological issues existed concerning the small number of studies, lack of objective quantification of food intake, and various definitions used to define active and inactive individuals.
Conclusion
Habitually active individuals showed improved compensation for the energy density of foods, but no consistent differences in appetite or absolute energy intake, in comparison with inactive individuals. This review supports a J-shaped relationship between physical activity level and energy intake. Further studies are required to confirm these findings.
PROSPERO Registration Number
CRD42015019696
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Habitual physical activity and appetite control are not independent of each other; they are interconnected. |
The relationship between physical activity level and energy intake is J-shaped. |
Objective assessment of all components of energy balance is necessary to improve understanding of this relationship. |
1 Introduction
The importance of physical activity in reducing morbidity and all-cause mortality [1] and in weight management [2] has become apparent. There has been increasing interest in the relationship between physical activity and appetite control, as both play an integral part in energy balance (e.g. [3–7]). Regular physical activity and exercise training are associated with several physiological adaptations, such as improved insulin sensitivity [8], leptin sensitivity [9, 10], blood pressure [11], blood lipids [12], substrate metabolism [13] and body composition [14], some of which have been proposed as mechanisms involved in eating behaviour [15, 16]. Scientific studies have tended to focus on the appetite responses to exercise rather than habitual physical activity levels per se. This distinction is important to make, as physical activity encompasses occupational, household, transportation and other activities, in addition to structured exercise [17], and the physiological adaptations to exercise and physical activity may differ. Few studies have specifically focused on the appetite control differences between physically active and inactive individuals, but there is some evidence suggesting that habitual physical activity improves appetite control by enhancing satiety signalling [18, 19]. Two recent reviews included secondary analyses on whether the relationship between acute or long-term exercise and energy intake is influenced by physical activity level [20, 21]. From their meta-analysis, Schubert et al. [21] found that absolute energy intake after acute exercise was greater in active individuals than in less active individuals, whereas Donnelly et al. [20] concluded from their systematic review that increased physical activity or exercise, regardless of physical activity level, had no consistent effect on acute or long-term energy intake. However, these reviews included only energy and macronutrient intake as their main outcome measures. As appetite control involves complex co-ordination of a range of homeostatic and non-homeostatic signals in the overall expression of food intake [22], in addition to energy intake, it is important to consider other components, such as appetite-related peptides, subjective appetite sensations, food choice and hedonic reward.
It has been proposed that regulation of the appetite control system and energy intake is improved with increasing levels of physical activity [23]. This issue has yet to be systematically reviewed, and the potential mechanisms behind any improvement in appetite control are unclear. The aims of this systematic review were to examine whether physically active individuals have more sensitive control over appetite than their inactive counterparts and if this confers on them the ability to better match energy intake to energy expenditure, and to identify behavioural or physiological mechanisms underlying any observed differences.
2 Methods
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (Electronic Supplementary Material Appendix S1) and is registered in the PROSPERO database (registration number CRD42015019696).
2.1 Search Strategy
A search was conducted in the databases Ovid Medline, Ovid Embase and SPORTDiscus (EBSCOHost), which included articles published between 1 January 1996 and 15 April 2015, using the strategy (physical activity AND (appetite AND (food intake OR appetite-related peptides))). Previous systematic reviews were screened to identify relevant subject headings and key words to include within each subject category. The specific key words used for the search are listed in Table 1, and the full search strategy for one of the databases that were consulted can be found in Electronic Supplementary Material Appendix S2. Limits were set to include articles published in the English language and studies conducted in human adults aged 18–64 years. Reference lists from the resulting articles were also screened to identify any additional articles.
2.2 Study Selection, Inclusion and Exclusion
Articles were included if they involved healthy adults participating in cross-sectional studies and examined appetite control in physically active and inactive individuals. Longitudinal studies assessing appetite control before and after an exercise-training intervention in previously inactive individuals were also included if the intervention lasted greater than 4 weeks (to allow sufficient time for adaptations from regular physical activity to emerge; e.g. see Cornelissen and Smart [11]) and did not include any concurrent dietary intervention (e.g. energy restriction, supplementation). Articles were excluded if they involved animals, children, adolescents, athletes or older adults (>65 years old) and participants who smoked. Abstracts and full-text articles were assessed for eligibility independently by two authors, with uncertainty regarding eligibility being discussed with an additional author.
2.3 Data Extraction and Synthesis
One author extracted the following information into a spreadsheet: authors, date of publication, sample size, participant characteristics (age, sex, body mass index [BMI], percentage body fat, maximal aerobic capacity [VO2max], physical activity details), criteria used to assess physical activity status (cross-sectional studies) or training intervention (longitudinal studies), setting, outcome measures (energy intake, appetite ratings and appetite-related peptides) and results. To determine any statistical relationship between habitual physical activity level and energy intake, where data were available, energy intake values were standardized (z-scores) and, from the definitions provided in the studies, physical activity levels were graded into low (<150 min/week, <1000 kcal/week or physical activity level 1.4–1.69), medium (150–419 min/week, 1000–2500 kcal/week or physical activity level 1.7–1.99), high (420–839 min/week or 2500 < 3500 kcal/week) or very high (>840 min/week or ≥3500 kcal/week). One-way analysis of variance (ANOVA) was then used to test for a main effect of graded physical activity level on energy intake score, followed by trend analyses for linear and non-linear functions. Other outcome measures are presented as a qualitative synthesis.
2.4 Risk of Bias
Risk of bias was assessed using the Cochrane Collaboration’s tool for assessing risk of bias for sequence generation, allocation concealment, blinding of participants, personnel and outcome assessors, incomplete outcome data, selective outcome reporting and other sources of bias [24] (Electronic Supplementary Material Table S1). Study inclusion was not influenced by the results of the risk of bias assessment.
3 Results
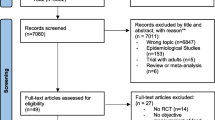
Figure 1 illustrates the systematic review flow diagram. The database search yielded 2078 articles, 1640 of which were eliminated on the basis of their titles and abstracts alone. The full text was retrieved for 77 articles, and 28 satisfied the inclusion criteria.
3.1 Cross-Sectional Studies
The results from the cross-sectional studies (n = 14) are presented in Table 2.
3.1.1 Study Characteristics: Physical Activity Definitions
The median (range) sample size of the included studies was 15 (7–968) for the active group and 14 (9–910) for the inactive group. Men and women were included in eight studies, of which the median percentage of men was 42.2 % (21.5–63.6 %) in the active group and 50 % (21.6–61.6 %) in the inactive group [25, 26, 28–32, 37]. Five studies included only men [19, 27, 33–35], and one study included only women [36].
Physical activity status was determined by self-report (a physical activity questionnaire, physical activity level question or physical activity recall) in 11 studies [19, 26, 28–30, 32, 33, 37], by a VO2max test in three studies [25, 27, 34] or from total daily energy expenditure (TDEE) and resting energy expenditure or basal metabolic rate (BMR) in three studies [31, 35, 36]. Only three studies used a combination of self-reported and objectively measured physical activity status [25, 35, 36].
The active groups were defined as participating in moderate to vigorous physical activity for at least 150 min/week [33, 35–37], 4 h/week [30], 5 h/week with a VO2max greater than 45 mL/kg/min [27], 3 days/week with a VO2max greater than 60 mL/kg/min [34], 4 days/week and >2500 kcal/week with a VO2max above average for age [25], or 1000 kcal/week [26]. A TDEE/BMR value between 1.70 and 1.99 was utilized in two studies [35, 36]. Moderate exercisers participated in 2–3 sessions/week of at least 40 min of moderate- to high-intensity physical activity [19] or expended between 1000 and 2500 kcal/week [26]. High exercisers participated in four or more structured exercise sessions/week of at least 40 min of moderate- to high-intensity physical activity [19] or expended 2500 > 3500 kcal/week [26], whereas very high exercisers expended 3500 kcal/week or greater [26].
The inactive groups were defined as having not exercised over the previous 6 months and VO2max values less than 50 mL/kg/min [34] or less than 1 session/week of moderate- to high-intensity physical activity [19], 20 min/day and 2 days/week [25], 60 min/week [33], 1000 kcal/week [26], 150 min/week of moderate-intensity physical activity [35, 36], 3 h/week of moderate- to high-intensity physical activity with a VO2max less than 45 mL/kg/min [27] or 4 h/week [30]. Two studies used a TDEE/BMR value between 1.4 and 1.69 [35, 36].
On the basis of the physical activity definitions above, for the purposes of statistical treatment, we distinguished physical activity levels as low (<150 min/week, <1000 kcal/week or physical activity level 1.4–1.69), medium (150–419 min/week, 1000–2500 kcal/week or physical activity level 1.7–1.99), high (420–839 min/week or 2500 > 3500 kcal/week) and very high (>840 min/week or ≥3500 kcal/week) for analysis of standardized energy intake.
3.1.2 Study Characteristics: Appetite-Related Measures
Five studies evaluated appetite measures in a laboratory [19, 30, 31, 33, 34], five studies did so in free-living conditions [25, 26, 28, 29, 32] and four studies combined laboratory and free-living measures [27, 35–37]. Four studies included exercise (45–60 min cycling at 50–75 % of VO2max or maximal heart rate [HRmax]) during the laboratory session [27, 33, 35, 36]. Ten studies included fasting and/or daily (area under the curve) subjective appetite ratings, all of which included hunger [19, 25, 27, 30, 31, 33–37]. Other appetite ratings assessed were fullness [25, 27, 30, 31, 33, 34, 37], prospective food consumption (PFC) [30, 31, 34], desire to eat [25, 27, 31], satiety [19, 30, 34], liking [33] and palatability [30]. One study reported restraint, disinhibition and susceptibility to hunger [26]. Eleven studies assessed energy intake, via either a food frequency questionnaire (FFQ) [26, 32], a food record [25], laboratory-based test meals [19, 31, 34] or a combination of laboratory-based test meals and food records [27, 33, 35–37]. Six studies reported energy compensation following either a preload [19, 37] or a single bout of exercise [27, 33, 35, 36]. Eight studies reported macronutrient intake [25–27, 32, 33, 35–37]. Three studies assessed food choices via an FFQ [28, 29, 32]. Two studies included assessment of appetite-related peptides [34, 37].
3.1.3 Participant Characteristics
The median (range) age was 23 (21–48) years for the active group and 22 (21–49) years for the inactive group.
In the ten studies that reported BMI for the active and inactive groups separately, the median (range) was 23.5 (21.9–25.2) kg/m2 for the active group and 24.1 (21.6–26.6) kg/m2 for the inactive group [19, 25–27, 29, 33–37]. In three studies, the inactive group had a significantly greater BMI than the active group [25, 27, 35]. In the studies that reported BMI for the groups combined, the median (range) was 24.8 (22.4–27.3) kg/m2 [28, 30–32].
In the seven studies that reported percentage body fat, the median (range) was 14.3 (12.0–22.5) % for the active group and 22.2 (15.0–27.2) % for the inactive group [25, 27, 33–37]. In all studies, the inactive group had a significantly greater percentage body fat than the active group.
In the six studies that reported VO2max, the median (range) was 49.6 (36.8–67.0) mL/kg/min for the active group and 36.3 (29.9–42.0) mL/kg/min for the inactive group [25, 27, 34–37]. In all studies, the active group had a significantly greater VO2max than the inactive group.
3.1.4 Study Results: Appetite Ratings
Of the ten studies that measured appetite ratings, three found differences between the physically active and inactive groups. Harrington et al. [31] reported greater fasting appetite and lower satiety quotient (SQ) [calculated as (pre-meal appetite rating minus post-meal appetite rating) divided by energy intake] for hunger, fullness, desire to eat and PFC in men in the high activity tertile compared to the moderate activity tertile, whereas Long et al. [19] reported greater fasting appetite in the inactive group. Gregersen et al. [30] found greater postprandial appetite in the active group, however differences became non-significant when age and sex were added as covariates.
3.1.5 Study Results: Energy and Macronutrient Intake
Ten of 11 studies found differences in energy intake between active and inactive individuals. Two studies found greater energy intake (habitual energy intake [37] or with a test meal [34]) in the active compared to the inactive group, whereas one study observed greater energy intake in inactive women over 4 days than active women [36]. Furthermore, two studies observed a non-linear relationship in energy intake, whereby energy intake was highest in the groups with the lowest and highest levels of physical activity [26, 31], while Jago et al. [32] only observed a greater energy intake in the very active group compared to the moderately active group. In studies assessing energy intake following a preload, Long et al. [19] found that energy intake at an ad libitum test meal following a high-energy preload was significantly lower than following the low-energy preload in regular exercisers. The same study showed that compared to non-exercisers, energy intake following the high-energy preload was significantly lower in exercisers. Moreover, Van Walleghen et al. [37] found that the active group consumed more throughout the day following the no-preload condition than the inactive group, leading to significantly more accurate short-term energy compensation. Of note, however, there were no differences in energy compensation between groups at the test meal after the preload [37]. In studies measuring energy intake after exercise, two of three studies in men observed energy compensation in the active group, where energy intake following an exercise session was greater compared to rest at test meal [33] or throughout the day (but not at the test meal in this study) [35]. One of these studies observed negative energy compensation in the inactive group, where energy intake was lower following the exercise session compared to rest, suggesting an effect of exercise-induced anorexia [33]. Of the above studies that observed differences between groups, only four were based on objectively measured (test meal) energy intake [19, 31, 33, 34].
As for macronutrient intake, compared to the inactive group, two studies found that the active group consumed a greater percentage of energy from carbohydrates [26, 37], three found a lower percentage of energy from fat [26, 32, 37], while one study found a greater percentage of energy from protein [33]. In terms of food choices, active individuals reported a greater intake of nutrient-dense, low-fat foods [29], fruits and 100 % fruit juices [28], and dairy products [32], and a lower intake of burgers and sandwiches [28] and fried foods [32] than inactive.
3.1.6 Study Results: Standardized Energy Intake
To further examine the relationship between energy intake and physical activity level, the available energy intake data from the cross-sectional studies [25–27, 31–37] were extracted and transformed into standardized scores then plotted according to physical activity level (low, medium, high, very high) as described in Sect. 3.1.1. In the studies that included a preload or an exercise bout [27, 33, 35, 36], energy intake was taken from the control condition. Of these ten studies, eight were based on self-reported daily energy intake [25–27, 32, 33, 35–37] while two were based on energy intake at a test meal [31, 34]. The pattern of means revealed a J-shaped curve for energy intake as habitual physical activity level increased (Fig. 2). One-way ANOVA confirmed a main effect of graded physical activity level on energy intake score [F(3,21) = 3.57, P = 0.03]. Post hoc trend analyses revealed significant effects for linear [F = 5.79, P = 0.03] and curvilinear (quadratic) [F = 8.10, P = 0.01] functions.
Standardized energy intake by physical activity level from the ten cross-sectional studies reporting energy intake (n = 25 data points). Trend analysis confirmed significant linear (P < 0.05) and quadratic (P < 0.01) relationships between the graded physical activity level and energy intake scores. The thick black line indicates the mean of the z-scores. SEM standard error of the mean
3.1.7 Study Results: Appetite-Related Peptides
Van Walleghen et al. [37] found greater insulin sensitivity in the active group. Lund et al. [34] found that in active individuals, glucagon-like peptide-1 (GLP-1) and acylated ghrelin were higher at baseline (insulin tended to be lower), and following a liquid meal, GLP-1 was higher and insulin was lower in active. No group differences were found for peptide YY (PYY) and pancreatic polypeptide.
3.2 Exercise-Training Interventions
The results from the exercise-training interventions (n = 14) are presented in Table 3.
3.2.1 Study Characteristics: Exercise Intervention
The median (range) duration of the interventions was 12 (4–72) weeks of exercise 5 (3–7) days/week. Exercise duration was prescribed in minutes or energy expenditure (kcal), at intensities in percentage of VO2max or percentage of HRmax. The median exercise prescription was 43.8 (30–60) min or 500 (300–600) kcal/session at 68.5 (45–90) % of VO2max or 70 (70–75) % of HRmax. Eleven training interventions involved aerobic exercise [18, 39–43, 45–49], two interventions involved resistance exercise [43, 50] and one intervention compared moderate-intensity interval training and high-intensity interval training in a crossover design [38]. One study did not specify the exercise modality [44]. In 11 of the 14 interventions the exercise was supervised [18, 39–43, 45, 47, 48]. Nine studies collected appetite-related measures in a laboratory [18, 38–41, 43, 45, 47, 49], two studies in free-living conditions [44, 50], and three studies in a combination of laboratory and free-living conditions [42, 46, 48].
3.2.2 Study Characteristics: Appetite-Related Measures
Ten studies included fasting and/or daily (area under the curve) appetite ratings, all of which included hunger [18, 38, 40, 42, 43, 45–49]. Fullness [18, 38, 41, 43, 45–49], PFC [18, 42, 45, 47–49], desire to eat [18, 38, 41, 45, 47, 48], satiety [42, 49], liking and palatability [46, 49] were also assessed. Three studies measured restraint, disinhibition and susceptibility to hunger [39, 42, 49]; one study included the Power of Food Scale, the Craving and Mood Questionnaire and the Food Craving Inventory [42]; one study included the Eating Behaviour Inventory [44]; and one study assessed liking and wanting for foods varying in fat and sweetness [38]. Eleven studies assessed energy intake, via an FFQ [44], food record [42, 50], test meals [38–41, 45, 49], or combination of test meals and food records [46, 48]. Two studies measured energy intake following high- and low-energy preloads [46, 48] and one at high- and low-energy density meals [40]. Seven studies reported macronutrient intake [38, 42, 44–46, 48, 50]. Six studies assessed appetite-related peptides in the fasting state [42, 43, 46–49] and three in response to food ingestion [43, 47, 48].
3.2.3 Participant Characteristics
The median (range) age was 38 (28–49) years and the sample size of the included studies was 18 (10–88). Men and women were included in nine studies, of which the median percentage of men was 33.7 (23.5–53.3) % [18, 39–42, 45–48]. Four studies only included men [38, 43, 49, 50] and one study only included women [44].
Nine studies reported BMI before and after the intervention [39–41, 43, 44, 46–49], the median (range) was 30.5 (22.7–31.8) kg/m2 at baseline and 30.1 (22.8–31.1) kg/m2 post-intervention. Seven of these reported a significantly lower BMI after the exercise intervention [39, 41, 43, 44, 47–49]. In the four studies that only reported baseline BMI [18, 38, 42, 45], the median (range) was 31.8 (30.7–33.3) kg/m2.
Eight studies reported percentage body fat values before and after the intervention, the median (range) was 34.3 (23.6–44.1) % at baseline and 32.4 (23.0–42.5) % post-intervention [39–41, 44, 46–48, 50]. Seven of these reported a significantly lower percentage body fat after the intervention [39–41, 44, 47, 48, 50]. In the three studies that reported only baseline percentage body fat, the median (range) was 34.6 (31.2–37.2) % [38, 42, 45].
In the five studies that reported VO2max before and after the intervention, the median (range) was 32.9 (29.1–36.2) mL/kg/min at baseline and 37.7 (34.3–43.3) mL/kg/min post-intervention [41, 46–49]. In all studies, the increase in VO2max with training was significant. In the four studies that only reported baseline VO2max, the median (range) was 28.8 (28.4–29.1) mL/kg/min [18, 38, 39, 45].
3.2.4 Study Results: Appetite Ratings
Exercise training led to differences in appetite ratings in five of ten studies. Three studies found an increase in fasting hunger [18, 41, 47], desire to eat and PFC [47], and a decrease in fullness [47]. However, two studies found that fasting fullness increased following aerobic [43] and high-dose aerobic (600 kcal/day) [49] exercise training. King et al. [18] reported a greater daily hunger, desire to eat and lower fullness post-training in a subsample of non-responders to exercise-induced weight loss (i.e. individuals with changes in body composition below that expected based on the total exercise-induced energy expenditure). In response to a standardized breakfast, Martins et al. [47] found an increase in hunger and desire to eat following exercise training, whereas Guelfi et al. [43] found an increase in fullness after an oral glucose tolerance test following aerobic training.
The two studies that included the SQ found increases post-training [18, 41]. Only one of three studies found a reduction in disinhibition and an increase in restraint post-training [39].
3.2.5 Study Results: Energy and Macronutrient Intake
Five of 11 studies found differences in energy intake after the exercise-training intervention. Daily energy intake was lower post-training in one study [42], while it increased in a subsample of compensators in another study [45]. As for high-energy test meal challenges, Caudwell et al. [40] showed a reduction in meal size containing high-energy density foods, and two studies demonstrated that energy intake was lower throughout the day after a high-energy preload compared to a low-energy preload [46, 48].
One study showed an increase in the percentage of energy from fat in a subsample of compensators (individuals whose weight loss after exercise training was less than predicted on the basis of the total exercise-induced energy expenditure) [45] and another after moderate-intensity interval training [38]. Training led to an increase in the percentage of energy from protein in another study [46].
3.2.6 Study Results: Appetite-Related Peptides
Of the studies that assessed fasting peptides, five found differences following exercise training, where leptin [42, 43, 48] and insulin decreased [47, 49], and ghrelin increased [47]. Insulin sensitivity improved after training in two of three studies [43, 47]. Of note, the study that found no improvement in insulin sensitivity was half the duration of the two others (6 vs 12 weeks) [46]. All three studies that assessed the peptide response to food ingestion found training effects, where postprandial leptin [43, 48] and insulin decreased [43, 47] after aerobic training, while there was a tendency for GLP-1 in the late postprandial period to increase with training [47].
4 Discussion
4.1 Appetite Control in Active and Inactive Individuals
This systematic review investigated differences in appetite ratings, food intake and appetite-related peptides between active and inactive (or previously inactive) individuals in order to determine whether habitual physical activity improves appetite control. In terms of fasting, postprandial or daily appetite ratings, studies reported mixed results, such that no clear differences could be distinguished between physically active and inactive individuals. It has been suggested that combining appetite sensations with objectively measured energy intake to calculate parameters such as the SQ can provide a better indication of the ability of the energy consumed to affect appetite. One cross-sectional study [31] and two exercise-training studies [18, 41] assessed the SQ, with conflicting results; however, the former measured the SQ during an ad libitum meal while in the latter studies, the SQ was measured during a standardized meal. These differences, along with differences in the protocols in the other studies, may have accounted for the contradictory results in appetite ratings.
Several studies focused on the measurement of energy intake, but, again, no consistent differences were found between active and inactive individuals. However, these simple comparisons precluded the possibility that physical inactivity may lead to a dysregulation of appetite and subsequent overconsumption, meaning that differences between active and inactive individuals may not always be apparent. Indeed, we have recently argued that the relationship between physical activity level and energy intake may follow a curvilinear function [23]. After transforming absolute energy intake into standardized scores and distinguishing levels of physical activity from the definitions of the ‘active’ groups used in the cross-sectional studies, we were able to test this hypothesis. The results revealed a significant quadratic effect illustrated by a J-shaped curve across physical activity levels (see Fig. 2). A similar J-shaped relationship has recently been suggested by Shook et al. [51], who compared estimated energy intake, using an equation based on changes in body composition, across quintiles of physical activity in a large heterogeneous sample of young adults. Their analysis provides further support to our synthesis of the literature, which demonstrates that the relationship between physical activity level and energy intake is non-linear, as was postulated by Mayer et al. [52] almost 60 years ago. In Bengali jute mill workers whose daily occupations ranged from ‘sedentary’ to ‘very heavy’ work, daily energy expenditure and daily energy intake were closely matched at higher levels of daily physical activity, but at low levels of daily physical activity, this coupling was lost, such that daily energy intake exceeded expenditure in those performing ‘sedentary’ or ‘light’ work [52]. This relationship may explain why differences in energy intake may not be obvious between active and inactive individuals, as they stand at similar levels on the energy intake curve. As our findings are based on standardized scores from the results of studies using various methodologies and protocols [25–27, 31–37], and Shook et al. [51] inferred from changes in body composition, confirmation of this J-shaped relationship is required with objective measures of energy intake in studies designed to assess intake across well-defined physical activity levels.
Of interest to this review are the studies that used preload challenges or macronutrient manipulations to examine whether differences exist in the ability to adjust energy intake after previous food intake or in meals that vary in composition. Three studies demonstrated that physically active individuals have a better ability to make adjustments in energy intake following a high-energy preload [19, 46, 48], suggesting increased sensitivity to previous energy intake (e.g. greater satiety). Another preload study also found more accurate energy compensation in active individuals, where the no-preload condition led to an increase in energy intake in active individuals but not in inactive individuals [37]. In line with these studies, one study found that exercise training led to a reduction in meal size at a high-energy density meal but not at a low-energy density meal [40]. This also supports the proposition of increased sensitivity to the energy density of foods, but this time during a meal (e.g. greater satiation). Interestingly, in this study it appeared that women may have been more susceptible to the effect than men. Therefore, further studies in males and females are required to confirm this finding and the potential interaction between physical activity and energy density on the sensitivity of appetite control. Nonetheless, these data support a J-shaped relationship between physical activity level and energy intake, and suggest a better ability to regulate energy intake with increasing levels of physical activity.
Despite the effects observed following a preload, there was no consistent effect of physical activity level on energy compensation immediately after an exercise bout or over several hours or days after exercise [27, 33, 35, 36, 38]. These results do not support a recent meta-analysis that found that absolute energy intake after acute exercise was greater in active individuals than in those who were less active [21]. However, this analysis reported only absolute energy intake and not energy compensation. In fact, Charlot and Chapelot [27] report in their study on lean/fit and fat/unfit men that energy compensation after exercise was highly variable, and they found no clear differences between groups. This raises the concern of the reliability of the measure of energy compensation (discussed in Sect. 4.3). Nevertheless, in the short-term, it appears that in physically active individuals, the regulation of energy intake may be more sensitive to previous food intake than to exercise.
4.2 Differences in the Proposed Mechanisms of Appetite Control
Eating behaviour is influenced by several proposed mechanisms, one of which is appetite-related peptides. Acute exercise and exercise training also affect these peptides [53, 54]. The studies that measured the peptide response to food intake found lower postprandial insulin levels [34, 43, 47, 49] and higher postprandial GLP-1 levels [34] (and tendency [47]) in active individuals. An emphasis on insulin will be given, as it was the most commonly measured hormone in the studies within the review. Interestingly, the same subjects who showed a preload effect in the study by Martins et al. [48] also showed an improvement in insulin sensitivity [47]. Additionally, the aerobic training group in the study by Guelfi et al. [43] had significantly lowered postprandial insulin and improved insulin sensitivity, with concomitant changes in postprandial fullness. However, the resistance-training group in the same study had a tendency for lower postprandial insulin (P = 0.066) and also had improved insulin sensitivity after training, without an effect on postprandial appetite ratings, while another study that showed a preload effect after 6 weeks of training did not find a significant improvement in insulin sensitivity [46]. Despite the relationship between insulin and appetite control not being consistent in the above studies, a meta-analysis by Flint et al. [55] proposed that insulin resistance could lead to disrupted satiety signalling. This meta-analysis showed that postprandial insulin was associated with satiety in individuals with a healthy weight but not in overweight individuals; however, it did not take into account the physical activity status of the participants, nor their body composition (fat mass and fat-free mass).
Measuring body composition, rather than just BMI, has become important in understanding the mechanisms affecting eating behaviour, as fat-free mass (but not fat mass) was found to be associated with daily energy intake and meal size in overweight and obese individuals [56]. In addition to appetite signals from adipose tissue and gut hormones, Blundell et al. [56] proposed a role for fat-free mass and resting metabolic rate as drivers of food intake. Differences in body composition were apparent in the cross-sectional studies, as six reported lower body fat percentage in active individuals [25, 33–37], despite only two reporting a lower BMI [25, 35]. Three of the former studies reported enhanced appetite control in terms of more accurate energy compensation [33, 35, 37]. No cross-sectional studies compared lean and overweight active individuals, thus a question arises as whether ‘fat but fit’ individuals would have enhanced appetite control. Four training studies conducted in overweight participants reported improvements in appetite control post-intervention (but also showed significant reductions in fat mass) [40, 41, 43, 48]. Overall, these studies indicate that differences in body composition and insulin sensitivity may be factors promoting more sensitive appetite control in active individuals. Furthermore, a recent study found faster gastric emptying in active males than in inactive males [57], proposing another mechanism by which appetite control (i.e. satiety signalling) could be better regulated in physically active individuals. More studies are required to elucidate the mechanisms involved in the appetite control differences between active and inactive individuals, such as body composition, postprandial satiety and hunger peptides, insulin (and possibly leptin [9, 10]) sensitivity and gastric emptying, in addition to resting metabolic rate [40, 56] and substrate oxidation [58], which were not covered in this review.
4.3 Methodological Considerations
A number of points regarding the methodologies used in the studies included in this review need addressing. In the cross-sectional studies, the definitions used for active and inactive individuals varied markedly. For example, some studies used only a self-rated measure (‘yes or no’ question [29] or a Likert scale [28, 30, 32]) or a self-reported measure (physical activity questionnaires [26, 37] or diaries/recalls [19, 33]) instead of objectively assessing physical activity via accelerometry. This may have confounded the results of the active groups from participants overestimating their physical activity habits [59, 60]. Moreover, some studies only used VO2max [27, 34] to define the active groups, which may not have reflected all aspects of physical activity (e.g. low- to moderate-intensity activity) [61]. Clear definitions of activity levels should be set in place to allow future studies to investigate appetite and energy intake across these defined levels. Along these lines, the studies in this review preclude us from distinguishing the effects of the several aspects of physical activity—such as time spent in low, moderate and vigorous activities, cardiovascular fitness and activity-related energy expenditure—on appetite control. In addition, future studies should assess all components of energy intake and energy expenditure in order to determine their influence on eating behaviour, particularly in light of recent evidence suggesting a plateau in daily energy expenditure above a certain threshold of physical activity [62]. This would allow us to tease out whether changes in cardiovascular fitness and/or physical activity energy expenditure are most important for appetite control. Secondly, food intake was assessed both in laboratory conditions (using test meals) and in free-living conditions (using an FFQ or food diaries). Test meals are known to be a rigorous method of assessing energy intake (under controlled laboratory conditions), but food diaries—despite providing a longer window of observation of ‘real world’ feeding patterns—may lead to underreporting and biased results [59]. It should be noted that the short-term results (daily energy intake) observed in the preload studies were based on food diaries [19, 37, 46, 48]. These data should be replicated in more rigorous conditions to confirm the observed effects. Thirdly, the within-subject consistency (i.e. test–retest reliability) and between-subject consistency (i.e. interindividual variability) in energy compensation following preload intake is often not acknowledged in studies, and this should be addressed in light of recent studies demonstrating marked interindividual variability [27, 63–65] and modest test–retest reliability [66] in energy compensation following acute exercise. The composition of the preloads and tests meals should also be further examined to determine whether physical activity enhances the sensitivity to energy density or to specific macronutrients. Finally, the sample size in most of the studies was small, which may have resulted in non-significant results and caused relatively small but important effects to be overlooked. The studies were also not designed to test the effects of sex, body composition (lean versus overweight) and exercise mode; therefore, this does not allow us to determine specific criteria or characteristics eliciting the reported effects (or lack thereof).
4.4 Review Limitations
This review included a limited number of studies assessing a broad range of appetite-related measures between active and inactive individuals, using various definitions. This may have led to some of the inconsistent patterns or lack of effects observed. Physical activity encompasses not only exercise training but also activities of daily living, and, as most definitions were based on a minimal level of moderate-intensity structured exercise, the studies included in this review leaned towards a comparison between exercise-trained and untrained individuals. Therefore, these results should be interpreted with caution while more studies assessing all facets of habitual physical activity become available. Clearly, there is a lot more work to be done to elucidate the effects of physical activity and exercise on the appetite control system.
5 Conclusion
It can be concluded from this review that habitually active individuals appear to have increased sensitivity to the energy density of foods, in comparison with inactive individuals, despite the lack of observable group differences in subjective appetite ratings. This review also supports the formulation that the relationship between physical activity level and energy intake may be non-linear, as reflected by the J-shaped curve obtained from analysis of standardized energy intake scores. The mechanisms underlying this effect are not known but could include differences in body composition (fat mass and fat-free mass), postprandial hunger or satiety peptides, or sensitivity to tonic peptides, such as insulin or leptin. This characteristic of active individuals could mitigate the risk of overconsumption in an energy-dense food environment. Further studies are required to confirm these findings.
References
Blair SN, Cheng Y, Holder JS. Is physical activity or physical fitness more important in defining health benefits? Med Sci Sports Exerc. 2001;33(6 Suppl):S379–99.
Donnelly JE, Blair SN, Jakicic JM, et al. American College of Sports Medicine position stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41(2):459–71. doi:10.1249/MSS.0b013e3181949333.
Caudwell P, Gibbons C, Finlayson G, et al. Physical activity, energy intake, and obesity: the links between exercise and appetite. Curr Obes Rep. 2013;2(2):185–90. doi:10.1007/s13679-013-0051-1.
Martins C, Robertson MD, Morgan LM. Effects of exercise and restrained eating behaviour on appetite control. Proc Nutr Soc. 2008;67(01):28–41. doi:10.1017/S0029665108005995.
Prentice A, Jebb S. Energy intake/physical activity interactions in the homeostasis of body weight regulation. Nutr Rev. 2004;62(7 Pt 2):S98–104.
Martins C, Morgan L, Truby H. A review of the effects of exercise on appetite regulation: an obesity perspective. Int J Obes. 2008;32(9):1337–47.
Riou M-È, Jomphe-Tremblay S, Lamothe G, et al. Predictors of energy compensation during exercise interventions: a systematic review. Nutrients. 2015;7(5):3677–704.
Goodyear LJ, Kahn BB. Exercise, glucose transport, and insulin sensitivity. Annu Rev Med. 1998;49:235–61.
Dyck DJ. Leptin sensitivity in skeletal muscle is modulated by diet and exercise. Exerc Sport Sci Rev. 2005;33(4):189–94.
Steinberg GR, Smith AC, Wormald S, et al. Endurance training partially reverses dietary-induced leptin resistance in rodent skeletal muscle. Am J Physiol Endocrinol Metab. 2004;286(1):E57–63.
Cornelissen VA, Smart NA. Exercise training for blood pressure: a systematic review and meta-analysis. J Am Heart Assoc. 2013;2:e004473. doi:10.1161/JAHA.112.004473.
Durstine JL, Haskell WL. Effects of exercise training on plasma lipids and lipoproteins. Exerc Sport Sci Rev. 1994;22:477–521.
Richter EA, Ruderman NB. AMPK and the biochemistry of exercise: implications for human health and disease. Biochem J. 2009;418(2):261–75.
Stiegler P, Cunliffe A. The role of diet and exercise for the maintenance of fat-free mass and resting metabolic rate during weight loss. Sports Med. 2006;36(3):239–62.
Blundell JE, Gibbons C, Caudwell P, et al. Appetite control and energy balance: impact of exercise. Obes Rev. 2015;16:67–76. doi:10.1111/obr.12257.
King NA, Horner K, Hills AP, et al. Exercise, appetite and weight management: understanding the compensatory responses in eating behaviour and how they contribute to variability in exercise-induced weight loss. Br J Sports Med. 2012;46(5):315–22.
Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126–31.
King NA, Caudwell PP, Hopkins M, et al. Dual-process action of exercise on appetite control: increase in orexigenic drive but improvement in meal-induced satiety. Am J Clin Nutr. 2009;90(4):921–7.
Long SJ, Hart K, Morgan LM. The ability of habitual exercise to influence appetite and food intake in response to high- and low-energy preloads in man. Br J Nutr. 2002;87(5):517–23.
Donnelly JE, Herrmann SD, Lambourne K, et al. Does increased exercise or physical activity alter ad-libitum daily energy intake or macronutrient composition in healthy adults? A systematic review. PloS One. 2014;9(1):e83498. doi:10.1371/journal.pone.0083498.
Schubert MM, Desbrow B, Sabapathy S, et al. Acute exercise and subsequent energy intake. A meta-analysis. Appetite. 2013;63:92–104. doi:10.1016/j.appet.2012.12.010.
Schwartz MW, Woods SC, Porte D, et al. Central nervous system control of food intake. Nature. 2000;404(6778):661–71. doi:10.1038/35007534.
Blundell JE. Physical activity and appetite control: can we close the energy gap? Nutr Bull. 2011;36(3):356–66. doi:10.1111/j.1467-3010.2011.01911.x.
Higgins J, Green S. Cochrane handbook for systematic reviews of interventions. Chichester, UK: Wiley Online Library; 2008.
Apolzan JW, Flynn MG, McFarlin BK, et al. Age and physical activity status effects on appetite and mood state in older humans. Appl Physiol Nutr Metab. 2009;34(2):203–11. doi:10.1139/h08-150.
Catenacci VA, Odgen L, Phelan S, et al. Dietary habits and weight maintenance success in high versus low exercisers in the National Weight Control Registry. J Phys Act Health. 2014;11(8):1540–8.
Charlot K, Chapelot D. Energy compensation after an aerobic exercise session in high-fat/low-fit and low-fat/high-fit young male subjects. Br J Nutr. 2013;110(6):1133–42.
Deshmukh-Taskar P, Nicklas TA, Yang SJ, et al. Does food group consumption vary by differences in socioeconomic, demographic, and lifestyle factors in young adults? The Bogalusa Heart Study. J Am Diet Assoc. 2007;107(2):223–34.
Georgiou C, Betts N, Hoos T, et al. Young adult exercisers and nonexercisers differ in food attitudes, perceived dietary changes, and food choices. Int J Sport Nutr. 1996;6(4):402–13.
Gregersen NT, Moller BK, Raben A, et al. Determinants of appetite ratings: the role of age, gender, BMI, physical activity, smoking habits, and diet/weight concern. Food Nutr Res. 2011. doi:10.3402/fnr.v55i0.7028.
Harrington DM, Martin CK, Ravussin E, et al. Activity related energy expenditure, appetite and energy intake. Potential implications for weight management. Appetite. 2013;67:1–7.
Jago R, Nicklas T, Yang S, et al. Physical activity and health enhancing dietary behaviors in young adults: Bogalusa Heart Study. Prev Med. 2005;41(1):194–202.
Jokisch E, Coletta A, Raynor HA. Acute energy compensation and macronutrient intake following exercise in active and inactive males who are normal weight. Appetite. 2012;58(2):722–9.
Lund MT, Taudorf L, Hartmann B, et al. Meal induced gut hormone secretion is altered in aerobically trained compared to sedentary young healthy males. Eur J Appl Physiol. 2013;113(11):2737–47.
Rocha J, Paxman J, Dalton C, et al. Effects of an acute bout of aerobic exercise on immediate and subsequent three-day food intake and energy expenditure in active and inactive men. Appetite. 2013;71:369–78.
Rocha J, Paxman J, Dalton C, et al. Effects of an acute bout of aerobic exercise on immediate and subsequent three-day food intake and energy expenditure in active and inactive pre-menopausal women taking oral contraceptives. Appetite. 2015;89:183–91. doi:10.1016/j.appet.2015.02.005.
Van Walleghen EL, Orr JS, Gentile CL, et al. Habitual physical activity differentially affects acute and short-term energy intake regulation in young and older adults. Int J Obes. 2007;31(8):1277–85.
Alkahtani SA, Byrne NM, Hills AP, et al. Interval training intensity affects energy intake compensation in obese men. Int J Sport Nutr Exerc Metab. 2014;24(6):595–604.
Bryant EJ, Caudwell P, Hopkins ME, et al. Psycho-markers of weight loss. The roles of TFEQ disinhibition and restraint in exercise-induced weight management. Appetite. 2012;58(1):234–41.
Caudwell P, Finlayson G, Gibbons C, et al. Resting metabolic rate is associated with hunger, self-determined meal size, and daily energy intake and may represent a marker for appetite. Am J Clin Nutr. 2013;97(1):7–14.
Caudwell P, Gibbons C, Hopkins M, et al. No sex difference in body fat in response to supervised and measured exercise. Med Sci Sports Exerc. 2013;45(2):351–8.
Cornier MA, Melanson EL, Salzberg AK, et al. The effects of exercise on the neuronal response to food cues. Physiol Behav. 2012;105(4):1028–34.
Guelfi KJ, Donges CE, Duffield R. Beneficial effects of 12 weeks of aerobic compared with resistance exercise training on perceived appetite in previously sedentary overweight and obese men. Metabolism. 2013;62(2):235–43.
Jakicic JM, Otto AD, Lang W, et al. The effect of physical activity on 18-month weight change in overweight adults. Obesity. 2011;19(1):100–9.
King NA, Hopkins M, Caudwell P, et al. Individual variability following 12 weeks of supervised exercise: identification and characterization of compensation for exercise-induced weight loss. Int J Obes. 2008;32(1):177–84.
Martins C, Truby H, Morgan LM. Short-term appetite control in response to a 6-week exercise programme in sedentary volunteers. Br J Nutr. 2007;98(4):834–42.
Martins C, Kulseng B, King NA, et al. The effects of exercise-induced weight loss on appetite-related peptides and motivation to eat. J Clin Endocrinol Metab. 2010;95(4):1609–16.
Martins C, Kulseng B, Rehfeld JF, et al. Effect of chronic exercise on appetite control in overweight and obese individuals. Med Sci Sports Exerc. 2013;45(5):805–12.
Rosenkilde M, Reichkendler MH, Auerbach P, et al. Appetite regulation in overweight, sedentary men after different amounts of endurance exercise: a randomized controlled trial. J Appl Physiol. 2013;115(11):1599–609.
Shaw BS, Shaw I, Brown GA. Relationship between resistance training and self-reported habitual macronutrient and energy intake. S Afr J Res Sport. 2010;32(2):109–16.
Shook RP, Hand GA, Drenowatz C, et al. Low levels of physical activity are associated with dysregulation of energy intake and fat mass gain over 1 year. Am J Clin Nutr. 2015;102(6):1332–8. doi:10.3945/ajcn.115.115360.
Mayer J, Roy P, Mitra KP. Relation between caloric intake, body weight, and physical work: studies in an industrial male population in West Bengal. Am J Clin Nutr. 1956;4(2):169–75.
Schubert MM, Sabapathy S, Leveritt M, et al. Acute exercise and hormones related to appetite regulation: a meta-analysis. Sports Med. 2014;44(3):387–403. doi:10.1007/s40279-013-0120-3.
Stensel D. Exercise, appetite and appetite-regulating hormones: implications for food intake and weight control. Ann Nutr Metab. 2010;57(Suppl 2):36–42. doi:10.1159/000322702.
Flint A, Gregersen NT, Gluud LL, et al. Associations between postprandial insulin and blood glucose responses, appetite sensations and energy intake in normal weight and overweight individuals: a meta-analysis of test meal studies. Br J Nutr. 2007;98(1):17–25. doi:10.1017/s000711450768297x.
Blundell JE, Finlayson G, Gibbons C, et al. The biology of appetite control: do resting metabolic rate and fat-free mass drive energy intake? Physiol Behav. 2015. doi:10.1016/j.physbeh.2015.05.031.
Horner KM, Byrne NM, Cleghorn GJ, et al. Influence of habitual physical activity on gastric emptying in healthy males and relationships with body composition and energy expenditure. Br J Nutr. 2015;114(3):489–96. doi:10.1017/s0007114515002044.
Hopkins M, Jeukendrup A, King NA, et al. The relationship between substrate metabolism, exercise and appetite control: does glycogen availability influence the motivation to eat, energy intake or food choice? Sports Med. 2011;41(6):507–21. doi:10.2165/11588780-000000000-00000.
Dhurandhar NV, Schoeller D, Brown AW, et al. Energy balance measurement: when something is not better than nothing. Int J Obes. 2014;39(7):1109–13. doi:10.1038/ijo.2014.199.
Sallis JF, Saelens BE. Assessment of physical activity by self-report: status, limitations, and future directions. Res Q Exerc Sport. 2000;71(Suppl 2):1–14.
Jacobs DR Jr, Ainsworth BE, Hartman TJ, et al. A simultaneous evaluation of 10 commonly used physical activity questionnaires. Med Sci Sports Exerc. 1993;25(1):81–91.
Pontzer H, Durazo-Arvizu R, Dugas LR, et al. Constrained total energy expenditure and metabolic adaptation to physical activity in adult humans. Curr Biol. 2016;26(3):410–7. doi:10.1016/j.cub.2015.12.046.
Finlayson G, Bryant E, Blundell JE, et al. Acute compensatory eating following exercise is associated with implicit hedonic wanting for food. Physiol Behav. 2009;97(1):62–7.
Unick JL, Otto AD, Goodpaster BH, et al. Acute effect of walking on energy intake in overweight/obese women. Appetite. 2010;55(3):413–9.
Hopkins M, Blundell JE, King NA. Individual variability in compensatory eating following acute exercise in overweight and obese women. Br J Sports Med. 2014;48(20):1–6.
Unick JL, O’Leary KC, Dorfman L, et al. Consistency in compensatory eating responses following acute exercise in inactive, overweight and obese women. Br J Nutr. 2015;113(7):1170–7. doi:10.1017/S000711451500046X.
Compliance with Ethical Standards
Funding
No sources of funding were used in the preparation of this article.
Conflict of interest
Kristine Beaulieu, Mark Hopkins, John Blundell and Graham Finlayson declare that they have no conflicts of interest that are relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Beaulieu, K., Hopkins, M., Blundell, J. et al. Does Habitual Physical Activity Increase the Sensitivity of the Appetite Control System? A Systematic Review. Sports Med 46, 1897–1919 (2016). https://doi.org/10.1007/s40279-016-0518-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-016-0518-9