Abstract
Background
Relapses are the hallmark of multiple sclerosis (MS). Analyses have shown that the cost of MS increases during periods of relapse. However, results are inconsistent between studies, possibly due to different study designs and the different implications of relapses with respect to patient characteristics.
Objectives
The aims were to estimate and describe direct and indirect relapse costs and to determine differences in costs with respect to patient characteristics. Furthermore, we describe the pharmacoeconomic impact during the relapse follow-up.
Methods
Data were extracted from two German, multicenter, observational studies applying a validated resource costs instrument. Relapse costs were calculated as the difference in quarterly costs between propensity score (PS)–matched patients with and without relapses (1:1 ratio). For relapse active patients, we additionally calculated the difference between quarterly costs prior to and during relapse and determined costs in the post-relapse quarter.
Results
Of 1882 patients, 607 (32%) presented at least one relapse. After PS-matching, 597 relapse active and relapse inactive patients were retained. Relapse costs (in 2019 values) ranged between €791 (age 50 + years) and €1910 (disease duration < 5 years). In mildly disabled and recently diagnosed patients, indirect relapse costs (range €1073–€1207) constantly outweighed direct costs (range €591–€703). The increase from prior quarter to relapse quarter was strongest for inpatient stays (+ 366%, €432; p < 0.001), day admissions (+ 228%, €57; p < 0.001), and absenteeism (127%, €463; p < 0.001). In the post-relapse quarter, direct costs and costs of absenteeism remained elevated for patients with relapse-associated worsening.
Conclusion
A recent diagnosis and mild disability lead to high relapse costs. The results suggest the necessity to incorporate patient characteristics when assessing relapse costs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
For the case of multiple sclerosis (MS), relapses increased the societal economic burden by €1388 on average, but costs varied substantially within patients, between €791 (age 50+) and €1910 (disease duration < 5 years). |
In patients with relapse-associated worsening, relapse-associated resource costs remain elevated in the post-relapse period, indicating the need for treatment of residual disability. |
Future health economic assessments might incorporate patients’ disease characteristics to appropriately assess relapse costs. |
1 Introduction
Relapsing remitting multiple sclerosis (MS) is a chronic disease of the central nervous system with an unpredictable course and relapses as a hallmark. Clinically, a relapse is defined as a worsening or appearance of new neurological symptoms in the absence of fever or infection lasting more than 24 h followed by a period of partial or complete recovery [1, 2]. However, it has been reported that 57% of relapse deficits do not recover completely and neurological deficits remain, contributing to so called relapse-associated worsening (RAW) [3].
From the patient's perspective, relapses are associated with sudden considerable impairments in health-related quality of life and substantial functional and mental impairments [4]. From a societal perspective, relapses lead to immense economic costs due to increased utilization of direct and indirect resources [5,6,7,8,9]. The intensity of a relapse determines the costs of managing relapse symptoms [10,11,12]. During relapse periods, increases in direct costs are driven by inpatient stays, medications, and ambulatory care, while indirect economic burden increases due to restrictions in work performance [10, 13].
A recent study estimated the mean societal costs of a relapse at €2468 (in 2015 values) for Germany, and costs ranged between €632 and €4569 (both in 2015 values) in 15 other European countries [6, 14]. These studies calculated relapse costs as the difference between the costs of patients with and without relapses, but did not consider any subgroups [6, 14]. However, differences in relapse costs between subgroups are likely since relapses and the associated costs might vary across and within individuals with respect to disease severity, disease duration, gender, or therapy [5, 15, 16]. The main objectives of our analyses were to estimate and describe relapse costs and to determine whether and how relapse costs differ with respect to disease and patient characteristics, using real-world evidence from Germany. Furthermore, we described the pharmacoeconomic impact during the relapse follow-up.
2 Methods
2.1 Study Design, Population, and Ethics
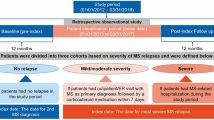
Two-year clinical and health resource utilization data on relapsing–remitting multiple sclerosis (RRMS) patients were extracted from two prospective, non-interventional, multicenter studies with observational periods ending in 2015 (PANGAEA) and 2013 (PEARL). Study design as well as inclusion criteria have been described in detail previously [17, 18]. The studies included patients either treated with beta-interferons or glatiramer acetate (PEARL) or fingolimod (PANGAEA), and no exclusion criteria except for the contraindications mentioned in the respective summary of the product information were applied. In both studies, health economic and clinical data were collected with equal regularity per quarter. Approval for both studies was obtained from independent, local competent ethics committees, and all patients provided written informed consent for the collection of clinical and health economic information. The current analyses were limited to patients with a baseline Expanded Disability Status Scale (EDSS) score lower than 6.0 to ensure a sufficiently large population in each EDSS step. Furthermore, we excluded 77 persons who had fewer than three visits. The excluded persons did not statistically significantly differ in their sociodemographic or disease characteristics.
2.2 Economic and Clinical Outcomes
The Multiple Sclerosis-Health Resource Survey (MS-HRS) was used in the PEARL and PANGAEA studies to holistically quantify and value resources using the most accurate form of bottom-up microcosting. We chose a societal perspective in which all resources are considered regardless of who bears them [19, 20]. Costs were categorized into direct medical (inpatient stays, day admissions, physician consultations, examinations, over-the-counter [OTC] medication, medical consumables, and professional care), direct non-medical costs (informal care, investments/purchases), and indirect costs. Within indirect costs, we considered absenteeism, either short term (sick leave) or long term (disability pension), but also presenteeism, which refers to impaired performance during work.
Resources were valued at the societal opportunity cost or the best possible approximation using official statistics and administrative data [19, 21]. Valuations of direct medical resources were taken from Bock et al. [21]. To value direct non-medical informal care, we used the opportunity cost method. The informal caregiver’s forgone benefits are approximated by multiplying the hours spent on informal care by the opportunity costs of leisure time (€23.30/h) [21, 22]. Indirect costs were valued using the human capital approach. For short-term absence (sick leave), we multiplied the hours of absence from work with the average labor costs in Germany (€33.04/h). For long-term absence (disability pension), we multiplied the average daily labor costs (€199.40/day) with the average number of working days (233 days) and the disability percentage (0–100%), leading to a maximum amount of €46,460.14 per year. Quarterly costs due to presenteeism were calculated by multiplying the quarterly average percentage of reduction in productivity with the quarterly labor costs. A list of all considered resources and their valuation is publicly accessible in Ness et al. [19]. Costs are reported per quarter according to the recall periods in the MS-HRS and are presented in 2019 Euros [19]. Adjustments for inflation were performed using the harmonized consumer price index published by the Federal Statistical Office as recommended for Germany [23, 24].
Relapses and EDSS were assessed every 3 and 6 months, respectively, according to the clinical judgment of physicians during routine clinical visits. RAW was defined as a relapse with incomplete recovery resulting in an increase in EDSS of ≥ 1.5 points, ≥ 1 point, or ≥ 0.5 points for a reference score of 0, 1.0–5.0, or ≥ 5.5, respectively, with confirmation after ≥ 6 months. Due to its higher sensitivity, we implemented a roving EDSS reference, so that the obtained EDSS increase is compared with the last EDSS assessment instead of the baseline score [25, 26].
Additionally, we reported costs stratified by body mass index (BMI). Therefore, we examined the weight and height of the patients and classified them as under- or normal weight (< 25 kg/m2), overweight, including pre-obesity (25–30 kg/m2), and obese (BMI ≥ 30 kg/m2) [27].
2.3 Data Processing
Patients were considered as ‘relapse active’ if they had at least one relapse during the 2-year follow-up, whereas ‘relapse inactive’ refers to patients without relapses during the follow-up time. We calculated relapse costs as the cross-sectional difference in the mean quarterly costs between relapse active patients during relapse quarters and relapse inactive patients. For the relapse costs analysis, we applied propensity score (PS) matching to compensate for differences between relapse active and relapse inactive patients. Patients were matched in a 1:1 ratio using nearest-neighbor matching. PSs were calculated as the probability to be part of the relapse active group according to the following baseline predictors: EDSS score, time since diagnosis of MS, disease-modifying therapies (DMTs), age, and gender. These variables were selected for matching as previous studies showed that a shorter disease duration and lower EDSS are associated with higher relapse activity and that women tend to experience more relapses [28]. Furthermore, DMTs are associated with varying effectiveness in reducing relapse rates [29]. More detailed information on the PS matching can be found in Supplement 1 and Supplement 2 in the electronic supplementary material. In addition to the cross-sectional approach of calculating relapse costs, we analyzed relapse-associated costs longitudinally. Each relapse quarter was re-baselined, and costs for corresponding intervals before (prior relapse) and after this interval (post relapse) were calculated additionally. On an intraindividual basis, we calculated the difference between quarterly costs prior to relapse and quarterly cost during relapse.
2.4 Statistical Analysis
All analyses were descriptive in nature. Frequency counts (n) and proportions (%) were used to summarize categorical variables. Means and standard deviations (± SD) were used as appropriate for continuous variables. Statistical differences between patient characteristics were analyzed using Chi-squared and Mann–Whitney U tests. P values for differences in relapse costs between patient and disease strata were derived from generalized linear models with a negative binomial distribution and the respective cost category as the dependent variable. The models included the characteristic (gender, age, BMI, disease duration, EDSS, or therapy) of interest as well as a variable indicating the relapse status (relapse active vs relapse inactive) and the interaction of both (Supplement 3; see the electronic supplementary material). For example, to assess statistical differences in total relapse costs regarding disease duration, we included the disease duration variable, the variable indicating the relapse status, and the interaction of these two variables as independent variables. All reported p values were compared to an alpha-error level of 5%. Statistical analyses were performed using SPSS statistics for Windows version 25 and PS matching was performed using the MatchIt package (version 3.0.2) in R (version 3.5.1).
3 Results
3.1 Patient Characteristics
Out of 1959 principal eligible subjects, we excluded 77 persons who had less than three visits, so that 1882 patients were included in the analysis. Of these, 607 (32%) presented at least one relapse, while 1275 patients (68%) were free of relapse activity during the 2-year study period (Table 1). Compared with relapse inactive patients, relapse active patients were younger (40.46 ± 9.98 years of age vs 42.09 ± 10.01 years of age, p < 0.01), more often female (76% vs 70%, p < 0.01), more severely disabled (2.41 ± 1.30 EDSS and 2.17 ± 1.39 EDSS, p < 0.001), and more often employed (64% vs 62%, p = 0.712). The cohorts did not differ in BMI, with average scores of 25.45 ± 5.14 and 25.66 ± 5.11 for the relapse inactive and relapse active populations, respectively. After PS matching, 597 out of 607 patients per cohort were retained for further analysis. The PS matching resulted in an alignment of the relapse inactive and relapse active populations (Supplement 1; see the electronic supplementary material). There were no statistically significant differences between the matched cohorts (Table 1). One quarter (25%) of the relapse active patients presented with RAW.
3.2 Comparison of Total Relapse Costs
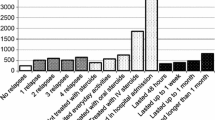
The relapse active cohort incurred significantly higher costs during relapses compared to the relapse inactive cohort (p < 0.001) (Fig. 1). More specifically, total relapse costs amounted to €1388, consisting of €618 (45%) direct and €770 (55%) indirect costs. Differences in direct costs arose essentially from inpatient treatment (relapse inactive €103 vs relapse active €550), day admissions (relapse inactive €10 vs relapse active €82), and physician consultations (relapse inactive €168 vs relapse active €257), while costs of absenteeism (relapse inactive €152 vs relapse active €820) were mainly responsible for indirect relapse costs.
Resource costs (3 months) for relapse inactive (n = 597) and relapse active (RAW n = 149; not RAW n = 448) patients (before, during, and after relapse). Relapse costs, defined as the difference in inactive and active patients during relapses, were significant (∆ €1388, p < 0.001). A significant increase was also observed between prior relapse and during relapse quarters Prior relapse 3 months before relapse quarter, during relapse relapse quarter, post relapse 3 months after relapse quarter. RAW relapse-associated worsening
3.3 Relapse Costs Stratified by Patient and Disease Characteristics
There were noticeable patterns in relapse costs regarding disease and patient characteristics (Table 2). Total relapse costs were higher in patients with shorter disease duration (< 5 years €1910 vs > 10 years €853; p < 0.001) and those being mildly disabled (EDSS 0–1.5 €1664 vs EDSS 4.5–5.5 €1071; p < 0.001). In mildly disabled patients and those with a short disease duration, indirect relapse costs (range €1073–€1207) constantly outweighed direct costs (€591–€703). Men incurred higher total relapse costs compared to women (€1603 vs €1322), which is mostly due to higher indirect work-related relapse costs (€1016 vs €694). However, differences were not statistically significant. Furthermore, patients on fingolimod incurred lower relapse costs throughout all cost categories compared to patients on beta-interferons and glatiramer acetate (BRACE) therapy (total relapse costs €1179 vs €1436), with noticeable differences in direct relapse costs (€350 vs €679). A detailed breakdown of these costs is provided in Supplement 4 (see the electronic supplementary material).
A higher economic burden for patients with short disease duration and those that are mildly disabled was also observed when assessing the increase in resource costs from prior relapse to relapse quarter in relapse active patients. However, relapse-associated costs were lower throughout all subgroups in this predisposed relapse active only population.
3.4 Longitudinal Intraindividual Relapse-Associated Costs
In the relapse active cohort, costs increased significantly from prior relapse to the relapse period (total €1148; p < 0.001). In detail, direct and indirect costs increased by €579 (p < 0.001) and €569 (p < 0.001), respectively. The increase was strongest for inpatient stays (+ 366%, €432; p < 0.001) and day admissions (+ 228%, €57; p < 0.001) in direct costs, while in indirect costs, the increase was higher in costs of absenteeism (127%, €463; p < 0.001) than in those of presenteeism (11%, €98; p = 0.002). Costs mostly decreased in the post-relapse quarter close to the initial level. However, in patients with RAW, direct costs and costs of absenteeism remained elevated (Fig. 2).
Quarterly costs (in €) of direct and indirect resource items before, during, and post relapse in relapse active patients (no RAW = 457; RAW = 150). Increases in costs are mainly due to inpatient stays and work absenteeism. Prior relapse 3 months before relapse quarter, during relapse relapse quarter, post relapse 3 months after relapse quarter. RAW relapse-associated worsening
4 Discussion
Our analysis revealed new detailed insights on the societal economic costs of MS relapses. We showed a great variability in societal economic costs associated with relapses. More precisely, patients with less severe burden of disease (young, mild to moderate disabled, short disease duration) are more likely to incur higher relapse costs. In these patients, indirect work-related costs accounted for the majority of costs. Furthermore, we confirmed that relapse costs arise essentially from inpatient stays and absenteeism. In future economic evaluations in MS, the accuracy of relapse costs can be improved if patient and disease characteristics are taken into account.
The study population corresponded in its patient characteristics to a typical clinical RRMS population. Nearly two thirds of the population were female, mild to moderately affected, reported an average age of about 41 years, and a mean disease duration of 7 years. Data were obtained from multiple study centers across Germany. A real-world setting was further supported by the fact that no explicit exclusion criteria were applied except contraindications associated with current DMTs. Additionally, our estimates are based on a validated health resource survey and applied quarterly in a clinical practice setting to generate robust cost estimates. The results of this analysis are expected to be largely transferable to a broader MS population in Germany. However, caution is needed when transferring the results to other countries due to different healthcare system structures.
Relapse costs have been investigated repeatedly in cross-sectional studies, with highly variable methodologies and results ranging from €503 to €8862 [10, 14]. Most commonly, relapse costs were calculated as the difference between costs of patients with and without relapses without presenting characteristics of these distinct populations [5, 14]. Less frequently, relapse-associated costs were directly enquired in interviews [10]. This methodology can be critically questioned because patients are required to differentiate between routinely utilized resources and relapse-related resources. We extended the first mentioned approach by applying PS matching. Each relapse active patient was assigned to a statistical twin which was relapse inactive. We reported relapse costs stratified by patient and disease characteristics, with estimated relapse costs ranging between €791 (age 50 + years) and €1910 (disease duration < 5 years). The matched and unmatched total relapse cost estimates differed by up to 23% due to the heterogeneity in patient and disease characteristics in the respective populations (Supplement 5; see the electronic supplementary material). The wide range of relapse costs in the PS-matched analysis suggests that the precision of cost estimates will increase if patient and disease characteristics are taken into account.
Former studies hypothesized that relapses may have a stronger effect in patients with limited permanent disability; however, a systematic investigation of this hypothesis remains unfulfilled [30]. With regard to our analysis, we can confirm that patients with a less severe burden of disease (mildly disabled, short disease duration) are more likely to incur high relapse costs, predominantly due to higher indirect costs. These patients are more frequently employed, resulting in higher costs due to sick leave. Our results complement the findings of O’Brien et al., who showed that relapse costs varied with relapse severity [11].
A recent systematic review found that the most notable increase in relapse costs were associated with inpatient treatments, physician consultations, informal care, and sick leave [31]. With regard to our analysis, we confirm these results. Inpatient treatment during a relapse might be indicative of a severe relapse [10, 11]. In the investigated population inpatient care costs were lower than previously assumed [14]. This could partly be explained by the inclusion criteria, which require patients to be treated with DMTs. DMTs can affect the disease course by slowing disease progression and reducing the frequency and severity of relapses [32,33,34,35]. Fingolimod in particular has been shown to reduce healthcare resource utilization during relapses [33].
Despite the increase in resource costs during relapses, we found that resource cost utilization decreases in the post-relapse phase. However, costs partially remained above the initial cost level. Interestingly, this was particularly observed in patients with RAW for resources that are notably increased during periods of relapses (inpatient stays, physician consultations, informal care, and sick leave). This might indicate an incomplete recovery with residual disability [28, 36]. This result is in line with recent findings showing the high frequency of post-relapse residual disability [37].
Our analyses provide valuable information about the diverse economic impact of MS and thus might help decision makers to allocate scarce resources. Furthermore, in the context of economic evaluation, the results have the potential to increase the validity of cost-effectiveness assessments of innovative therapies in relapsing MS. Given the large number of available and upcoming MS therapies of varying effectiveness and costs, economic evaluations become even more relevant.
4.1 Limitations
Our relapse costs calculation is based on patient-reported resource utilization. To increase the validity of our results we calculated the relapse costs on an interindividual and intraindividual basis. In the interindividual calculation we compensated for variation between patients with and without relapses by applying PS matching. PS matching is a powerful technique to reduce imbalances in observable outcomes between populations. However, PS matching cannot address unknown or unmeasured differences, which might partially explain the lower relapse costs in the intraindividual as compared to the interindividual calculation. Furthermore, we did not include corticosteroid medication costs, which could have led to an underestimation of relapse costs.
5 Conclusion
For the case of MS, relapses significantly increased the societal economic burden. In particular, patients with a recent diagnosis and a mild disability incur higher relapse costs. In patients with RAW, relapse-associated resource costs remain elevated in the post-relapse period, indicating the need for treatment of residual disability. Our results suggest that it is necessary to incorporate patients’ disease characteristics to appropriately assess relapse costs in economic evaluations and to optimize the provision of care from a clinical and societal perspective.
Data Availability Statement
The data can be provided by the authors upon reasonable request, with the permission of Novartis Pharma GmbH and after receipt of a signed confidentiality agreement.
Change history
13 May 2020
In the original version of this article, the title of the article has been published incorrectly.
References
Lublin FD, Reingold SC, Cohen JA, Cutter GR, Sørensen PS, Thompson AJ, Wolinsky JS, Balcer LJ, Banwell B, Barkhof F, Bebo B, Calabresi PA, Clanet M, Comi G, Fox RJ, Freedman MS, Goodman AD, Inglese M, Kappos L, Kieseier BC, Lincoln JA, Lubetzki C, Miller AE, Montalban X, O'Connor PW, Petkau J, Pozzilli C, Rudick RA, Sormani MP, Stüve O, Waubant E, Polman CH. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83(3):278–86. https://doi.org/10.1212/WNL.0000000000000560.
McDonald WI, Compston A, Edan G, Goodkin D, Hartung H-P, Lublin FD, McFarland HF, Paty DW, Polman CH, Reingold SC, Sandberg-Wollheim M, Sibley W, Thompson A, Van Den Noort S, Weinshenker BY, Wolinsky JS. Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50(1):121–7. https://doi.org/10.1002/ana.1032.
Lublin FD, Baier M, Cutter G. Effect of relapses on development of residual deficit in multiple sclerosis. Neurology. 2003;61(11):1528–32. https://doi.org/10.1212/01.wnl.0000096175.39831.21.
Repovic P, Lublin FD. Treatment of multiple sclerosis exacerbations. Neurol Clin. 2011;29(2):389–400. https://doi.org/10.1016/j.ncl.2010.12.012.
Hawton AJ, Green C. Multiple sclerosis: relapses, resource use, and costs. Eur J Health Econ. 2016;17(7):875–84. https://doi.org/10.1007/s10198-015-0728-3.
Kobelt G, Thompson A, Berg J, Gannedahl M, Eriksson J, MSCOI Study Group, European Multiple Sclerosis Platform. New insights into the burden and costs of multiple sclerosis in Europe. Mult Scler. 2017;23(8):1123–36. https://doi.org/10.1177/1352458517694432.
Raimundo K, Tian H, Zhou H, Zhang X, Kahler KH, Agashivala N, Kim E. Resource utilization, costs and treatment patterns of switching and discontinuing treatment of MS patients with high relapse activity. BMC Health Serv Res. 2013;13:131.
Ke X, Navaratnam P, Sasane R, Eisenberg Lawrence DF, Friedman HS, Tulsi BB, Vollmer T. Determinants of high cost in multiple sclerosis patients: a claims and chart review study. Curr Med Res Opin. 2016;32(9):1589–97. https://doi.org/10.1080/03007995.2016.1192529.
Ernstsson O, Gyllensten H, Alexanderson K, Tinghog P, Friberg E, Norlund A. Cost of Illness of multiple sclerosis—a systematic review. PLoS ONE. 2016;11(7):e0159129. https://doi.org/10.1371/journal.pone.0159129.
O'Connell K, Kelly SB, Fogarty E, Duggan M, Buckley L, Hutchinson M, McGuigan C, Tubridy N. Economic costs associated with an MS relapse. Mult Scler Relat Disord. 2014;3(6):678–83. https://doi.org/10.1016/j.msard.2014.09.002.
O'Brien JA, Ward AJ, Patrick AR, Caro J. Cost of managing an episode of relapse in multiple sclerosis in the United States. BMC Health Serv Res. 2003. https://doi.org/10.1186/1472-6963-3-17.
Parisé H, Laliberté F, Lefebvre P, Duh MS, Kim E, Agashivala N, Abouzaid S, Weinstock-Guttman B. Direct and indirect cost burden associated with multiple sclerosis relapses: excess costs of persons with MS and their spouse caregivers. J Neurol Sci. 2013;330(1):71–7. https://doi.org/10.1016/j.jns.2013.04.007.
Oleen-Burkey M, Castelli-Haley J, Lage MJ, Johnson KP. Burden of a multiple sclerosis relapse. Patient Patient-Cent Outcomes Res. 2012;5(1):57–69. https://doi.org/10.2165/11592160-000000000-00000.
Flachenecker P, Kobelt G, Berg J, Capsa D, Gannedahl M, European Multiple Sclerosis Platform. New insights into the burden and costs of multiple sclerosis in Europe: results for Germany. Mult Scler. 2017;23(Suppl_2):S78–S90. https://doi.org/10.1177/1352458517708141.
Turner B, Cree BAC, Kappos L, Montalban X, Papeix C, Wolinsky JS, Buffels R, Fiore D, Garren H, Han J, Hauser SL. Ocrelizumab efficacy in subgroups of patients with relapsing multiple sclerosis. J Neurol. 2019;266(5):1182–93. https://doi.org/10.1007/s00415-019-09248-6.
Derfuss T, Ontaneda D, Nicholas J, Meng X, Hawker K. Relapse rates in patients with multiple sclerosis treated with fingolimod: subgroup analyses of pooled data from three phase 3 trials. Mult Scler Relat Disord. 2016;8:124–30. https://doi.org/10.1016/j.msard.2016.05.015.
Ziemssen T, Kern R, Cornelissen C. The PANGAEA study design - a prospective, multicenter, non-interventional, long-term study on fingolimod for the treatment of multiple sclerosis in daily practice. BMC Neurol. 2015. https://doi.org/10.1186/s12883-015-0342-0.
Vormfelde SV, Ortler S, Ziemssen T. Multiple sclerosis therapy with disease-modifying treatments in Germany: the PEARL (ProspEctive phArmacoeconomic cohoRt evaluation) Noninterventional Study Protocol. JMIR Res Protoc. 2016;5(1):e23. https://doi.org/10.2196/resprot.4473.
Ness N-H, Haase R, Kern R, Schriefer D, Ettle B, Cornelissen C, Akgün K, Ziemssen T. Multiple Sclerosis Health Resource Utilization Survey (MS-HRS): development and validation study. J Med Internet Res. 2020;22(3):e17921.
D'Amico E, Haase R, Ziemssen T. Review: patient-reported outcomes in multiple sclerosis care. Mult Scler Relat Disord. 2019;33:61–6. https://doi.org/10.1016/j.msard.2019.05.019.
Bock JO, Brettschneider C, Seidl H, Bowles D, Holle R, Greiner W, Konig HH. Calculation of standardised unit costs from a societal perspective for health economic evaluation. Gesundheitswesen. 2015;77(1):53–61. https://doi.org/10.1055/s-0034-1374621.
Koopmanschap MA, van Exel NJA, van den Berg B, Brouwer WBF. An overview of methods and applications to value informal care in economic evaluations of healthcare. Pharmacoeconomics. 2008;26(4):269–80. https://doi.org/10.2165/00019053-200826040-00001.
IQWIG (2017) Allgemeine Methoden Version 5.0 vom 10.07.2017 [General Methods Version 5.0 from 10.07.2017].
Statistisches Bundesamt (2020) Verbraucherpreisindex für Deutschland - Lange Reihen ab 1948 - Februar 2020. https://www.destatis.de/DE/Themen/Wirtschaft/Preise/Verbraucherpreisindex/Publikationen/Downloads-Verbraucherpreise/verbraucherpreisindex-lange-reihen-pdf-5611103.html. Accessed 06 Apr 2020.
Kappos L, Butzkueven H, Wiendl H, Spelman T, Pellegrini F, Chen Y, Dong Q, Koendgen H, Belachew S, Trojano M. Greater sensitivity to multiple sclerosis disability worsening and progression events using a roving versus a fixed reference value in a prospective cohort study. Mult Scler. 2018;24(7):963–73. https://doi.org/10.1177/1352458517709619.
Inojosa H, Schriefer D, Ziemssen T. Clinical outcome measures in multiple sclerosis: a review. Autoimmun Rev. 2020. https://doi.org/10.1016/j.autrev.2020.102512.
WHO. Physical status: the use and interpretation of anthropometry physical status. Geneva: World Health Organization; 1995.
Kalincik T. Multiple sclerosis relapses: epidemiology, outcomes and management. Syst Rev Neuroepidemiol. 2015;44(4):199–21414. https://doi.org/10.1159/000382130.
Li H, Hu F, Zhang Y, Li K. Comparative efficacy and acceptability of disease-modifying therapies in patients with relapsing–remitting multiple sclerosis: a systematic review and network meta-analysis. J Neurol. 2019. https://doi.org/10.1007/s00415-019-09395-w.
Kobelt G, Berg J, Lindgren P, Fredrikson S, Jonsson B. Costs and quality of life of patients with multiple sclerosis in Europe. J Neurol Neurosurg Psychiatry. 2006;77(8):918–26. https://doi.org/10.1136/jnnp.2006.090365.
Naci H, Fleurence R, Birt J, Duhig A. Economic burden of multiple sclerosis: a systematic review of the literature. Pharmacoeconomics. 2010;28(5):363–79. https://doi.org/10.2165/11532230-000000000-00000.
Vercellino M, Romagnolo A, Mattioda A, Masera S, Piacentino C, Merola A, Chiò A, Mutani R, Cavalla P. Multiple sclerosis relapses: a multivariable analysis of residual disability determinants. Acta Neurol Scand. 2009;119(2):126–30. https://doi.org/10.1111/j.1600-0404.2008.01076.x.
Haas J, Jeffery D, Silva D, Meier DP, Meinert R, Cohen J, Hartung H-P. Early switch to fingolimod reduces rates of severe relapses over the long term: Post hoc analysis from the FREEDOMS, FREEDOMS II, and TRANSFORMS studies. Mult Scler Relat Disord. 2019;36:101335. https://doi.org/10.1016/j.msard.2019.07.011.
Sanchirico M, Caldwell-Tarr A, Mudumby P, Hashemi L, Dufour R. Treatment patterns, healthcare resource utilization, and costs among Medicare patients with multiple sclerosis in relation to disease-modifying therapy and corticosteroid treatment. Neurol Ther. 2019;8(1):121–33. https://doi.org/10.1007/s40120-018-0123-y.
Giovannoni G, Butzkueven H, Dhib-Jalbut S, Hobart J, Kobelt G, Pepper G, Sormani MP, Thalheim C, Traboulsee A, Vollmer T. Brain health: time matters in multiple sclerosis. Mult Scler Relat Disord. 2016;9(Suppl_1):S5–S48. https://doi.org/10.1016/j.msard.2016.07.003.
Ness N-H, Schriefer D, Haase R, Ettle B, Cornelissen C, Ziemssen T. Differentiating societal costs of disability worsening in multiple sclerosis. J Neurol (online first). 2019. https://doi.org/10.1007/s00415-019-09676-4.
Achiron A, Sarova-Pinhas I, Magalashvili D, Stern Y, Gal A, Dolev M, Menascu S, Harari G, Gurevich M. Residual disability after severe relapse in people with multiple sclerosis treated with disease-modifying therapy. Mult Scler. 2018;25(13):1746–53. https://doi.org/10.1177/1352458518809903.
Acknowledgement
Open Access funding provided by Projekt DEAL. There was no funding received for the conduct of the study.
Author information
Authors and Affiliations
Contributions
NHN wrote the first manuscript. DS, NHN, and RH contributed substantially to the statistical analysis. All authors made substantial contributions to study conception and design and the interpretation of the data. All authors reviewed the manuscript and approved the manuscript for publication.
Corresponding author
Ethics declarations
Conflict of interest
NHN has received funding for research from Novartis. DS has nothing to disclose. RH has received speaker fees from Sanofi and travel grants from Celgene. BE is an employee of Novartis. TZ received personal compensation from Almirall, Biogen, Bayer, Celgene, Novartis, Roche, Sanofi, and Teva for consulting and speaking services, and he is the section editor for BMC Neurology. Additionally, he received financial support for research activities from BAT, Biogen Novartis, Teva, and Sanofi.
Ethical approvals
Approval for PANGAEA and PEARL were obtained from independent, local, competent ethics committees.
Informed consent
All patients provided written informed consent for the collection of clinical and health economic information.
Additional information
The original version of this article was revised: The article title should have been published as "Real-World Evidence on the Societal Economic Relapse Costs in Patients with Multiple Sclerosis" rather than "Real-World Evidence on the Patterns of Increased Societal Economic Relapse Costs in Patients with Multiple Sclerosis"
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ness, NH., Schriefer, D., Haase, R. et al. Real-World Evidence on the Societal Economic Relapse Costs in Patients with Multiple Sclerosis. PharmacoEconomics 38, 883–892 (2020). https://doi.org/10.1007/s40273-020-00917-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-020-00917-3