Abstract
The National Institute for Health and Care Excellence (NICE) invited AstraZeneca, the manufacturer of ticagrelor (Brilique®), to submit evidence on the clinical and cost effectiveness of ticagrelor 60 mg twice daily (BID) in combination with low-dose aspirin [acetylsalicylic acid (ASA)] compared with ASA only for secondary prevention of atherothrombotic events in patients with a history of myocardial infarction (MI) and who are at increased risk of atherothrombotic events. Kleijnen Systematic Reviews Ltd (KSR), in collaboration with Maastricht University Medical Centre+, was commissioned as the evidence review group (ERG). This paper summarises the company submission (CS), the ERG report and the NICE guidance produced by the appraisal committee (AC) for the use of ticagrelor in England and Wales. The ERG critically reviewed the clinical- and cost-effectiveness evidence in the CS. The systematic review conducted as part of the CS identified one randomised controlled trial (RCT), PEGASUS-TIMI 54. This trial reported the time to first occurrence of any event from the composite of cardiovascular death, MI and stroke as the primary outcome (hazard ratio 0.84 ticagrelor 60 mg BID vs. placebo, 95% confidence interval 0.74–0.95). The population addressed in the CS was a subgroup of the PEGASUS-TIMI 54 trial population, i.e. the ‘base-case’ population, which comprised patients who had experienced an MI between 1 and 2 years ago, whereas the full trial population included patients who had experienced an MI between 1 and 3 years ago. While the ERG believed the findings of this RCT to be robust, doubts concerning the applicability of the trial to UK patients were raised. The company submitted an individual patient simulation model to estimate the cost-effectiveness of ticagrelor 60 mg BID + ASA versus ASA only. Parametric time-to-event models were used to estimate the time to first and subsequent (cardiovascular) events, time to treatment discontinuation and time to adverse events. The company’s base-case analysis resulted in an incremental cost-effectiveness ratio (ICER) of £20,098 per quality-adjusted life-year (QALY) gained. The main issues surrounding the cost effectiveness of ticagrelor 60 mg BID + ASA were the use of parametric time-to-event models estimated based on the full trial population instead of being fitted to the ‘label’ population (the ‘label’ population comprised the ‘base-case’ population and patients who started ticagrelor 60 mg BID within 1 year of previous adenosine diphosphate inhibitor treatment), the incorrect implementation of the probabilistic sensitivity analysis (PSA) of the individual patient simulation, and simplifications of the model structure that may have biased the health benefits and costs estimations of the intervention and comparator. The ERG believed the use of the full trial population to inform the parametric time-to-event models was not appropriate because the ‘label’ population was the main focus of the scope and CS. The ERG could not investigate the magnitude of the bias introduced by this assumption. The PSA of the individual patient simulation provided unreliable probabilistic results and underestimated the uncertainty surrounding the results because it was based on a single patient. The ERG used the cohort simulation presented in the cost-effectiveness model to perform its base-case and additional analyses and to obtain probabilistic results. The ERG amended the company cost-effectiveness model, which resulted in an ERG base-case ICER of £24,711 per QALY gained. In its final guidance, the AC recommended treatment with ticagrelor 60 mg BID + low-dose ASA for secondary prevention of atherothrombotic events in adults who have had an MI and are at increased risk of atherothrombotic events.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The only randomised controlled trial (RCT) identified in this scope found a statistically significant advantage (hazard ratio 0.84; 95% confidence interval 0.74–0.95) for ticagrelor 60 mg twice daily (BID) + low-dose aspirin compared with placebo + low-dose aspirin regarding the primary outcome (composite of cardiovascular death, myocardial infarction and stroke). |
The company implemented an individual patient simulation to account for model non-linearity. This type of simulation may be more flexible than a cohort simulation, but the technical implementation of the probabilistic sensitivity analysis did not provide reliable cost-effectiveness estimates. Results of analyses based on the individual patient simulation were not fit for purpose. |
The National Institute for Health and Care Excellence (NICE) appraisal committee considered that all expected cost-effectiveness estimates supported ticagrelor 60 mg BID + low-dose aspirin as a cost-effective use of the UK NHS resources. The NICE appraisal committee recommended ticagrelor, in combination with aspirin, within its marketing authorisation. |
1 Introduction
New health technologies need to represent a clinically effective and cost-effective use of the UK NHS resources to be recommended for use within the NHS. The National Institute for Health and Care Excellence (NICE) is an independent organisation responsible for providing national guidance on promoting good health and preventing and treating ill health in priority areas with a significant impact. Through the single technology assessment (STA) process, NICE appraises single health technologies within a single indication.
During the STA process, the manufacturer submits a document that compiles the clinical- and cost-effectiveness evidence concerning the health technology to be appraised. The evidence review group (ERG), an independent academic group, summarises and critically reviews this company submission (CS) and produces an ERG report.
Based on the CS, the ERG report and stakeholder consultations, the NICE appraisal committee (AC) produces an appraisal consultation document (ACD), which formulates the preliminary guidance and contains the initial decision on whether the technology will be recommended for use within the NHS. Subsequently, stakeholders may comment on the submitted evidence and the ACD. These comments may result in the production of a second ACD or a final appraisal determination (FAD), which are both open for appeal.
This paper summarises the CS, the ERG report and considerations of the AC for the appraisal of ticagrelor (Brilique®) 60 mg twice daily (BID) + low-dose aspirin [acetylsalicylic acid (ASA)] for the secondary prevention of atherothrombotic events in adults who have had a myocardial infarction (MI) and are at increased risk of atherothrombotic events [1]. All documents relating to this STA are available online [2].
2 The Decision Problem
Patients who survive an MI are at increased long-term risk of subsequent cardiovascular events such as MI, stroke, or death because of cardiovascular events [3,4,5]. NICE guidance recommends the use of dual antiplatelet therapy (i.e. an antiplatelet agent in combination with ASA) for up to 12 months after an MI, followed by low-dose ASA monotherapy indefinitely. For patients who are intolerant to ASA, clopidogrel monotherapy may be considered for long-term secondary prevention of atherothrombotic events (i.e. MI, stroke and death) [6]. However, patients with a history of MI (> 1 year previously) are still at increased risk of cardiovascular events, so health benefits may be gained from more intensive long-term secondary prevention. Post hoc analyses of the CHARISMA and DAPT trials suggested that longer-term secondary prevention with dual antiplatelet therapy (beyond 1 year following an MI) may reduce the number of cardiovascular events in patients with a history of MI, but this had not yet been confirmed prospectively [7,8,9]. In the PEGASUS-TIMI 54 trial, dual antiplatelet therapy (ticagrelor 60 mg BID + ASA) was compared with placebo + ASA, and results indicated that ticagrelor 60 mg BID + ASA decreased the risk of atherothrombotic events compared with placebo + ASA in patients with a history of MI (MI between 1 and 3 years previously) [10, 11]. Ticagrelor 60 mg BID is the first antiplatelet agent that can be administered in combination with ASA for long-term secondary prevention of atherothrombotic events in this patient population [12].
The current appraisal focussed on ticagrelor 60 mg BID + low-dose ASA for the treatment of “adults who have had a myocardial infarction and are at increased risk of atherothrombotic events”, as described in the final scope issued by NICE [13]. The CS considered “adults who have had a myocardial infarction between 1 and 2 years ago and are at increased risk of atherothrombotic events”. This population, referred to as the base-case population, is a subgroup of the full trial population included in the PEGASUS-TIMI 54 trial [12].
Ticagrelor received market approval in December 2015 for the treatment of adult patients with a history of MI and a high risk of developing an atherothrombotic event. Ticagrelor is administered at a dose of 60 mg BID in combination with low-dose ASA (75–150 mg daily). In February 2016, NICE issued a final scope concerning the appraisal of the clinical and cost effectiveness of ticagrelor 60 mg BID + low-dose ASA compared with low-dose ASA only or clopidogrel + ASA.
3 Independent Evidence Review Group (ERG) Report
The ERG was commissioned to review and critically assess the clinical- and cost-effectiveness evidence submitted by the company (AstraZeneca). The review process aimed to investigate whether the company’s analyses followed the NICE methodological guidelines [14]. The ERG also analysed the influence of alternative assumptions on the company’s results. Finally, the ERG produced an ERG base-case reflecting the ERG’s preferred assumptions, its interpretation of the evidence and considerations of alternative evidence when appropriate.
3.1 Clinical-Effectiveness Evidence Submitted by the Company
The CS presented results of a large randomised controlled trial (RCT), PEGASUS-TIMI 54, comparing ticagrelor 90 mg BID + ASA (7050 participants) versus ticagrelor 60 mg BID + ASA (7045 participants) versus placebo + ASA (7067 participants). Evidence was presented for the overall intention-to-treat (ITT) population and for the subgroup of patients who experienced the qualifying MI < 2 years previously who also had one or more additional atherothrombotic risk factor (4331 and 4333 participants, respectively), i.e. the base-case population [15]. This was consistent with the population set out in the final scope issued by NICE and with the licensed indication for the intervention.
The primary outcome in PEGASUS-TIMI 54 was time to first occurrence of any event from the composite of cardiovascular death, MI and stroke. This outcome showed a statistically significant result in favour of ticagrelor BID + ASA compared with placebo + ASA [hazard ratio (HR) 0.84; 95% confidence interval (CI) 0.74–0.95]. In addition, the CS presented results for the individual components of this composite primary outcome, which—in the view of the ERG—should be preferred. In principle, the individual component endpoints may lack sufficient power; however, given the number of patients enrolled in the study, it is likely that any clinically meaningful differences would be detected.
Compared with the control group, participants receiving ticagrelor BID + ASA had statistically significantly fewer MIs (HR 0.84; 95% CI 0.72–0.98). Similar results were reported for stroke (HR 0.75; 95% CI 0.57–0.98). Fewer cardiovascular deaths were reported in the intervention than in the comparison group, but this difference was not statistically significant (HR 0.83; 95% CI 0.68–1.01).
As mentioned, the CS included results for a subgroup matching the decision problem (i.e. the base-case population, defined as patients who had experienced an MI < 2 years previously). Further details were reported for this subgroup, e.g. patients with/without diabetes or a history of percutaneous coronary intervention. These results were marked as ‘commercial in confidence’ and are not reported in the public domain but were considered in the ERG and AC reports.
3.2 Critique of Clinical-Effectiveness Evidence and Interpretation
The company conducted a systematic review to identify relevant clinical data from the published literature. The review, which was conducted in September 2014 and last updated in December 2015, identified a single RCT directly comparing ticagrelor 60 mg BID + ASA with any of the included comparators: PEGASUS-TIMI 54. The ERG agreed with the CS that the trial was of high methodological quality.
The company did not report any indirect comparison or multiple treatment comparison. The systematic review identified two studies in addition to the PEGASUS-TIMI 54 study that were potentially eligible for inclusion in an indirect comparison (CHARISMA and DAPT). However, the overall study populations in each of the CHARISMA and DAPT studies were not consistent with the population specified in the final scope for the appraisal [8, 16].
The literature searches reported in the CS were well documented and easily reproducible. An appropriate range of databases were searched, and additional searches of clinical trials registers and conference proceedings were conducted. Searches were carried out in accordance with the NICE guidance for technology appraisal.
There was some uncertainty regarding the number of reviewers involved in relevant steps of the review process, e.g. title and abstract screening, data extraction and quality assessment. This potentially affects the robustness of the review process, but the ERG found no clear evidence that the quality of the systematic review was compromised.
It should be noted that the results from PEGASUS-TIMI 54 were based on small numbers of events for each outcome compared with the total number of patients in each arm and should be interpreted with a degree of caution.
Furthermore, the UK Clinical Pharmacy Association pointed out that the patients included in the PEGASUS-TIMI 54 trial were not representative of the UK patient population as patients are not actively sought out post-event to restart or redefine treatment [17]. Because of the mean length of treatment (25.3 months), the AC concluded that it could only consider a maximum duration of treatment of up to 3 years, in line with the evidence presented for ticagrelor.
3.3 Cost-Effectiveness Evidence Submitted by the Company
The company performed a systematic review to identify relevant cost-effectiveness, health-related quality of life, and resource use and costs studies. A de novo cost-effectiveness model was developed because the company did not identify any cost-effectiveness analyses of interest for the decision problem defined in the scope [13].
The company compared ticagrelor 60 mg BID + ASA with ASA only through a health state transition model with a 3-month cycle length and a 40-year time horizon. All patients started in the ‘no event’ state, in which patients were at risk of experiencing their first event, which were non-fatal cardiovascular events (MI or stroke), fatal cardiovascular events, and other fatal events. Patients experiencing a fatal (cardiovascular) event transited to the absorbing ‘death’ health state. Patients experiencing their first non-fatal cardiovascular event entered a series of five ‘post non-fatal MI’ or ‘post non-fatal stroke’ tunnel states. The first four tunnel states represented the first year following the first non-fatal event. In these tunnel states, the risk of experiencing subsequent fatal and non-fatal (cardiovascular) events was elevated but decreased over time. In the last tunnel state (≥ 12 months after the first non-fatal event), a constant risk for subsequent fatal and non-fatal (cardiovascular) events was applied (see Fig. 1).
A competing risk framework was used to estimate the time to first fatal or non-fatal event occurring in the model. Parametric time-to-event models were most often based on the full trial population of the PEGASUS-TIMI 54 clinical trial [11]. Because the cost-effectiveness analysis in the CS concentrated on the ‘label’ population (the ‘label’ population comprised the base-case population, as defined in Sect. 3.1 and patients who started ticagrelor 60 mg BID within 1 year of previous adenosine diphosphate inhibitor treatment), two of the 17 parametric time-to-event models were adjusted to represent this ‘label’ population. The remaining parametric time-to-event models were not adjusted because the covariate representing membership to the ‘label’ population was not statistically significant. The company adjusted the probability of ‘other fatal events’ with UK life tables by excluding cardiovascular-specific mortality because the parametric time-to-event model resulted in lower probabilities of death than in the UK general population.
Costs and utility decrements were associated with subsequent non-fatal events for the duration of one cycle. The model included adverse events, which were assumed to occur only when patients were receiving treatment. Adverse events, i.e. major and minor TIMI bleeding and dyspnoea (grade 1–2 or 3–4), incurred costs and utility decrements for the duration of one cycle (i.e. were assumed to be reversible). Treatment duration was capped at 36 months, after which treatment effects were not modelled and patients were assumed to receive ASA monotherapy.
Health state utility values were obtained from the PEGASUS-TIMI 54 clinical trial, in which utilities were elicited through the EuroQoL 5-Dimensions, 3 Levels (EQ-5D-3L) at set time intervals. Utility decrements associated with adverse events and non-fatal events were estimated through a linear random effects panel data analysis model. This methodology estimates utility decrements by calculating the difference in utility values pre- and post-event based on the effects of events occurring in a pre-determined time period. This method allows for utility values > 1. Hence, baseline utility values were capped at 1. This resulted in a health state utility value above the UK general population utility values for patients in the ‘no event’ health state. This seemed implausible to the company, so they decided to use the UK general population age- and sex-specific utility values for the ‘no-event’ health state.
Costs data were obtained from the ERG assessment of technology appraisal (TA) 317 (inflated to year 2015 values) [18], the ERG assessment report of TA210 [19], NHS reference costs [20] and the British National Formulary [11]. Costs included in the analysis were treatment costs (i.e. ticagrelor 60 mg and ASA) and health state costs. The health state costs comprised inpatient costs, outpatient costs, maintenance costs, and adverse event costs. One-off inpatient costs were associated with the occurrence of fatal and non-fatal events.
Both an individual patient simulation and a cohort simulation were performed. In the individual patient simulation, all patients from the ‘label’ population (N = 10,779) went through the model one at a time, all risk equations (parametric time-to-events models, probability of experiencing adverse events and being hospitalised) were applied to each patient individually. Individual results were then aggregated for all simulated patients. In the cohort simulation, all patients went through the model simultaneously. The population in the cohort simulation represented the ‘average patient’, i.e. with averaged risk factors, on whom all risk equations were applied. The individual patient population resulted in an incremental cost-effectiveness ratio (ICER) of £20,098 per quality-adjusted life-year (QALY) gained for ticagrelor 60 mg BID + ASA versus ASA only. The cohort simulation resulted in an ICER of £24,070 per QALY gained. The company explained that the cohort simulation overestimated the ICER because it ignored model non-linearity.
The probabilistic sensitivity analysis (PSA) of the individual patient simulation was performed on a single patient from the ‘label’ population. This patient was selected because this individual patient profile produced the closest ICER to the mean expected ICER of the individual patient simulation.
3.4 Critique of Cost-Effectiveness Evidence Submitted by the Company
The main concerns identified by the ERG when reviewing the company’s cost-effectiveness evidence were the simplified model structure, the use of parametric time-to-event models fitted to the full trial population instead of the ‘label’ population, and the incorrect implementation of the PSA of the individual patient simulation.
The ERG noticed that several assumptions underlying the model structure might have influenced the company’s cost-effectiveness results. First, the cost-effectiveness model did not explicitly incorporate third and further non-fatal events because the consequences of these events on survival, quality of life and costs only lasted for the duration of one cycle. However, third and further events, such as non-fatal strokes, may have long-term consequences. The company argued that the possible under-prediction of third and further events in the model was a conservative assumption “owing to the treatment effect observed for first events in PEGASUS-TIMI 54 and the influence of first events on subsequent events” [21]. The ERG agreed with the company that the non-explicit modelling of third and subsequent events resulted in a likely conservative assumption because it may have underestimated the consequences on costs and health outcomes of these events, which were more likely to occur in the ASA monotherapy group. Second, the long-term consequences of major TIMI bleedings were not incorporated in the model, which favoured ticagrelor 60 mg BID + ASA. The ERG considered that this assumption did not fully capture the long-term consequences of this adverse event and used an alternative utility decrement for major bleedings. Finally, the model did not differentiate between non-fatal disabling and non-disabling strokes even though they have different long-term impacts on quality of life and costs. Based on data from PEGASUS-TIMI 54, the company argued that this assumption was conservative because of the higher percentage of non-fatal disabling strokes in the ASA monotherapy group compared with the ticagrelor 60 mg BID + ASA group. The ERG agreed with this argument. Although several of these simplifications seem to be conservative, they lead to biased estimations of health outcomes and costs.
Most parametric time-to-event models incorporated in the cost-effectiveness model were based on the full trial population instead of being fitted to the ‘label’ population, which was the population included in the scope and CS. The company argued that fitting parametric time-to-event models to the full trial population would “maintain the level of precision of the model” [22]. However, the ERG disagreed with this argument because “even a perfectly precise model is useless if it is not valid” [23]. The ERG argued that using parametric time-to-event models fitted to the ‘label’ population would have increased the validity of these models as well as the validity of the cost-effectiveness model, even though it probably would have decreased the precision of the parametric time-to-event models. The ERG could not investigate the extent of bias introduced by using parametric time-to-event models fitted to the full trial population because the CS did not provide parameter estimates relating to the ‘label’ population. Therefore, the uncertainty surrounding the effectiveness of ticagrelor in the ‘label’ population was likely underestimated, and a risk remains that results may not be conservative to ticagrelor.
The PSA of the individual patient simulation was only performed on a single patient, who provided the closest ICER to the mean expected ICER of the complete individual patient simulation. However, it was observed that the individual patient selected by the company did not provide the closest ICER to the mean expected ICER of the complete individual patient simulation. A comparison of the characteristics of this individual patient and the ‘average’ patient used in the cohort simulation is provided in Table 1. The ERG considered the use of a single patient for the PSA to be incorrect because this implementation of the PSA does not adequately represent first and second order uncertainty [24]. The PSA estimates provided by the company were thus unreliable, and the uncertainty surrounding the probabilistic results was underestimated. The ERG therefore requested PSA results based on the cohort simulation instead of the individual patient simulation. The company responded that the PSA results based on the cohort simulation did not take model non-linearity into account and were incorrect. The ERG would have preferred that the PSA of the individual patient simulation included all patients (N = 10,799) or a sufficient number of patients to produce reliable probabilistic results.
3.5 Additional Work Undertaken by the ERG
The ERG corrected the parameterisation of the log-logistic time-to-event models estimating the time to the first and subsequent non-fatal cardiovascular events, first fatal cardiovascular event and other fatal events because the parameterisation used by the company was unclear. In addition, the ERG corrected violations that decreased the adherence of the cost-effectiveness model to the NICE reference case or good modelling practices [25]. These violations concerned the following:
-
the non-inclusion of the quality of life and economic consequences of gout;
-
the choice of the time-to-event model used to estimate the probability of experiencing adverse events;
-
healthcare costs, of which the following were adjusted: the outpatient and inpatient costs of the tunnel states representing the first year after a non-fatal MI or stroke; the inpatient costs associated with MI, stroke, dyspnoea (grade 3–4), and TIMI bleeds (major and minor); and the inpatient costs associated with the ‘no event’ health state;
-
the exclusion of uncertainty surrounding costs in the PSA, based on NHS reference costs.
Additionally, the ERG preferred using higher disutility values for major bleeds. The ERG recalculated inpatient costs for the ‘no event’ health state, based on NHS reference costs, because the inpatient costs in the ‘no event’ health state were assumed to be equal to the inpatient costs incurred by fatal events (which seemed implausible to the ERG). These adjustments resulted in the ERG base-case ICER of £24,711 per QALY gained, based on the cohort simulation. These results should be interpreted with caution because (1) the cohort simulation likely overestimated the ICER, (2) the uncertainty associated with the use of the full trial population instead of the ‘label’ population to inform the effectiveness parameters was not captured in the PSA, and (3) the influence on the results of the simplifications underlying the model structure (i.e. non-inclusion of long-term consequences of subsequent and adverse events) were also not captured in the model. These results may consequently not represent the ‘true’ cost-effectiveness of ticagrelor. Additionally, the uncertainty surrounding the cost-effectiveness results is likely underestimated, which leads to misspecification of the risk associated with the reimbursement of ticagrelor.
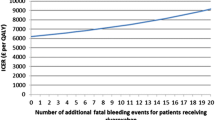
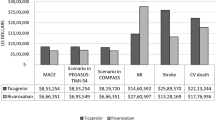
The ERG explored the influence of alternative assumptions through different scenario analyses. These analyses resulted in ICERs ranging from £24,989 to £33,676 per QALY gained. The most influential assumption was assuming treatment discontinuation because of non-fatal events or after 3 years of treatment (Table 2) [23].
3.6 Conclusion of the ERG Report
The clinical evidence presented in the CS was based on a large RCT, PEGASUS-TIMI 54, comparing ticagrelor 60 mg BID + ASA versus placebo + ASA in patients who had experienced an MI between 1 and 3 years previously. The population included in the CS was a subgroup of the population of the trial, comprising patients who had experienced an MI < 2 years previously, i.e. the ‘label’ population. Results of PEGASUS-TIMI 54 showed differences in the relative risk of experiencing events, but these differences were based on a small number of observed events. Hence, the absolute risk of experiencing events remains low.
The CS did not include a comparison of ticagrelor 60 mg BID + ASA versus clopidogrel + ASA, whereas the latter was included as a comparator in the final scope [13]. Indirect comparison between these treatments was deemed impossible by the company because of differences in study design and patient characteristics in the available studies.
Simplifications underlying the model structure (i.e. no long-term consequences of third and subsequent non-fatal events and adverse events) may potentially influence health outcomes and costs. The ERG could not explore the impact on the results of using parametric time-to-event models fitted to the full trial population instead of the ‘label’ population. Because of the inadequate implementation of the PSA of the individual patient simulation, the ERG used the cohort simulation to obtain probabilistic cost-effectiveness estimates.
The ERG amended the company’s cost-effectiveness model to increase its adherence to the NICE reference case, final scope and modelling best practices. A more conservative utility decrement was used for major bleeds to account for the non-inclusion of long-term consequences of this event. The ERG recalculated inpatient costs for the ‘no event’ health state. These adjustments resulted in an ERG base-case ICER of £24,711 per QALY gained, based on the PSA of the cohort simulation. These results may not be appropriate to support decision making for the following reasons. First, the use of the cohort simulation may likely overestimate the ICER, but it probably underestimates the uncertainty surrounding the results. Second, the effect and resulting uncertainty of using indirect evidence (i.e. not obtained from the population of interest) to inform the effectiveness of ticagrelor has not been explored and is not incorporated in the PSA (i.e. effectiveness parameters were not based on the ‘label’ population but on the full trial population). This may be a conservative assumption, but no evidence was shown to support it. Third, the model structure does not account for the long-term consequences of subsequent and adverse events, which leads to flawed estimations of the health benefits and costs obtained by the comparators.
In conclusion, given these concerns, the results of these analyses may not reveal the ‘true’ cost-effectiveness of ticagrelor. The uncertainty surrounding the cost-effectiveness results is likely underestimated, which results in a misspecification of the risk associated with the reimbursement of ticagrelor.
4 Key Methodological Issues
The main methodological issue was the incorrect implementation of the PSA of the individual patient simulation. The company judged that it was impossible to perform deterministic sensitivity analyses (DSA) and a PSA on the complete individual patient simulation (N = 10,779) because of the computational time required by such analyses. Therefore, DSA and PSA were performed on the patient with the profile that provided the closest ICER to the mean expected ICER of the complete individual patient simulation. In its clarification response, the company included 11 patients in the PSA of the individual patient simulation [22]. The ERG thinks this updated PSA was still incorrect.
The ERG agreed that performing DSA and PSA on the complete individual patient population is computationally intensive. However, the ERG did not consider that this was a valid argument to select a single patient to perform the DSA and PSA. To correctly represent the uncertainty surrounding the results of an individual patient simulation, PSA should include both variability (i.e. stochastic variation in the results between patients, or first-order uncertainty) and parameter uncertainty (i.e. uncertainty surrounding the ‘true’ parameter value, or second-order uncertainty) [24]. The NICE decision support unit technical support document 15 describes how to estimate the number of patients who should be included in the PSA to obtain reliable cost-effectiveness estimates [26]. By using a single patient (or 11), the company mainly focussed on parameter uncertainty and mostly ignored the variability in outcomes across patients. This led to an underestimation of the uncertainty surrounding the probabilistic outcomes of the individual patient simulation.
In addition, selecting a single patient (or 11) for the PSA produces biased mean cost-effectiveness results because “probabilistic methods provide the best estimates of mean costs and outcomes” according to the NICE methodological guidelines [14]. Hence, the probabilistic results of the company were not fit to support decision making because they did not provide reliable mean cost-effectiveness estimates. In conclusion, the ERG acknowledged the advantages of individual patient-level simulation and that this might be necessary in certain situations. However, to ensure that assessments are fit for purpose, the use of this technique and its computational burden should not be regarded as a justification to violate methodological guidelines (e.g. not performing appropriate PSA). Hence, it is recommended that these aspects are considered when planning and developing individual patient-level simulations.
5 National Institute for Health and Care Excellence Guidance
In November 2016, the AC produced a final guidance concerning the use of ticagrelor 60 mg BID + ASA. The AC recommended ticagrelor 60 mg BID + ASA within its marketing authorisation as secondary prevention of atherothrombotic events in adults who have had an MI and who are at high risk of a subsequent event. Treatment with ticagrelor should be stopped when clinically indicated or after 3 years of treatment.
5.1 Decision Problem—Comparator
The AC considered the comparison of ticagrelor 60 mg BID + ASA with ASA monotherapy as the most appropriate comparison for the decision problem. Clopidogrel + ASA was not considered to be an appropriate comparator “because it does not have a marketing authorisation for use more than 12 months after a myocardial infarction and is not considered established clinical practice at that point in the treatment pathway” [15].
5.2 Consideration of Clinical Effectiveness
The AC noted that the company base-case analysis was based on a pre-specified subgroup of the PEGASUS TIMI-54 trial, i.e. the ‘base-case’ population (patients who had an MI between 1 and 2 years previously). The AC accepted the use of this ‘base-case’ population because of the size of the subgroup even though the PEGASUS TIMI-54 trial was not powered to detect differences in outcomes within this specific subgroup [15].
The AC concluded that treatment with ticagrelor 60 mg BID + ASA was clinically effective.
The AC concluded that ticagrelor 60 mg BID + ASA should last for a maximum duration of up to 3 years, based on the evidence presented.
5.3 Consideration of Cost-Effectiveness
The AC concluded that the use of parametric time-to-event models based on the full trial population in the cost-effectiveness model was likely to underestimate the effect of ticagrelor 60 mg BID + ASA. Hence, the AC established that the ICERs were likely to be overestimated.
When deliberating about the most plausible ICER, the AC considered both the company and the ERG base-case analyses. The AC was reassured that only one scenario analysis performed by the ERG, which was incidentally considered as implausible by the AC, resulted in an ICER above the £30,000 per QALY threshold.
The AC concluded that all cost-effectiveness estimates were within a range considered to be a cost-effective use of NHS resources (£20,000–30,000 per QALY). Therefore, the AC could recommend treatment with ticagrelor 60 mg BID + ASA within its marketing authorisation.
6 Conclusions
This paper describes the STA of ticagrelor 60 mg BID + ASA for the secondary prevention of atherothrombotic events after an MI. The AC considered ticagrelor 60 mg BID + ASA as a clinically effective and cost-effective use of NHS resources.
References
European Medicines Agency. Brilique (ticagrelor): EPAR—product information. Annex I. Summary of product characteristics. London: European Medicines Agency; 2011. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001241/WC500100494.pdf. Accessed 1 Aug 2017.
National Institute for Health and Care Excellence (NICE). Ticagrelor for preventing atherothrombotic events after myocardial infarction. NICE technology appraisal guidance 420. London: NICE; 2017. https://www.nice.org.uk/guidance/ta420. Accessed 1 Aug 2017.
Garcia-Garcia C, Subirana I, Sala J, Bruguera J, Sanz G, Valle V, et al. Long-term prognosis of first myocardial infarction according to the electrocardiographic pattern (ST elevation myocardial infarction, non-ST elevation myocardial infarction and non-classified myocardial infarction) and revascularization procedures. Am J Cardiol. 2011;108(8):1061–7. https://doi.org/10.1016/j.amjcard.2011.06.003.
Kikkert WJ, Hoebers LP, Damman P, Lieve KV, Claessen BE, Vis MM, et al. Recurrent myocardial infarction after primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Am J Cardiol. 2014;113(2):229–35. https://doi.org/10.1016/j.amjcard.2013.08.039.
Nakatani D, Sakata Y, Suna S, Usami M, Matsumoto S, Shimizu M, et al. Incidence, predictors, and subsequent mortality risk of recurrent myocardial infarction in patients following discharge for acute myocardial infarction. Circ J. 2013;77(2):439–46.
National Institute for Health and Care Excellence (NICE). Myocardial infarction: cardiac rehabilitation and prevention of further MI. NICE clinical guideline 172. London: NICE; 2013. https://www.nice.org.uk/guidance/cg172. Accessed 4 Aug 2017.
Bhatt DL, Flather MD, Hacke W, Berger PB, Black HR, Boden WE, et al. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol. 2007;49(19):1982–8. https://doi.org/10.1016/j.jacc.2007.03.025.
Bhatt DL, Topol EJ. Clopidogrel added to aspirin versus aspirin alone in secondary prevention and high-risk primary prevention: rationale and design of the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial. Am Heart J. 2004;148(2):263–8. https://doi.org/10.1016/j.ahj.2004.03.028.
Yeh RW, Kereiakes DJ, Steg PG, Windecker S, Rinaldi MJ, Gershlick AH, et al. Benefits and risks of extended duration dual antiplatelet therapy after PCI in patients with and without acute myocardial infarction. J Am Coll Cardiol. 2015;65(20):2211–21. https://doi.org/10.1016/j.jacc.2015.03.003.
Bonaca MP, Bhatt DL, Braunwald E, Cohen M, Steg PG, Storey RF, et al. Design and rationale for the prevention of cardiovascular events in patients with prior heart attack using ticagrelor compared to placebo on a background of aspirin-thrombolysis in myocardial infarction 54 (PEGASUS-TIMI 54) trial. Am Heart J. 2014;167(4):437–45. https://doi.org/10.1016/j.ahj.2013.12.020.
Joint Formulary Committee. British National Formulary. 73. London: BMJ Group and Pharmaceutical Press; 2017. http://www.bnf.org. Accessed 11 Dec 2017.
AstraZeneca. Ticagrelor for secondary prevention of atherothrombotic events after myocardial infarction [ID813]: AstraZeneca evidence submission to National Institute of Health and Clinical Excellence: Single technology appraisal (STA). Cambridge: AstraZeneca; 2016.
National Institute for Health and Care Excellence (NICE). Ticagrelor for secondary prevention of atherothrombotic events after myocardial infarction [ID813]. Final scope. London: NICE; 2016. https://www.nice.org.uk/guidance/ta420/documents/final-scope. Accessed 26 July 2017.
National Institute for Health and Care Excellence (NICE). Guide to the methods of technology appraisal 2013. London: NICE; 2013. http://publications.nice.org.uk/pmg9. Accessed 1 Aug 2017.
National Institute for Health and Care Excellence (NICE). Ticagrelor for preventing atherothrombotic events after myocardial infarction [ID813]. Final appraisal determination. London: NICE; 2016. https://www.nice.org.uk/guidance/ta420/documents/final-appraisal-determination-document. Accessed 26 July 2017.
Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001–15.
United Kingdom Clinical Pharmacy Association (UKCPA)—Cardiac Group. Ticagrelor for secondary prevention of atherothrombotic events after myocardial infarction [ID813]: UKCPA professional organisation submission to National Institute of Health and Clinical Excellence. Single technology appraisal (STA). 2016.
National Institute for Health and Care Excellence (NICE). Prasugrel with percutaneous coronary intervention for treating acute coronary syndromes. NICE technology appraisal guidance 317. London: NICE; 2014. https://www.nice.org.uk/guidance/ta317. Accessed 26 July 2017.
National Institute for Health and Care Excellence (NICE). Clopidogrel and modified-release dipyridamole for the prevention of occlusive vascular events. NICE technology appraisal guidance 210. London: NICE; 2010. https://www.nice.org.uk/guidance/ta210. Accessed 26 July 2017.
Department of Health. Reference costs 2014–15. London: Department of Health; 2015. https://www.gov.uk/government/publications/nhs-reference-costs-2014-to-2015. Accessed 26 July 2017.
National Institute for Health and Care Excellence (NICE). Ticagrelor for secondary prevention of atherothrombotic events after myocardial infarction [ID813]. Clarification letter. London: NICE; 2016.
AstraZeneca. Ticagrelor for secondary prevention of atherothrombotic events after myocardial infarction [ID813]. Response to request for clarification from the ERG. Cambridge: AstraZeneca; 2016.
Wolff R, Pouwels X, Ramaekers BLT, Van Giessen A, Birnie R, Lang S, et al. Ticagrelor for secondary prevention of atherothrombotic events after myocardial infarction: a single technology assessment. York: Kleijnen Systematic Reviews Ltd; 2016.
Halpern EF, Weinstein MC, Hunink MG, Gazelle GS. Representing both first- and second-order uncertainties by Monte Carlo simulation for groups of patients. Med Decis Mak. 2000;20(3):314–22. https://doi.org/10.1177/0272989x0002000308.
Kaltenthaler E, Carroll C, Hill-McManus D, Scope A, Holmes M, Rice S, et al. The use of exploratory analyses within the National Institute for Health and Care Excellence single technology appraisal process: an evaluation and qualitative analysis. Health Technol Assess. 2016;20(26):1–48.
Davis S, Stevenson M, Tappenden P, Wailoo A. NICE DSU technical support document 15: cost-effectiveness modelling using patient-level simulation. Sheffield: NICE Decision Support Unit; 2014. http://www.nicedsu.org.uk/TSD15_Patient-level_simulation.pdf. Accessed 4 Aug 2017.
Acknowledgements
The authors acknowledge Richard Birnie for his critique of the clinical-effectiveness evidence.
Author information
Authors and Affiliations
Contributions
All authors commented on the submitted manuscript and gave approval for the final version to be published. RW, SL, GW and JK reviewed the clinical-effectiveness evidence; SD reviewed the search methods; XP, BR, AvG, SR, NA and MJ reviewed the cost-effectiveness evidence. XP acts as overall guarantor for the manuscript. This summary was not externally peer reviewed by PharmacoEconomics.
Corresponding author
Ethics declarations
Funding
This project was funded by the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) Programme. See the HTA programme website for further project information (http://www.hta.ac.uk). This summary of the ERG report was compiled after NICE issued the FAD. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of NICE or the Department of Health.
Conflict of interest
Xavier Pouwels, Robert Wolff, Bram Ramaekers, Anoukh van Giessen, Shona Lang, Steve Ryder, Gill Worthy, Steven Duffy, Nigel Armstrong, Jos Kleijnen and Manuela Joore have no conflicts of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Pouwels, X.G.L.V., Wolff, R., Ramaekers, B.L.T. et al. Ticagrelor for Secondary Prevention of Atherothrombotic Events After Myocardial Infarction: An Evidence Review Group Perspective of a NICE Single Technology Appraisal. PharmacoEconomics 36, 533–543 (2018). https://doi.org/10.1007/s40273-017-0607-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-017-0607-0