Abstract
Background
To date, evidence has been lacking regarding bevacizumab pharmacokinetics in the cerebrospinal fluid (CSF).
Objective
This study assessed the penetration of bevacizumab, as part of a metronomic antiangiogenic treatment regimen, into the CSF of children, adolescents, and young adults with recurrent brain tumors.
Patients and Methods
Serum and CSF concentrations, malignant cells, and vascular endothelial growth factor A (VEGF-A) were analyzed in 12 patients (5–27 years) following 10 mg/kg bevacizumab intravenous biweekly administration (EudraCT number 2009-013024-23). A population pharmacokinetic model including body weight, albumin, and tumor type as influential factors was extended to quantify the CSF penetration of bevacizumab.
Results
Apart from in serum (minimum concentration/maximum concentration [Cmin/Cmax] 77.0–305/267–612 mg/L, median 144/417 mg/L), bevacizumab could be quantified in the CSF (0.01–2.26 mg/L, median 0.35 mg/L). The CSF/serum ratio was 0.16 and highly variable between patients. Malignant cells could be detected in CSF before initiation of treatment in five of 12 patients; after treatment, the CSF was cleared in all patients. VEGF-A was detected in three patients before treatment (mean ± SD: 20 ± 11 pg/mL), and was still measurable in one of these patients despite treatment (16 pg/mL).
Conclusions
This pharmacokinetic pilot study indicated penetration of bevacizumab into the CSF in a population of children, adolescents, and young adults with recurrent brain tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The study presents first evidence on the penetration of bevacizumab into the cerebrospinal fluid (CSF), with a CSF/plasma ratio of 0.16. |
Malignant cells were present in CSF before (5/12 patients), but not after treatment. |
Vascular endothelial growth factor A (VEGF-A) was detected in three patients before treatment and in one out of these three patients despite treatment. |
1 Introduction
Brain tumors represent the most common form of solid malignancies in the pediatric population and substantially contribute to cancer-related morbidity and mortality in children and adolescents [1]. Despite multimodal treatment strategies including surgery, radiotherapy, and chemotherapy, approximately 25% of pediatric brain tumor patients experience relapse or progression, often leading to fatal outcomes. The histological categories most prone to progression or recurrence comprise high-grade glioma, ependymoma, medulloblastoma, and atypical teratoid rhabdoid tumor (ATRT) [2].
Treating recurrent brain tumors is complicated by their tendency to leptomeningeal dissemination. Drug concentrations within the central nervous system (CNS) are influenced by multiple factors, including the permeability of the blood–brain barrier (BBB) and the blood–cerebrospinal fluid (CSF) barrier to the therapeutic agent. Despite the critical role of CNS distribution in the treatment of brain tumors, scarce information exists for many administered drugs, particularly monoclonal antibodies, regarding their concentration–time profiles in the CSF. The penetration of antibodies into the CSF has been reported to be approximately 0.1% of the serum concentration in patients [3,4,5]. Given the wide use of antibodies as antineoplastic agents, understanding their distribution in the CNS and CSF is imperative for the effective use of antibody-based therapeutics against brain tumors [6].
Malignant cells often exhibit a propensity to develop drug resistance, posing a major challenge in managing recurrent malignancies. Beyond cancer cells themselves, the tumor microenvironment plays a critical role in tumor progression and response to treatment across various cancer types. Metronomic antiangiogenic therapy exerts its antitumor effects through multiple mechanisms, by targeting the tumor microenvironment via the inhibition of angiogenesis, by modulating tumor immunity, and by directly targeting cancer cells [7,8,9,10,11,12]. The development of metronomic antiangiogenic therapy as a treatment option emerged from the observation that tumors resistant to dose-intense therapy responded to the same agents at low continuous dosing [7, 8]. Metronomic antiangiogenic therapy regimens including bevacizumab are increasingly applied in the treatment of various pediatric CNS malignancies, particularly in recurrent tumors, and have shown encouraging treatment effects [13, 14]. Bevacizumab is a recombinant humanized monoclonal IgG1 antibody that binds to and inactivates vascular endothelial growth factor A (VEGF-A), thereby inhibiting angiogenesis [15]. A multicenter, controlled trial recently showed prolonged survival in patients with recurrent medulloblastoma using a metronomic antiangiogenic regimen supplemented by intravenous bevacizumab [16].
Children and adolescents and young adults (AYA) frequently exhibit different drug exposure and may require varied drug dosages compared to adults to achieve the desired therapeutic effect. Despite the evident need for investigated safe treatment regimens for children and adolescents with malignant diseases, most drugs have exclusively been approved for adult patients. Furthermore, AYA aged between 15 and 39 years represent a frequently overlooked group [17,18,19].
Ethical considerations and practical challenges frequently constrain the inclusion of children and AYA in clinical trials [20], resulting in a lack of comprehensive data regarding the pharmacological behavior of drugs within these age groups. Furthermore, sampling is limited in clinical studies involving children, leading to a scarcity of data that poses challenges for data analysis. Population pharmacokinetic models have proven particularly useful in situations with sparse and/or unbalanced data (i.e., with few observations per patient sampled at different times of the dosing interval), as often encountered in pediatric clinical trials [21]. These models do not only allow determination of pharmacokinetic parameters for a population and individual patients, but also quantification of variability in the pharmacokinetic parameters and concentration–time profiles between patients.
Several studies have investigated the pharmacokinetics of bevacizumab in children in serum [22, 23]. In pediatric cancer patients, bevacizumab exhibited linear pharmacokinetics and a half-life of 19.6 days [23]. However, little is known about the pharmacokinetics and activity of bevacizumab in the CNS. Because an intact BBB restricts the penetration of monoclonal antibodies into the CNS, it has been assumed that bevacizumab might be unable to enter the CSF.
The present study aimed to address this question and quantified bevacizumab concentrations in serum and CSF in children and adolescents with recurrent malignant brain tumors. A population pharmacokinetic model was employed to assess the extent of distribution into the CSF. We further investigated the impact of bevacizumab on VEGF-A levels in CSF and the presence of cytological leptomeningeal metastases.
2 Patients and Methods
2.1 Patients and Treatment
Pediatric patients with a histologically confirmed diagnosis of a malignant brain tumor receiving metronomic antiangiogenic therapy were eligible for the study. All enrolled patients had an Ommaya reservoir in place. Bevacizumab (Avastin®, 500 mg, concentrate for preparation of an infusion solution, Roche, Switzerland) was administered as intravenous infusion at a dosage of 10 mg/kg over 30 min every 2 weeks. Patients further received thalidomide (3 mg/kg/day daily, orally), celecoxib (50–400 mg twice daily, orally), fenofibrate (90 mg/m2 daily, orally), alternating 21-day courses of etoposide (35–50 mg/m2 daily, orally), and cyclophosphamide (2.5 mg/kg daily, orally). Additionally, intraventricular etoposide was administered (0.5 mg × 5 days in 1 week out of every 4 weeks), alternating with intraventricular liposomal cytarabine (25–50 mg every 4 weeks) [13, 14, 16].
Further inclusion criteria encompassed (1) a life expectancy of at least 8 weeks, (2) the acquisition of written informed consent from the parents and/or the patients themselves, (3) serum creatinine concentrations < 1.5 mg/dL, (4) total serum bilirubin concentrations < 2.0 mg/dL and alanine aminotransferase concentrations < 5 times upper limit of normal, and (5) platelet counts > 40,000/mm3 within 48 h preceding the first treatment. The study was approved by the Ethics Committee of the Medical University of Vienna, carried out in accordance with the Declaration of Helsinki, and registered in the European Clinical Trials Database (EudraCT number 2009-013024-23).
2.2 Sampling, Bioanalysis, and Cytology
For the pharmacokinetic study, blood and CSF samples were collected under real-world conditions defined by the patient’s treatment protocol. Approximately 1.2 mL of venous blood were collected in tubes without previously added anticoagulant before and within 3 h following bevacizumab administration. CSF (2 mL) was collected in separate tubes after puncture of the Ommaya reservoir at steady state, immediately before the intraventricular administration of antineoplastic drugs (etoposide or liposomal cytarabine). To avoid any additional risks for the patients, CSF samples were only taken on occasions when the Ommaya reservoir was punctured for routine reasons. Blood samples were kept on ice for a maximum duration of 60 min and centrifuged at +4 °C and 3000 rpm for 10 min. CSF and serum samples were snap frozen and stored at −80 °C until analysis.
Bevacizumab concentrations were determined using an enzyme-linked immunosorbent assay (ELISA), according to the manufacturer’s instructions. Briefly, snap frozen serum and CSF aliquots were thawed on ice before analysis in triplicates. The lowest quantifiable concentration in human serum and CSF was 30 ng/mL (Matriks Biotech, Ankara, Turkey), and detected values were validated with a calibration curve.
CSF concentrations of human VEGF-A taken before the start of recurrence treatment and the last available sample at the end of treatment (eight patients at the end of planned treatment, four patients due to progression) were determined using a specific sandwich ELISA kit (RayBiotech, GA, USA; catalog number ELH-VEGF) following the standard procedure specified by the manufacturer. The lowest quantifiable concentration in human serum and CSF was 10 pg/mL. Samples were used without dilution, and the absorption was measured at 450 nm.
A cytological examination of CSF was performed using cytospins stained with a May-Grünwald-Giemsa staining. The results were reviewed by an experienced neuropathologist to identify any presence of malignant cells.
2.3 Pharmacokinetic Assessment of Serum and CSF Concentrations
Population pharmacokinetic modelling was used to describe CSF concentrations of bevacizumab relative to serum concentrations. The population approach has demonstrated substantial utility in analyzing scenarios with sparse and unevenly distributed data. A robust and externally validated population model for pediatric cancer patients was used to describe bevacizumab concentration–time profiles in serum [23]. The two-compartment model was developed based on a large patient population (152 patients, 1427 observations) spanning broad ranges of age (0.5–21 years) and body weight (5.9–125 kg). The patients underlying the model received a similar dosing regimen (5–15 mg/kg biweekly or triweekly) as the current study population.
To obtain predictions of serum concentrations for the study participants, parameters from children with primary CNS tumors (displaying similar characteristics as the enrolled patients) were used and fixed to their final estimates (Table 1). The model contained covariate effects (i.e., patient characteristics influencing the pharmacokinetics of bevacizumab) of albumin, sex, and tumor type on clearance, tumor type on central volume of distribution, and body weight on all distribution and elimination parameters (using allometric or power functions). Individual pharmacokinetic parameters and, consequently, serum concentration–time profiles were obtained post hoc for each patient, based on their individual covariate values and the available two serum concentrations.
To assess drug penetration into the CSF, the model was adapted to accommodate CSF concentrations of bevacizumab by adding an additional peripheral compartment to the serum-based pharmacokinetic model (Fig. 1). Consequently, the parameters volume of distribution of CSF (VCSF), intercompartmental clearance between the central and CSF compartment (QCSF), and an uptake factor, accounting for bevacizumab penetration from the central compartment into the CSF (‘CSF/serum ratio’), were introduced [26].
Schematic depiction of the population pharmacokinetic model used to characterize serum and CSF concentrations of bevacizumab. CL clearance; CSF cerebrospinal fluid; Q/QCSF intercompartmental clearance parameters; UPTK uptake factor capturing drug penetration into CSF; VC volume of distribution of the central compartment; VP volume of distribution of the peripheral compartment; VCSF volume of distribution of the CSF compartment
Between-patient variability of the model parameters was described assuming a log-normal distribution. Proportional, additive, and combined error models were explored to describe residual variability. For model discrimination, a decrease in the objective function value (OFV) of ≥ 3.84 was deemed significant (given 1 degree of freedom and α = 0.05 according to the χ2 distribution). Model evaluation included standard goodness-of-fit plots (e.g., observed vs [individual] predicted concentrations, conditional weighted residuals vs predictions and time), as well as a visual predictive check (n = 1000) to assess the predictive performance of the model. A non-parametric bootstrap (n = 500) was performed on the final model to assess parameter uncertainty.
Stochastic simulations (n = 500) were performed with the final model to assess bevacizumab penetration into CSF considering interindividual variability in the pharmacokinetic parameter estimates. Areas under the concentration–time curve (AUCs) were compared for a dosing interval of 2 weeks after 20 doses of 10 mg/kg for 12 individuals with patient characteristics of the study participants.
All modelling activities were conducted using NONMEM®7.4 (ICON Development Solutions, Hanover, MD, USA; first-order conditional estimation method with interaction [FOCEI]) and supported by PsN (version 4.9.6, Uppsala University, Uppsala, Sweden). Model evaluation and graphical illustrations were carried out using R (v3.6.2., R Foundation for Statistical Computing, Vienna, Austria; https://www.R-project.org).
3 Results
3.1 Patients
The pharmacokinetic study was performed in 12 patients (five female, seven male) with a median age of 13 years (range 5–27 years; see Table 2 for further patient characteristics). Diagnoses were medulloblastoma (n = 5), ependymoma (n = 1), choroid plexus carcinoma (n = 1), pineoblastoma (n = 2), CNS germ cell tumor (n = 1), CNS embryonal tumor, not otherwise specified (n = 1), and ATRT (n = 1). All patients received treatment for recurrent disease.
3.2 Pharmacokinetic Assessment of Serum and CSF Concentrations
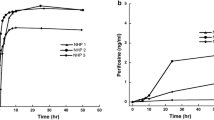
Serum and CSF concentrations were obtained following 22 (median) (min–max: n = 7–41) prior doses of bevacizumab. Bevacizumab administration (10 mg/kg) and measurements in serum were performed 14 days (range 12–15 days) after the previous infusion of bevacizumab. Trough (minimum concentration [Cmin]) and peak (maximum concentration [Cmax]) serum samples (before and after intravenous infusion of bevacizumab) were available for all patients (nsample = 24). Cmin ranged from 77.0 to 305 mg/L (median 144 mg/L), and Cmax encompassed values between 267 and 612 mg/L (median 417 mg/L; Fig. 2).
Left panel: Serum concentrations, measured before and after bevacizumab (BVZ) infusion (vertical red line). Right panel: concentrations in cerebrospinal fluid (CSF), measured on different days following BVZ administration (partly in different dosing intervals). BLQ below the lower limit of quantification
CSF samples were taken when intraventricular therapy was scheduled. In each patient, three (median) (min–max: 3–4) CSF concentrations were obtained during different treatment cycles of bevacizumab within 1–15 days after dosing (see Fig. S1 in the Online Resource [electronic supplementary material] for individual CSF and serum sampling). In CSF, concentrations of 0.01–2.26 mg/L (median 0.35 mg/L) were quantified (ntotal = 38; 3–4 samples/patient; Fig. 2).
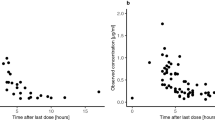
Individual pharmacokinetic parameters were determined for each patient using a previously developed two-compartment model for bevacizumab [23]. The model predicted Cmin and Cmax concentrations reasonably well (Fig. 3; individual profiles: Fig. S2, Online Resource) and was used to describe the serum concentration–time profiles of the patients also at later time points (Fig. 4).
Measured serum concentrations (blue circles) and predicted serum concentration–time profiles of bevacizumab (BVZ) based on a previously published population pharmacokinetic model for pediatric cancer patients [23]
To quantify bevacizumab penetration into CSF, an additional compartment was added to the model (Fig. 1). The difference between serum and CSF concentrations was modelled using an uptake factor (UPTK), which was 0.16 for the typical patient in the population. The model significantly improved when allowing interindividual variability on the uptake factor (ΔOFV 48.5) and revealed a broad range of individual estimates between 0.02 and 0.66 (median 0.17). Simulations considering between-patient variability in the pharmacokinetic parameters revealed a median ratio of AUCPL/AUCCSF of 0.156 (95% prediction interval 0.00282–0.897).
Due to the sparse nature of the data, two CSF parameters were fixed to physiology-based values. VCSF was set to 150 mL/70 kg with linear weight scaling [27]. Intercompartmental clearance to CSF (QCSF) was described as the blood flow rate to brain (12% of cardiac output, corrected by hematocrit to represent plasma flow) [24, 28]. Furthermore, a blood flow correction factor (FCSF) was added to the model, but did not lead to significant model improvement. FCSF was estimated as 0.33, indicating permeation restriction. Residual unexplained variability was described using a proportional model, which was superior to an additive model (ΔOFV 37.3) and not inferior to a combined variability model. Model parameter estimates are summarized in Table 1. To assess a potential influence of one CSF concentration below the lower limit of quantification on the results, the model was rerun after exclusion of this specific sample. Model parameters remained virtually unchanged (UPTK 0.0016; relative standard error 27.2→25.2%), with just a slight reduction of interindividual variability associated with UPTK, as expected (coefficient of variation [CV%] 107→101%).
Goodness-of-fit plots indicated good agreement between the observed and predicted data (Fig. 5), without any striking trends and with random scatter around the line of identity or reference line (conditional weighted residuals: Fig. S3, Online Resource). Individual predicted profiles (Fig. S4, Online Resource) were reasonably predicted given the sparse data situation, and the visual predictive check showed acceptable predictive performance of the model, with slight overestimation of interindividual variability (Fig. 6).
Visual predictive check of the model for cerebrospinal fluid concentrations of bevacizumab (BVZ). Blue circles represent measured BVZ concentrations; the blue solid line shows median observed concentrations, and the blue dashed lines indicate the 10th and 90th percentiles of the observed data. The green solid line shows the median of model predictions. Shaded areas depict the 95% confidence intervals for the prediction lines (median, 10th and 90th percentile)
3.3 VEGF-A Concentrations in the CSF
VEGF-A was detected before the start of treatment for the recurrent tumor in three patients (25%), diagnosed with medulloblastoma, plexus carcinoma, and pineoblastoma. VEGF-A concentrations ranged from 12 to 33 pg/mL (mean ± SD: 20 ± 11 pg/mL). After the treatment, VEGF-A levels were below the detection limit in all but one patient (Table 3).
3.4 Cytology
Upon recurrence, before initiation of treatment, malignant cells in the CSF were detectable in five out of 12 patients. At the time of pharmacokinetic sampling as well as at the last available examination at the end of treatment, all patients attained treatment response, with CSF samples tested negative for malignant cells. Clearance of VEGF-A levels and clearance of malignant cells in CSF cytology correlated in two cases (Table 3). Overall, detection of tumor cells in CSF and detectable VEGF-A levels were not significantly associated (Fisher’s exact test, p = 0.5).
4 Discussion
This study presents a comprehensive analysis of bevacizumab in serum and CSF in children and AYA with recurrent brain tumors using population pharmacokinetic modeling and simulation. Metronomic antiangiogenic therapy including bevacizumab is increasingly used in recurrent brain tumors in children and AYA [13, 14].
The BBB prevents the entry of many therapeutic agents to the CNS. The tight junctions interconnecting the endothelial cells of the BBB restrict the free passage of water-soluble molecules and cells [29], prevent the leakage of substances from the bloodstream into the CNS, and thereby protect the brain against circulating potentially neurotoxic substances. The BBB serves multiple functions in neuroimmunology by acting as a barrier, isolating the CNS from the immune components of the periphery. This unique function grants the brain its status as an immunologically privileged organ [30]. Although the intact BBB is expected to restrict the penetration of molecules with high molecular weight into the CNS, there is some evidence suggesting that the permeability of the BBB may be increased in patients with impaired BBB, e.g., those having undergone cranial radiotherapy [31].
To date, no direct assessment of the extent to which intravenous bevacizumab can reach the CSF has been performed. To address this important clinical question, we measured bevacizumab in serum and CSF and assessed the extent of distribution using population pharmacokinetic modelling and simulation. Serum concentration measurements were available only shortly before and after bevacizumab administration (Cmin 77.0–305 mg/L; Cmax 267–612 mg/L). The observed serum concentrations were in the range of previous studies in children with osteosarcoma [32] and patients with advanced non‑squamous non‑small cell lung cancer [33]. These studies showed variable serum concentrations up to 800 mg/L (even in the first cycles) given 15 mg/kg dosing over 30–90 min, corresponding to 530 mg/L for 10 mg/L assuming dose linearity, as had been shown for bevacizumab within a dose range of 1–20 mg/kg [34]. In a study with dosing ≤ 20 mg/kg infused over 30–90 min (600 mg/L assuming 10 mg/kg dosing), serum concentrations even up to 1200 mg/L have been reported [34]. A previous study with eight children with refractory solid tumors demonstrated lower Cmax of bevacizumab than in the present investigation (230–425 mg/L after 15 mg/kg, corresponding to 153–283 mg/L after 10 mg/kg) [35]. However, like in the aforementioned studies, longer infusion durations (30–90 min vs 30 min), leading to lower Cmax, and sampling in early phases rather than at steady state could explain this difference. None of the studies investigated CSF concentrations.
The current study found measurable bevacizumab concentrations of 0.01–2.26 mg/L (median 0.35 mg/L) in CSF. Unlike serum concentrations, CSF samples were not exclusively taken at the exact time points of the serum concentrations, which covered merely a small segment of the concentration–time profile. Thus, serum pharmacokinetics of the study participants were predicted using a previously published two-compartment model of a pediatric and adolescent population with primary CNS tumors, and CNS concentrations were described based on individual predicted serum profiles. An uptake factor of 0.16 was estimated as a measure of the fraction of bevacizumab concentrations penetrating from the central compartment into the CSF. This magnitude was in agreement with penetration ratios derived from raw data of serum and CSF concentrations measured on the same day. The estimated uptake factor aligns well with physiological CSF/serum ratios (0.16–0.32 [36, 37]) and the limited uptake of therapeutic antibodies (0.1–0.2% of injected dose) into the brain [38]. CSF penetration has been demonstrated for several antibodies, including ponezumab (0.5% to < 1% [39, 40]), elezanumab (0.1–0.4% [41]), nivolumab (0.3–1.9% [42, 43]), atibuclimab (0.03% [44]), GNbAC (0.12–0.42 [45]), and rituximab (0.1–0.3%) [4]. For bevacizumab, previous evidence on penetration into the CNS is scarce. A study with mice showed bevacizumab distribution within brain structures and particularly within glioblastoma tumor areas after intraperitoneal injection [46]. However, the transferability of these results to humans is not clear, and although also a surrogate for CNS penetration, distribution to brain or tumor tissue cannot be directly compared to CSF penetration.
The measured CSF concentrations (0.01–2.26 mg/L) partly exceeded the inhibitory concentrations reported in several previous experimental studies, suggesting a potential for effects in this peripheral compartment. For example, investigations of the binding affinity of bevacizumab for VEGF isoforms demonstrated concentration for 50% of maximal effect (EC50) values ranging from 0.15 nM (0.0224 mg/L given a molar mass of 149,196.8162) [47] to 0.18 mg/L [48]. Fifty percent inhibitory concentration (IC50) values for inhibiting vascular endothelial growth factor receptor 1 (VEGFR1) and VEGFR2 binding/activation lay between 0.63 nM (~ 0.0940 mg/L), 1.476 nM (~ 0.220 mg/L), and 2.661 nM (0.397 mg/L) [15, 49]. Furthermore, bevacizumab inhibited the proliferation of bovine retinal microvascular endothelial cells with an average IC50 of 0.668 nM (~ 0.0997 mg/L) [50]. Similarly, assays with human umbilical vein endothelial cells (HUVEC) showed that VEGF-induced proliferation was blocked with IC50 = 0.047 ± 0.0081 mg/L [48], and VEGF-A165-induced calcium mobilization was inhibited with IC50 = 70.8 pM (0.0106 mg/L) [15], thus inhibitory concentrations were within the magnitude of the measured CSF concentrations.
The detection of tumor cells in the CSF remains the gold standard for a definite diagnosis of leptomeningeal dissemination. However, the cytological analysis of the CSF suffers from several important shortcomings [51]. These limitations are especially relevant in children, in whom only small CSF volumes can be collected.
The secretion of the proangiogenic molecule VEGF-A has been recognized as a crucial mechanism through which cancer cells stimulate neoangiogenesis in tumor tissue [15]. Patients with malignant tumors and leptomeningeal dissemination showed high values of VEGF in CSF [52, 53]. During treatment with radiation and intrathecal chemotherapy in adult patients with carcinomatous meningitis, responders showed a decrease in elevated CSF VEGF concentrations below the detection limit, while non-responders maintained high concentrations [52]. In our study’s patients with recurrent brain tumors, VEGF-A could be detected in the CSF of three patients, all of whom also exhibited positive CSF cytology, reflecting the metastatic nature of their disease. While the CSF could be cleared of malignant cells during treatment in all patients, detectable VEGF-A persisted in one patient. This might be a further indication of the limitation of CSF cytology in diagnosing leptomeningeal disease, even with repeated sampling.
Several limitations should be acknowledged in the context of this study. First, the number of patients in the study was rather small (n = 12). Second, given the limited number of blood draws to reduce the burden on children, the available data were sparse, with merely two serum concentrations (both collected right at the initiation of the dosing interval) and two to three CSF samples per patient. A previously published model built upon rich data (n = 1427 observations) was thus used to predict complete serum concentration–time profiles as the basis for describing CSF concentrations relative to serum concentrations [23]. The assumption that serum pharmacokinetics follow the model described by Han et al. [23] was deemed reasonable, as the population underlying the model was largely comparable with the study population, with both pediatric and adolescent patients with primary CNS tumors, similar administered dosages (5–15 mg/kg biweekly or triweekly ↔ 10 mg/kg), and virtually identical median body weight (44 ↔ 47 kg) and albumin concentrations (39 ↔ 39 g/L). Two patients displayed higher ages (26 and 27/0.5–21 years). However, pharmacokinetic differences in this narrow age range of young adults are likely small and model evaluation did not reveal worse performance for these individuals. As a further limitation, the CSF data obtained in this study were not sufficiently rich to support the estimation of the volume of distribution of CSF and the intercompartmental clearance between CSF and the central compartment. Thus, VCSF and QCSF were fixed to physiology-based values in line with multiple previous studies on CSF pharmacokinetics [26, 54]. Studies with more rich data and more patients would be useful to describe CSF penetration of bevacizumab in more detail and with higher precision, as well as to assess a potential impact on tumor type on CSF penetration.
5 Conclusions
In conclusion, this pharmacokinetic pilot study indicated bevacizumab penetration into the CSF in a population of children and AYA with recurrent brain tumors. Further studies involving more patients and more rich data will be interesting to confirm results and to investigate factors influencing drug distribution to the CSF in children and adolescents with recurrent brain tumors.
References
Ostrom QT, Price M, Neff C, Cioffi G, Waite KA, Kruchko C, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2016–2020. Neuro-Oncology. 2023;25:iv1-99. https://doi.org/10.1093/neuonc/noad149.
Cohen AR. Brain tumors in children. N Engl J Med. 2022;386:1922–31. https://doi.org/10.1056/NEJMra2116344.
Kaschka WP, Theilkaes L, Eickhoff K, Skvaril F. Disproportionate elevation of the immunoglobulin G1 concentration in cerebrospinal fluids of patients with multiple sclerosis. Infect Immun. 1979;26:933–41.
Petereit H, Rubbert-Roth A. Rituximab levels in cerebrospinal fluid of patients with neurological autoimmune disorders. Mult Scler. 2009;15:189–92. https://doi.org/10.1177/1352458508098268.
Rubenstein JL, Combs D, Rosenberg J, Levy A, McDermott M, Damon L, et al. Rituximab therapy for CNS lymphomas: targeting the leptomeningeal compartment. Blood. 2003;101:466–8. https://doi.org/10.1182/blood-2002-06-1636.
Tabrizi M, Bornstein GG, Suria H. Biodistribution mechanisms of therapeutic monoclonal antibodies in health and disease. AAPS J. 2010;12:33–43. https://doi.org/10.1208/s12248-009-9157-5.
Browder T, Butterfield CE, Kräling BM, Shi B, Marshall B, O’Reilly MS, et al. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60:1878–86.
Klement G, Baruchel S, Rak J, Man S, Clark K, Hicklin DJ, et al. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest. 2000;105:R15-24. https://doi.org/10.1172/JCI8829.
Pasquier E, Kavallaris M, André N. Metronomic chemotherapy: new rationale for new directions. Nat Rev Clin Oncol. 2010;7:455–65. https://doi.org/10.1038/nrclinonc.2010.82.
Peereboom DM, Alban TJ, Grabowski MM, Alvarado AG, Otvos B, Bayik D, et al. Metronomic capecitabine as an immune modulator in glioblastoma patients reduces myeloid-derived suppressor cells. JCI Insight. 2019. https://doi.org/10.1172/jci.insight.130748.
André N, Orbach D, Pasquier E. Metronomic maintenance for high-risk pediatric malignancies: one size will not fit all. Trends Cancer. 2020;6:819–28. https://doi.org/10.1016/j.trecan.2020.05.007.
Chen Y-L, Chang M-C, Cheng W-F. Metronomic chemotherapy and immunotherapy in cancer treatment. Cancer Lett. 2017;400:282–92. https://doi.org/10.1016/j.canlet.2017.01.040.
Peyrl A, Chocholous M, Kieran MW, Azizi AA, Prucker C, Czech T, et al. Antiangiogenic metronomic therapy for children with recurrent embryonal brain tumors. Pediatr Blood Cancer. 2012;59:511–7. https://doi.org/10.1002/pbc.24006.
Slavc I, Mayr L, Stepien N, Gojo J, Aliotti Lippolis M, Azizi AA, et al. Improved long-term survival of patients with recurrent medulloblastoma treated with a “MEMMAT-like” metronomic antiangiogenic approach. Cancers. 2022;14:5128. https://doi.org/10.3390/cancers14205128.
Papadopoulos N, Martin J, Ruan Q, Rafique A, Rosconi MP, Shi E, et al. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis. 2012;15:171–85. https://doi.org/10.1007/s10456-011-9249-6.
Peyrl A, Chocholous M, Sabel M, Lassaletta A, Sterba J, Leblond P, et al. Sustained survival benefit in recurrent medulloblastoma by a metronomic antiangiogenic regimen: a nonrandomized controlled trial. JAMA Oncol. 2023;26: e234437. https://doi.org/10.1001/jamaoncol.2023.4437.
Zapotocky M, Ramaswamy V, Lassaletta A, Bouffet E. Adolescents and young adults with brain tumors in the context of molecular advances in neuro-oncology. Pediatr Blood Cancer. 2018;65: e26861. https://doi.org/10.1002/pbc.26861.
Geiger AM, Castellino SM. Delineating the age ranges used to define adolescents and young adults. JCO. 2011;29:e492–3. https://doi.org/10.1200/JCO.2011.35.5602.
Sender L, Zabokrtsky KB. Adolescent and young adult patients with cancer: a milieu of unique features. Nat Rev Clin Oncol. 2015;12:465–80. https://doi.org/10.1038/nrclinonc.2015.92.
Shnorhavorian M, Doody DR, Chen VW, Hamilton AS, Kato I, Cress RD, et al. Knowledge of clinical trial availability and reasons for nonparticipation among adolescent and young adult cancer patients: a population-based study. Am J Clin Oncol. 2018;41:581. https://doi.org/10.1097/COC.0000000000000327.
Mould D, Upton R. Basic concepts in population modeling, simulation, and model-based drug development-part 2: introduction to pharmacokinetic modeling methods. CPT Pharmacometr Syst Pharmacol. 2013;2:38. https://doi.org/10.1038/psp.2013.14.
Gojo J, Sauermann R, Knaack U, Slavc I, Peyrl A. Pharmacokinetics of bevacizumab in three patients under the age of 3 years with CNS malignancies. Drugs R D. 2017;17:469–74. https://doi.org/10.1007/s40268-017-0190-z.
Han K, Peyret T, Quartino A, Gosselin NH, Gururangan S, Casanova M, et al. Bevacizumab dosing strategy in paediatric cancer patients based on population pharmacokinetic analysis with external validation. Br J Clin Pharmacol. 2016;81:148–60. https://doi.org/10.1111/bcp.12778.
International Commission on Radiological Protection. Basic anatomical and physiological data for use in radiological protection: reference values. Ann ICRP. 2006. https://doi.org/10.1016/j.icrp.2006.06.001.
Ramakrishnan V, Yang QJ, Quach HP, Cao Y, Chow ECY, Mager DE, et al. Physiologically-based pharmacokinetic-pharmacodynamic modeling of 1,25-dihydroxyvitamin D3 in mice. Drug Metab Dispos. 2016;44:189–208. https://doi.org/10.1124/dmd.115.067033.
Kane Z, Gastine S, Obiero C, Williams P, Murunga S, Thitiri J, et al. IV and oral fosfomycin pharmacokinetics in neonates with suspected clinical sepsis. J Antimicrob Chemother. 2021;76:1855–64. https://doi.org/10.1093/jac/dkab083.
Johanson CE, Duncan JA, Klinge PM, Brinker T, Stopa EG, Silverberg GD. Multiplicity of cerebrospinal fluid functions: new challenges in health and disease. Cerebrospinal Fluid Res. 2008;5:10. https://doi.org/10.1186/1743-8454-5-10.
Krishnan K, Crouse LCB, Bazar MA, Major MA, Reddy G. Physiologically based pharmacokinetic modeling of cyclotrimethylenetrinitramine in male rats. J Appl Toxicol. 2009;29:629–37. https://doi.org/10.1002/jat.1455.
Engelhardt B, Sorokin L. The blood–brain and the blood–cerebrospinal fluid barriers: function and dysfunction. Semin Immunopathol. 2009;31:497–511. https://doi.org/10.1007/s00281-009-0177-0.
Banks WA. The blood–brain barrier in neuroimmunology: tales of separation and assimilation. Brain Behav Immun. 2015;44:1–8. https://doi.org/10.1016/j.bbi.2014.08.007.
Stemmler H-J, Schmitt M, Willems A, Bernhard H, Harbeck N, Heinemann V. Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood–brain barrier. Anticancer Drugs. 2007;18:23. https://doi.org/10.1097/01.cad.0000236313.50833.ee.
Turner DC, Navid F, Daw NC, Mao S, Wu J, Santana VM, et al. Population pharmacokinetics of bevacizumab in children with osteosarcoma: implications for dosing. Clin Cancer Res. 2014;20:2783–92. https://doi.org/10.1158/1078-0432.CCR-13-2364.
Li CSW, Sweeney K, Cronenberger C. Population pharmacokinetic modeling of PF-06439535 (a bevacizumab biosimilar) and reference bevacizumab (Avastin®) in patients with advanced non-squamous non-small cell lung cancer. Cancer Chemother Pharmacol. 2020;85:487–99. https://doi.org/10.1007/s00280-019-03946-8.
Lu J-F, Bruno R, Eppler S, Novotny W, Lum B, Gaudreault J. Clinical pharmacokinetics of bevacizumab in patients with solid tumors. Cancer Chemother Pharmacol. 2008;62:779–86. https://doi.org/10.1007/s00280-007-0664-8.
Bender JLG, Adamson PC, Reid JM, Xu L, Baruchel S, Shaked Y, et al. Phase I trial and pharmacokinetic study of bevacizumab in pediatric patients with refractory solid tumors: a children’s oncology group study. JCO. 2008;26:399–405. https://doi.org/10.1200/JCO.2007.11.9230.
Male DK. Immunology of brain endothelium and the blood brain barrier. In: Bradbury MWB, editor. Physiology and pharmacology of the blood–brain barrier. Berlin: Springer; 1992. p. 397–415.
Bickel U. Antibody delivery through the blood–brain barrier. Adv Drug Deliv Rev. 1995;15:53–72. https://doi.org/10.1016/0169-409X(95)00005-R.
Kouhi A, Pachipulusu V, Kapenstein T, Hu P, Epstein AL, Khawli LA. Brain disposition of antibody-based therapeutics: dogma, approaches and perspectives. IJMS. 2021;22:6442. https://doi.org/10.3390/ijms22126442.
Landen JW, Andreasen N, Cronenberger CL, Schwartz PF, Börjesson-Hanson A, Östlund H, et al. Ponezumab in mild-to-moderate Alzheimer’s disease: randomized phase II PET-PIB study. Alzheimer’s Dementia Transl Res Clin Intervent. 2017;3:393–401. https://doi.org/10.1016/j.trci.2017.05.003.
Landen JW, Zhao Q, Cohen S, Borrie M, Woodward M, Billing CBJ, et al. Safety and pharmacology of a single intravenous dose of ponezumab in subjects with mild-to-moderate Alzheimer disease: a phase I, randomized, placebo-controlled, double-blind, dose-escalation study. Clin Neuropharmacol. 2013;36:14. https://doi.org/10.1097/WNF.0b013e31827db49b.
Kalluri HV, Rosebraugh MR, Misko TP, Ziemann A, Liu W, Cree BAC. Phase 1 evaluation of elezanumab (anti–repulsive guidance molecule A monoclonal antibody) in healthy and multiple sclerosis participants. Ann Neurol. 2023;93:285–96. https://doi.org/10.1002/ana.26503.
van Bussel MTJ, Beijnen JH, Brandsma D. Intracranial antitumor responses of nivolumab and ipilimumab: a pharmacodynamic and pharmacokinetic perspective, a scoping systematic review. BMC Cancer. 2019;19:519. https://doi.org/10.1186/s12885-019-5741-y.
Pluim D, Ros W, van Bussel MTJ, Brandsma D, Beijnen JH, Schellens JHM. Enzyme linked immunosorbent assay for the quantification of nivolumab and pembrolizumab in human serum and cerebrospinal fluid. J Pharm Biomed Anal. 2019;164:128–34. https://doi.org/10.1016/j.jpba.2018.10.025.
Henderson RD, Agosti JM, McCombe PA, Thorpe K, Heggie S, Heshmat S, et al. Phase 1b dose-escalation, safety, and pharmacokinetic study of IC14, a monoclonal antibody against CD14, for the treatment of amyotrophic lateral sclerosis. Medicine. 2021;100: e27421. https://doi.org/10.1097/MD.0000000000027421.
Curtin F, Vidal V, Bernard C, Kromminga A, Lang AB, Porchet H. Serum pharmacokinetics and cerebrospinal fluid concentration analysis of the new IgG4 monoclonal antibody GNbAC1 to treat multiple sclerosis: a phase 1 study. mAbs. 2016;8:854–60. https://doi.org/10.1080/19420862.2016.1168956.
Ait-Belkacem R, Berenguer C, Villard C, Ouafik L, Figarella-Branger D, Beck A, et al. Monitoring therapeutic monoclonal antibodies in brain tumor. mAbs. 2014;6:1385–93. https://doi.org/10.4161/mabs.34405.
Wang Q, Li T, Wu Z, Wu Q, Ke X, Luo D, et al. Novel VEGF decoy receptor fusion protein conbercept targeting multiple VEGF isoforms provide remarkable anti-angiogenesis effect in vivo. PLoS ONE. 2013;8: e70544. https://doi.org/10.1371/journal.pone.0070544.
Wang Q, Yang J, Tang K, Luo L, Wang L, Tian L, et al. Pharmacological characteristics and efficacy of a novel anti-angiogenic antibody FD006 in corneal neovascularization. BMC Biotechnol. 2014;14:17. https://doi.org/10.1186/1472-6750-14-17.
Walker A, Chung C-W, Neu M, Burman M, Batuwangala T, Jones G, et al. Novel interaction mechanism of a domain antibody-based inhibitor of human vascular endothelial growth factor with greater potency than ranibizumab and bevacizumab and improved capacity over aflibercept. J Biol Chem. 2016;291:5500–11. https://doi.org/10.1074/jbc.M115.691162.
Yu L, Liang XH, Ferrara N. Comparing protein VEGF inhibitors: In vitro biological studies. Biochem Biophys Res Commun. 2011;408:276–81. https://doi.org/10.1016/j.bbrc.2011.04.014.
Chamberlain MC. Leptomeningeal metastasis. Semin Neurol. 2010;30:236–44. https://doi.org/10.1055/s-0030-1255220.
Stockhammer G, Poewe W, Burgstaller S, Deisenhammer F, Muigg A, Kiechl S, et al. Vascular endothelial growth factor in CSF: a biological marker for carcinomatous meningitis. Neurology. 2000;54:1670–6. https://doi.org/10.1212/WNL.54.8.1670.
Sampath P, Weaver CE, Sungarian A, Cortez S, Alderson L, Stopa EG. Cerebrospinal fluid (vascular endothelial growth factor) and serologic (recoverin) tumor markers for malignant glioma. Cancer Control. 2004;11:174–80. https://doi.org/10.1177/107327480401100305.
König C, Grensemann J, Czorlich P, Schlemm E, Kluge S, Wicha SG. A dosing nomograph for cerebrospinal fluid penetration of meropenem applied by continuous infusion in patients with nosocomial ventriculitis. Clin Microbiol Infect. 2022;28:1022.e9-1022.e16. https://doi.org/10.1016/j.cmi.2022.02.017.
Acknowledgements
The study was supported by Forschungsgesellschaft für cerebrale Tumore.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open access funding provided by Medical University of Vienna. This study was merely supported by internal funding. AP received administrative support from Forschungsgesellschaft für cerebrale Tumore.
Conflict of Interests
Iris K. Minichmayr, Ursula Knaack, Johannes Gojo, Daniel Senfter, Christine Haberler, Amedeo A. Azizi, Lisa Mayr, Markus Zeitlinger, and Andreas Peyrl declare that they have no conflicts of interest that might be relevant to the contents of this article.
Availability of Data
Data obtained in the study are presented in the article. The datasets generated during and/or analyzed during the current study are available from the study center on reasonable request.
Ethics Approval
The study was approved by the Ethics Committee of the Medical University of Vienna, carried out in accordance with the Declaration of Helsinki, and registered in the European Clinical Trials Database (EudraCT number 2009-013024-23).
Consent to Participate
Written informed consent was obtained from the parents and/or the participants themselves.
Consent for Publication
Not applicable.
Code Availability
The NONMEM code was based on a previously published population pharmacokinetic model. The control stream used for the current analysis is available on reasonable request.
Author Contributions
IKM: Conceptualization; analysis and interpretation of data; methodology; visualization of results; writing the original draft; writing—review and editing. UK: Conceptualization; data acquisition; formal analysis; writing—review and editing. JG: Conceptualization; data curation; formal analysis; methodology; supervision; writing the original draft; writing—review and editing. DS: Data curation; formal analysis; writing—review and editing. CH: Data curation; formal analysis; writing—review and editing. AA: Conceptualization; data curation; investigation; writing—review and editing. LM: Data curation; investigation; writing—review and editing. MZ: Supervision; interpretation of results; writing—review and editing. AP: Conceptualization; data curation; investigation; project administration; supervision; validation; writing the original draft; writing—review and editing. All authors critically reviewed and approved the final version of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Minichmayr, I.K., Knaack, U., Gojo, J. et al. Distribution of Bevacizumab into the Cerebrospinal Fluid of Children and Adolescents with Recurrent Brain Tumors. Pediatr Drugs 26, 429–440 (2024). https://doi.org/10.1007/s40272-024-00624-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-024-00624-y