Abstract
Aim
We aimed to evaluate the efficacy of intravenous administration of recombinant activated factor VIIa (rFVIIa) for acute pulmonary hemorrhage treatment in very-low-birth-weight (VLBW) premature infants.
Patients and Methods
This study was carried out retrospectively in premature infants with pulmonary hemorrhage that were ≤30 weeks gestational age or <1250 g birth weight. The data of all VLBW premature infants with pulmonary hemorrhage who were hospitalized in our neonatal intensive care unit between 01 January 2013 and 31 December 2015 were evaluated. Group 1 (n = 21) received rFVIIa support within the first 30 min of pulmonary hemorrhage plus conventional treatment, while Group 2 (n = 21) received conventional treatment only.
Results
The number of patients whose pulmonary hemorrhage was stopped within the first 2 h was significantly higher in Group 1 than Group 2 (n = 14 vs n = 4; p = 0.002). After pulmonary hemorrhage, hemoglobin values of Group 1 were higher than Group 2 (11.12 ± 1.06 vs 10.14 ± 1.59 g/dL; p = 0.024). Erythrocyte suspension (1.43 ± 4.51 vs 5.71 ± 7.46 mL/kg; p = 0.030) and fresh frozen plasma use (5.71 ± 8.10 vs 19.52 ± 12.44 mL/kg; p < 0.001) in Group 1 were lower than those of Group 2. Prothrombin time, activated partial thromboplastin time, and international normalized ratio values in Group 1 were lower than those of Group 2 (p < 0.05). No statistically significant difference was identified in recurrence of pulmonary hemorrhage after 72 h, overall mortality, mortality from pulmonary hemorrhage, surfactant use, intubation time, hospitalization duration, intraventricular hemorrhage (IVH), severe IVH, patent ductus arteriosus rates, or short-term complication rates.
Conclusion
rFVIIa administration was observed to be effective in stopping pulmonary hemorrhage, reducing blood product requirement, and improving coagulation test parameters. Prospective studies are needed to evaluate the efficacy, reliability, and long-term results of rFVIIa in the prevention and treatment of pulmonary hemorrhage in premature infants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In this retrospective study, very-low-birth-weight (VLBW) premature infants with pulmonary hemorrhage were treated with recombinant activated factor VIIa (rFVIIa). |
rFVIIa administration was effective in stopping pulmonary hemorrhage and reducing the need for blood products, although it did not decrease intraventricular hemorrhage rate and mortality. |
rFVIIa could be used safely without increasing short-term complications in VLBW premature infants with pulmonary hemorrhage. |
1 Introduction
Severe pulmonary hemorrhage is a life-threatening acute event appearing in 10% of premature infants [1–3]. It is characterized by acute deterioration in pulmonary function, bleeding from the lungs as evidenced by suctioning or aspiration of fresh blood, cardiovascular instability, and appearance of new pulmonary shadows on chest radiographs [4, 5]. Injuries of the premature lung due to the treatment of respiratory distress syndrome, mechanical ventilation, or an impairment in pulmonary circulation can cause pulmonary hemorrhage [2, 6, 7]. Mortality rates are high in severe pulmonary hemorrhage and can be as high as 50% in premature infants [2, 3, 6]. Conventional treatment of pulmonary hemorrhage includes high positive end-expiration pressure with mechanical ventilation, transfusion with blood products, and the administration of surfactant, to restore impaired oxygenation [1, 2, 4].
Through its interaction with tissue factor, activated factor VIIa acts locally in injured endothelial areas. The complex causes thrombin formation and fibrin clotting by activating platelets [8, 9]. Recombinant activated factor VIIa (rFVIIa) (rFVIIa; NovoSeven® Novo Nordisk, Princeton, NJ, USA) is approved for use in severe, life-threatening hemorrhages and perioperative prevention in patients wıth inherited or acquired hemophilia with inhibitors to coagulation factors VIII and IX and inherited factor VII deficiency [2, 10].
At birth, activities of the vitamin K-dependent clotting factors (II, VII, IX, and X) and the concentrations of the contact factors XI and XII are reduced to about 50% of normal adult values [11]. rFVIIa is used off-label to stop life-threatening bleeding unresponsive to conventional treatment with cryoprecipitate and fresh frozen plasma (FFP) in adults, children, and newborns [9].
Evidence for rFVIIa use in newborns, particularly in premature infants, is limited to few case reports and uncontrolled prospective patient studies [2, 4, 9, 10, 12–21]. To get a better understanding of the efficacy and safety of rFVIIa in premature infants with pulmonary hemorrhage, we retrospectively analyzed patient data of very-low-birth-weight (VLBW) infants with life-threatening acute pulmonary hemorrhage that either were treated with rFVIIa in addition to conventional treatment or received conventional treatment alone.
2 Patients and Methods
This retrospective study was carried out in a level III neonatal intensive care unit (NICU) (Izmir, Turkey) and approved by the local Institutional Review Board. The investigators complied with the ethical principles stated in the Helsinki Declaration of the World Health Organization in all stages of the study. This study was carried out retrospectively in premature infants that were ≤30 weeks gestational age or <1250 g birth weight (n = 56) with pulmonary hemorrhage. The data of all VLBW premature infants with pulmonary hemorrhage who were hospitalized between 1 January 2013 and 31 December 2015 were evaluated. In our NICU, conventional treatment of pulmonary hemorrhage includes high positive end-expiration pressure with mechanical ventilation, transfusion with blood products, and the administration of surfactant, to restore impaired oxygenation.
Life-threatening acute pulmonary hemorrhage was diagnosed as the presence of the following criteria: (1) acute deterioration in pulmonary function; (2) bleeding from the lungs as evidenced by suctioning or aspiration of fresh blood; (3) cardiovascular instability; (4) appearance of new pulmonary shadows on chest radiographs [4, 5].
Inclusion criteria were gestational age ≤30 weeks or <1250 g birth weight, the diagnosis of life-threatening acute pulmonary hemorrhage, and for the group with rFVIIa treatment administration, rFVIIa must have been administered within the first 30 min of pulmonary hemorrhage. Exclusion criteria were gestational age >30 weeks or ≥1250 g birth weight, gestational age <23 weeks or <500 g birth weight, rFVIIa use after first 30 min of pulmonary hemorrhage, those hospitalized after the first postnatal 24 h, and newborns with major congenital anomaly, metabolical disease, or congenital infection related to the TORCH complex (toxoplasmosis, other, rubella, cytomegalovirus, herpes simplex virus).
A total of 42 premature infants met the inclusion criteria and were evaluated retrospectively. All premature infants were diagnosed with life-threatening acute pulmonary hemorrhage on the basis of radiologic and clinical findings. Group 1 (n = 21) had received rFVIIa support within the first 30 min of pulmonary hemorrhage plus conventional treatment, while Group 2 (n = 21) had received conventional treatment only. Importantly, these patients (Group 2) were seen in the time before rFVIIa was available for our hospital. In Group 1, a single dose of 50 μg/kg rFVIIa had been administered intravenously within the first 30 min of pulmonary hemorrhage and prior to FFP support. Gestational age and birth weight were matched in both groups. Data such as gestational age, birth weight, gender, delivery mode, use of antenatal corticosteroids, risk factor in mother, transportation from external center, presence of patent ductus arteriosus (PDA) requiring treatment, duration of intubation, duration of hospitalization, dose of surfactant, and blood product use within the first 72 h were noted. Chorioamnionitis, early membrane rupture, urinary tract infection, preeclampsia and eclampsia were regarded as risk factors in the mother.
Adverse events such as intraventricular hemorrhage (IVH), retinopathy of prematurity (stage 2, defined as per international classification [22], ROP), bronchopulmonary dysplasia (oxygen dependency at 36 weeks postmenstrual age, BPD), necrotizing enterocolitis (Bell’s staging criteria, stage II or greater [23], NEC), sepsis, thrombosis, and mortality were noted. Sepsis was defined by a positive blood culture. The grade of IVH was defined by using Papile’s classification. Severe IVH included grade III and IV IVH [24].
2.1 Statistical Analysis
SPSS version 16.0 was used for the statistical analysis of data. Normality was tested for by 1-sample Kolmogorov–Smirnov test. To compare independent groups, the non-parametric Mann–Whitney U test was used. Categorical variables were compared using the χ 2 test. Continuous data are presented as mean ± SD. Statistical significance was determined as p < 0.05.
3 Results
A total of 42 premature infants who met the inclusion criteria were evaluated. Birth weight (822.00 ± 173.87 vs 877.14 ± 180.25 g; p = 0.322), gestational age (26.33 ± 1.39 vs 26.43 ± 1.50 weeks; p = 0.832), gender, delivery method, maternal risk factors, antenatal steroid treatment, multiple pregnancy, and transportation rate from external centers were similar in both groups (Table 1). The number of patients whose pulmonary hemorrhage could be stopped within the first 2 h was higher in Group 1 than Group 2 (n = 14 vs n = 4; p = 0.002), and the number of patients whose pulmonary hemorrhage had continued in the first 72 h (n = 7 vs n = 17; p = 0.002) was significantly lower in Group 1. The number of patients whose pulmonary hemorrhage had recurred later than 72 h (n = 2 vs n = 6; p = 0.116), however, was not statistically different between the two groups. There were no statistical differences in overall mortality (n = 9 vs n = 14; p = 0.121) and mortality from pulmonary hemorrhage (n = 5 vs n = 10; p = 0.107) (Table 2). Two patients died from NEC and two patients from sepsis in Group 1; and one patient died from NEC, two patients from sepsis, and one patient from ventilator-associated pneumonia in Group 2.
No statistically significant differences were identified in surfactant use, intubation time, hospitalization time, IVH, severe IVH, and PDA rates. Moreover, short-term complications including sepsis, NEC, BPD, and ROP rates were similar in both groups (Table 2). Neither acute adverse events nor thromboembolic complications were observed.
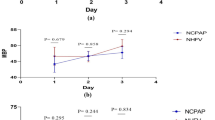
While there were no differences in hemoglobin and platelet values at the begining of hospitalization, hemoglobin value was higher in Group 1 after the treatment of pulmonary hemorrhage (11.12 ± 1.06 vs 10.14 ± 1.59 g/dL; p = 0.024). Furthermore, erythrocyte suspension (1.43 ± 4.51 vs 5.71 ± 7.46 mL/kg; p = 0.030) and FFP (5.71 ± 8.10 vs 19.52 ± 12.44 mL/kg; p < 0.001) use in Group 1 was lower than in Group 2 (Table 3).
Vitamin K was administered to all patients in the delivery rooms. There were no patients whose prothrombin time (PT), activated partial thromboplastin time (aPTT), and international normalized ratio (INR) values had been studied prior to pulmonary hemorrhage. However, 12 patients in Group 1 and 16 in Group 2 had PT, aPTT, and INR values measured following pulmonary hemorrhage. After the treatment of pulmonary hemorrhage, PT (17.51 ± 1.60 vs 19.28 ± 2.23; p = 0.047), aPTT (56.93 ± 11.18 vs 66.36 ± 9.59; p = 0.029), and INR (1.67 ± 0.14 vs 1.84 ± 0.20, p = 0.042) values in Group 1 were statistically lower compared with Group 2.
4 Discussion
In this paper, we present the first clinical study comparing the outcomes in two groups of VLBW preterm infants with life-threatening acute pulmonary hemorrhage that either did or did not receive rFVIIa. Although there have been a few studies presenting off-label use of rFVIIa in refractory bleeding in premature infants, data concerning rFVIIa use in pulmonary hemorrhage in premature infants are restricted to a limited number of case reports [2, 4, 9, 15, 16]. Poralla et al. [2] reported the successful use of rFVIIa to treat pulmonary hemorrhage in three premature infants with VLBW. Two other case reports have described the successful use of rFVIIa, Cetin et al. [15] reported on a single case and Olomu et al. [4] on two infants, using doses of 50–120 µg/kg. In a series involving 18 infants with refractory bleeding, five of these being premature infants with pulmonary hemorrhage (gestational age range 24–32 weeks), rFVIIa was administered as 1–3 doses of 90 μg/kg/dose within the first 72 h, and bleeding was successfully treated in 13 patients [9].
In our own study, pulmonary hemorrhage was effectively treated within 2 h in 14 of 21 (66.6%) premature infants who had been administered a single dose of 50 µg/kg/dose rFVIIa, whereas in the group who had not been administered rFVIIa, this was only the case for four (19%) patients (p = 0.002). In previous studies of rFVIIa administration to neonates with hemorrhage, it was reported that rFVIIa had mostly been administered to neonates resistant to FFP treatment. In our present study, a single dose of 50 µg/kg rFVIIa had been administered within the first 30 min of pulmonary hemorrhage and prior to FFP support. Gkiougki et al. [16] noted that 13 of 14 neonates who survived intractable bleeding or severe coagulation disturbances had received treatment with rFVIIa within 24 h of the beginning of bleeding (93%), whereas only 6 of the 15 neonates who died had received treatment with rFVIIa on the first day of hemorrhage (40%) (p = 0.009).
In two studies carried out in neonates with unresponsive hemorrhage, it was stated that FFP replacement could be reduced after rFVIIa treatment [9, 16]. In our study, less of both FFP and erythrocyte suspension was used in the group that had received rFVIIa. In addition, hemoglobin value assessed after the treatment was higher in the group to whom rFVIIa had been administered (p < 0.05, Table 3). We noticed that rFVIIa administration in VLBW premature infants with pulmonary hemorrhage decreased the number of patients whose pulmonary hemorrhage recurred in the first 72 h of the condition (n = 7 vs n = 17; p = 0.002, Table 2) and thereby the need for blood product use. The use of rFVIIa in pulmonary hemorrhage can be an alternative for large volumes of plasma that may create volume burden, especially in VLBW premature infants (Table 4).
Mortality following pulmonary hemorrhage is high. Gkiougki et al. [16] reported that 8 (53.3%) of 15 premature infants with rFVIIa administration due to pulmonary hemorrhage died. In our study, overall mortality was 54.7% and this value is comparable with the rates in the critical premature patient population with hemorrhage in the literature. Although the mortality rate was lower in the patient group with rFVIIa administration (42.8%; n = 9) compared with that of the patient group with no rFVIIa administration (66.6%; n = 14), no statistical difference was identified (p = 0.121). The mortality due to acute pulmonary hemorrhage tended to be lower in the rFVIIa treatment group (23.8%; n = 5) compared with the group with no administration (47.6%; n = 10; p = 0.107). Because only a single dose of rFVIIa was administered to our patients, it could be speculated that multiple doses of rFVIIa for patients whose pulmonary hemorrhage continued may improve survival rates.
No evident reduction in IVH and severe IVH (p > 0.05) was seen in patients who received rFVIIa (Table 2). Veldman et al. reported the results of a study of rFVIIa for the prophylaxis of IVH including ten extremely premature infants who were administered 100 µg/kg rVIIa every 4 h, for the first 72 h of life. Twenty percent of the premature infants developed grade III or IV IVH [12]. In another study involving three VLBW premature infants, it was reported that rFVIIa treatment was unable to inhibit preexisting IVH progression [2]. There is insufficient data to evaluate the efficacy of rFVIIa in preventing IVH formation and progression.
Moreover, no increase in short-term complications such as sepsis, NEC, BPD, and ROP was seen with the use of rFVIIa. rFVIIa was well tolerated by all preterm infants in our study. In previous studies, rFVIIa was administered to VLBW premature infants at 50–120 µg/kg/dose every 4–24 h [2, 4, 9, 10, 12, 14–16]. We administered a single low dose (50 µg/kg) because of the risk of thromboembolism in rFVIIa administration. We did not observe any signs of thrombosis or embolism. We are of the opinion that randomized controlled studies are needed in this patient group to evaluate the optimal dosing of rFVIIa in VLBW premature infants.
The most important restrictions of our study are its retrospective design and small numbers. Another restriction is the fact that the patients were not randomized to the treatment but rather, once rFVIIa became available, all patients were treated with it. However, our study indicates successful hemostasis and a reduction in the need for blood products with rFVIIa use in VLBW premature infants with acute pulmonary hemorrhage. Since the number of patients whose PT, aPTT, INR, and fibrinogen values had been controlled, particularly before pulmonary hemorrhage was limited, we could not assess how these values changed with rFVIIa treatment and this is another restriction of our study. Although the number of patients whose coagulation parameters had been examined after pulmonary hemorrhage was limited, we found that PT, aPTT, and INR values were lower in the group that had received rFVIIa when coagulation parameters were compared between the two groups (p < 0.05). Gkiougki et al. [16] showed in their study that there was an obvious reduction in PT, aPTT, and INR values with rFVIIa treatment in 29 neonates with hemorrhage. In another study, it was demonstrated that rFVIIa was more efficient than FFP in diminishing PT in ten premature infants with prolonged PT [21].
5 Conclusion
Early after pulmonary hemorrhage in VLBW premature infants, the administration of a single dose of 50 µg/kg rFVIIa was effective in stopping pulmonary hemorrhage, reducing the need for blood products and improving coagulation test parameters. It was seen that rFVIIa administration did not statistically decrease IVH rate or mortality. However, it could be used safely without increasing short-term complications in VLBW premature infants. We are of the opinion that randomized controlled studies are needed in this patient group to evaluate the efficacy, reliability, and long-term results of rFVIIa in preventing and treating severe pulmonary hemorrhage in VLBW premature infants.
References
Raju TN, Langenberg P. Pulmonary hemorrhage and exogenous surfactant therapy: a metaanalysis. J Pediatr. 1993;123:603–10.
Poralla C, Hertfelder HJ, Oldenburg J, et al. Treatment of acute pulmonary haemorrhage in extremely preterm infants with recombinant activated factor VII. Acta Paediatr. 2010;99:298–300.
Pandit PB, O’Brien K, Asztalos E, Colucci E, Dunn MS. Outcome following pulmonary hemorrhage in very low birthweight neonates treated with surfactant. Arch Dis Child Fetal Neonatal Ed. 1999;81:F40–4.
Olomu N, Kulkarni R, Manco-Johnson M. Treatment of severe pulmonary hemorrhage with activated recombinant factor VII (rFVIIa) in very low birth weight infants. J Perinatol. 2002;22:672–4.
Tomaszewska M, Stork E, Minich NM, Friedman H, Berlin S, Hack M. Pulmonary hemorrhage: clinical course and outcomes among very lowbirth-weight infants. Arch Pediatr Adolesc Med. 1999;153:715–21.
Kluckow M, Evans N. Ductal shunting, high pulmonary blood flow, and pulmonary hemorrhage. J Pediatr. 2000;137:68–72.
Trompeter R, Yu VU, Ansley-Green A, Roberton NR. Massive pulmonary hemorrhage in the newborn infant. Arch Dis Child. 1975;50:123–7.
Lindley CM, et al. Pharmacokinetics and pharmacodynamics of recombinant Factor VIIa. Clin Pharmacol Ther. 1994;55(6):638–48.
Dang CN, Katakam LI, Smith PB, et al. Recombinant activated factor VIIa treatment for refractory hemorrhage in infants. J Perinatol. 2011;31:188–92.
Robertson JD. Prevention of intraventricular haemorrhage: a role for recombinant activated factor VII? J Paediatr Child Health. 2006;42:325–31.
Pichler E, Pichler L. The neonatal coagulation system and the vitamin K deficiency bleeding—a mini review. Wien Med Wochenschr. 2008;158(13–14):385–95.
Veldman A, Josef J, Fischer D, Volk WR. A prospective pilot study of prophylactic treatment of preterm neonates with recombinant activated factor VII during the first 72 hours of life. Pediatr Crit Care Med. 2006;7:34–9.
Mitsiakos G, Papaioannou G, Giougi E, Karagianni P, Garipidou V, Nikolaidis N. Is the use of rFVIIa safe and effective in bleeding neonates? J Pediatr Hematol Oncol. 2007;29:145–50.
Brady KM, Easley RB, Tobias JD. Recombinant activated factor VII (rFVIIa) treatment in infants with hemorrhage. Pediatr Anesth. 2006;16:1042–6.
Cetin H, Yalaz M, Akisu M, Karapinar DY, Kavakli K, Kultursay N. The use of recombinant activated factor VII in the treatment of massive pulmonary hemorrhage in a preterm infant. Blood Coagul Fibrinolysis. 2006;17:213–6.
Gkiougki E, Mitsiakos G, Chatziioannidis E, Papadakis E, Nikolaidis N. Predicting response to rFVIIa in neonates with intractable bleeding or severe coagulation disturbances. J Pediatr Hematol Oncol. 2013;35:221–6.
Filan PM, Mills JF, Clarnette TD, et al. Spontaneous liver hemorrhage during laparotomy for necrotizing enterocolitis: a potential role for recombinant factor VIIa. J Pediatr. 2005;147:857–9.
Hunseler C, Kribs A, Eifinger F, et al. Recombinant activated factor seven in acute life-threatening bleeding in neonates: report on three cases and review of literature. J Perinatol. 2006;26:706–13.
Fischer D, Schloesser R, Buxmann H, et al. Recombinant activated factor VII as a hemostatic agent in very low birth weight preterms with gastrointestinal hemorrhage and disseminated intravascular coagulation. J Pediatr Hematol Oncol. 2008;30:337–42.
Faust K, Troger B, Kahl F, et al. Successful use of recombinant factor VIIa in a preterm infant with life-threatening haematuria. Blood Coagul Fibrinolysis. 2009;20:601–4.
Greisen G, Andreasen RB. Recombinant factor VIIa in preterm neonates with prolonged prothrombin time. Blood Coagul Fibrinolysis. 2003;14:117–20.
International Committee for Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity revisited. Arch Ophthalmol. 2005;123:991–9.
Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187:1–7.
Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1500 g. J Pediatr. 1978;92:529–34.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
None.
Conflict of interest
Hese Cosar, Halil Isik, Salih Cagrı Cakır, Nese Yar, Bulent Goksen, Hakan Tokbay, Hasan Kertmen, Nihal Erdoğan, and Ikbal Durak declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Cosar, H., Isik, H., Cakır, S.C. et al. Recombinant Activated Factor VIIa (rFVIIa) Treatment in Very-Low-Birth-Weight (VLBW) Premature Infants with Acute Pulmonary Hemorrhage: A Single-Center, Retrospective Study. Pediatr Drugs 19, 53–58 (2017). https://doi.org/10.1007/s40272-016-0203-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-016-0203-3