Abstract
The emergence of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the resulting coronavirus disease 2019 (COVID-19) pandemic has led to rapid vaccine development and emergency use (EU) rollout. Six vaccines, including two using novel mRNA technology, are EU-listed by the World Health Organisation, and promising published trial data are available for nine more. While efficacy is good, there are various barriers to their global use. Long-term safety and immunogenicity data are being collected along the way.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Many aspects define future-fit SARS-CoV-2 vaccines
Since wild-type severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was identified in China in 2019, the resulting coronavirus disease 2019 (COVID-19) pandemic has prompted the rapid development, emergency use listing or approval (EUL or EUA) and rollout of vaccines [1, 2]. This paper summarises promising COVID-19 vaccines as of 7 September 2021, based on available data, with emphasis on published phase 3 trial results. Data are sourced from peer-reviewed journals, press releases, public health organisations such as the World Health Organisation (WHO), the European Medicines Association (EMA) and the US Communicable Diseases Centre (CDC), review articles (Kyriakidis et al, McDonald et al and Sadarangani et al. [1, 3, 4]) and vaccine tracking websites [2, 5].
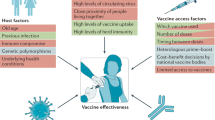
An ideal vaccine provides long-term protection in all populations after one dose, and is safe, affordable and easy to mass manufacture, store and distribute [1]. It must also be accepted; at present, WHO includes vaccine hesitancy (outside of the scope of this article) in its top 10 threats to global health [6]. While scientific opinions initially predicted that it would take at least a year to a year and a half for a COVID-19 vaccine to be approved for use in the USA, advances in the field allowed the issuing of EUAs for various vaccines by national and international drug regulation agencies within a year of the SARS-CoV-2 genome sequence being released [4]. By 7 September 2021, six COVID-19 vaccines had received WHO EULs, one of which (the AstraZeneca/University of Oxford formulation) has two versions (VaxzevriaTM and CovishieldTM, the latter manufactured by the Serum Institute of India) (Tables 1, 2). Nine more vaccines (Table 3) had published acceptable or excellent phase 3 efficacy results and ≥ 20 others had reached phase 3 trials (Table 5) [2, 5]. However, among the plethora of potential efficacious options, few meet the various ideal-vaccine [1] criteria.
Varied technology, varied pros and cons
Some vaccine technologies are well established [e.g. inactivated or live-attenuated whole virus, or viral subunit (protein or virus-like particles)], but COVID-19 vaccines have employed technologies such as novel modified viral mRNA or DNA approaches [4] and adenovirus (AD) vectors, generally with good efficacy (Tables 1, 3) [4, 7]. Immunology (Table 4) is not yet clear, but predictable issues with various technologies include:
-
inactivated, non-replicating virus and protein subunit vaccines (e.g. Sinovac’s CoronaVac and Sinopharm’s BBIBP-CorV) usually need booster shots and/or adjuvants as they typically prompt either no, or a weak, short-lived cellular immune response [4, 7];
-
potential reversion to virulent or wild-type strains mean all whole virus vaccines need regular testing [7];
-
mRNA is very unstable, necessitating freezer storage of the Moderna mRNA-1273 and Pfizer BNT162b2 vaccines (Table 1), and booster doses are probably needed [2, 7];
-
DNA vaccines could potentially integrate into the human genome, and probably need booster shots [7];
-
the efficacy and tolerability of AD vector vaccines can be affected by recipients’ previous exposure and antibodies (Abs) to common ADs, although this risk has been mitigated by the use of chimp AD (e.g. AstraZeneca’s AZD1222; Table 1), rare human AD26 (Janssen’s Ad26.COV2.S, [4]; Table 1) and different AD vectors in doses one and two (Gamaleya’s Sputnik vaccines; Table 3); and
-
novel technologies may cost more and increase vaccine hesitancy [6].
Although proven, eventually cheap to make and conferring long-lasting immunity, live-attenuated viruses (used, e.g., to prevent measles) seem unsuited to the COVID-19 pandemic, as they may take years to develop, do not suit fast-changing viruses and may be affected by coronavirus cross-immunity [4].
WHO approval linked to COVAX, others are needed
Equitable distribution of vaccines is proving difficult and the COVID-19 Vaccines Global Access (COVAX) initiative, co-led by WHO, aims to provide doses to lower-income countries [8]. However, COVAX may only distribute vaccines with WHO EULs (Table 1) [8] and the six that qualify so far are not logistically ideal. All are IM formulations requiring frozen or refrigerated storage, most are expensive and all but one require two initial doses (Table 1) [4, 8].
Good efficacy, especially for severe COVID-19
Several WHO EUL vaccines demonstrate ≥ 75% efficacy against symptomatic COVID-19 infection, and all showed excellent efficacy against severe illness (Table 2) [14]. Longer-term safety data now reveal anaphylaxis and very rare, but serious, vaccine adverse events (e.g. myocarditis, predominantly affecting men aged < 30 years, with the mRNA vaccines; thrombosis with thrombocytopenia syndrome, most common in women aged < 55 years and 30–49 years with the ChAdOx1 and Ad26.COV2.S vaccines, respectively [16, 17]) [Table 2], leading to some rollout reviews (Table 1). More positively, after the administration of well over 180 and 133 million doses of the BNT162b2 and mRNA-1273 vaccines, BNT162b2 has now received full US FDA approval [18] and Moderna has filed for full approval for mRNA-1273 [19].
Pregnant women and children last…
Only the BNT162b2 vaccine is WHO-listed for adolescents (Table 2), but increased COVID-19 knowledge confirms children and adolescents, as well as pregnant women, need safe vaccines. Several developments are in progress:
-
Moderna is seeking FDA approval for people aged ≥ 12 years, as its TeenCOVE phase 2/3 study in 3732 adolescents met its primary endpoint of non-inferior immunogenicity versus that in adult comparators (vaccine efficacy 100%) [29];
-
Pfizer, Moderna and China National Biotec Group with Beijing Institute of Biological Products (CNBG/BIBP) have all registered Phase 2/3/4 trials in children aged 6 months to 12 years (Moderna; NCT04796896/KidCOVE, and Pfizer; NCT04816643), those aged 3 to 17 years (CNBG/BIBP; NCT04863638, NCT04917523) or adolescents (Pfizer dose-boost study; NCT04368728) [30];
-
Phase 2/3 studies in younger people have been registered for the non-WHO EUL vaccines (Table 3) Covaxin (ages 2–18 years), Sputnik V and Novavax NVX-CoV2373 (both ages 12–17 years) [30]; and
-
Pfizer is conducting a phase 2/3 study of the BNT162b2 vaccine in pregnant women (NCT04754594) [30].
While preliminary US surveillance system and registry data have not revealed any obvious safety signals among pregnant women administered mRNA COVID-19 vaccines, more extensive long-term data are needed [31].
… along with immunocompromised patients
Immunocompromised patients, including those with autoimmune disorders or on immunosuppressive medications, have typically been excluded from vaccine trials and require particular attention, given that infections are a common cause of mortality in this group [32]. Although further research is warranted to determine the effects of immunocompromising medical conditions and immunosuppressing medications on COVID-19 vaccine efficacy, the benefits of vaccination are expected to outweigh any possible risks [33]. Additional doses may be required to achieve adequate protection; in August 2021, the US FDA approved an update to the EUAs for BNT162b2 and mRNA-1273 to include a third dose for certain immunocompromised patients [34].
Other promising vaccines yet to be WHO-listed
Table 3 shows currently COVAX-ineligible vaccines with reported phase 3 trial efficacy of 62–93%, plus other benefits [2, 35]. Two are stable for weeks at room temperature (Table 3), and two developed in India (one needle-free) appear effective against the delta strain [36]; the phase 3 trial of the needle-free ZyCoV-D vaccine also included adolescents [36]. Most of these vaccines are already in use (Table 3) [2, 5].
Understanding of immunogenicity just beginning…
Understanding the immunological mechanisms of current vaccines, the related correlates of protection (COPs) and the durability of immunity is essential to optimise the efficacy and practicality of COVID-19 vaccines and limit the development of viral “escape mutants” [1, 3]. At present, immunological data are short-term and very limited, trial assays vary and immunogenicity is not well understood [3, 40]. Questions around the need for booster doses, the ideal dose interval, and mucosal immunity and responses are largely unanswered [3].
… but humoral and cell-mediated immunity involved
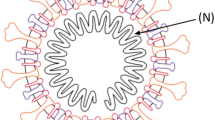
The genome of SARS-CoV-2 encodes the spike (S) protein (among others), which includes the S1 subunit containing the receptor-binding domain (RBD) and the S2 subunit that mediates membrane fusion and cell entry [7]. SARS-CoV-2 uses the RBD to engage with the host cells’ receptor angiotensin-converting enzyme 2 (ACE-2). The S protein can trigger both humoral and cell-mediated (i.e. neutralising Abs and T- and B-cell) immune responses [7]; both types appear to mediate recovery from COVID-19 infection (Table 4 [3]).
Most vaccines are designed to generate neutralising Abs (NAbs) against S proteins (Table 4), with several studies identifying a strong correlation between vaccine efficacy and mean NAb, even at very low NAb levels [13]. For example, the vaccine-generated NAb levels for 50% and full protection against detectable COVID-19 were 20.2% and 28.6% of the mean convalescent level and 50% protection against severe COVID-19 occurred at 3.0% [13]. After two doses, both mRNA and AD-vectored vaccines elicit NAb levels equivalent to, or higher than, those of patients who are in convalescence (with NAb levels relative to those in convalescent plasma being somewhat greater with mRNA vaccines than with AD-vectored vaccines) [3].
However, other evidence and experience with other coronavirus infections, e.g. SARS-CoV-1 and Middle East respiratory syndrome coronavirus (MERS-CoV), strongly suggest that NAbs alone are unlikely to provide such significant immunity [3, 40]. Cellular immunity, non-neutralising Abs and innate mechanisms, e.g. type I and II interferons, are all likely to be involved [3, 40].
The many functions of cytotoxic T-cells include recognising and killing infected cells, releasing cytokines and supporting the antibody response of B-cells [3, 40]. Clinical evidence for their involvement in COVID-19 immunity includes milder or asymptomatic infection in people with a strong T-cell response [3, 40] and the presence of T-cells in people with undetectable SARS-CoV-2 Abs [40]. More T-cell data are needed [40]. The NAb titre also correlates with anti-RBD immunoglobulin (Ig)G levels [7] and Ab activity in this region is also of interest (Table 4 [3]).
Very virulent variants may still respond
Vaccines were initially developed for protection against COVID-19 strains identified in Wuhan, China, but SARS-CoV-2’s fast mutation rate means efficacy against more transmissible variants of concern (VOCs) and perhaps additional variations of interest is required [41]. VOCs are:
-
alpha (⍺, or B.1.1.7 +/- E484K), which spreads faster and may cause more severe illness;
-
beta (β or B.1.351);
-
gamma (ɣ or P.1), which spreads faster; and
-
delta (δ or B.1.617.2), which spreads much faster and may cause more severe illness, now present in almost 100 countries.
An Indian study (preprint) found the δ variant dominated in breakthrough symptomatic COVID-19 in vaccinated healthcare workers [42]. Relative to wild-type virus, it showed an 8-fold reduction in sensitivity to vaccine-generated Abs. ChAdOx1 recipients had significantly lower serum neutralizing titers against the δ variant than BNT162b2 recipients [42]. However, severe COVID-19 in fully vaccinated people was rare [42]. The β, ɣ and δ variants seem to reduce convalescent immunity [30].
Other analyses [43,44,45] suggest VOCs are still susceptible to several vaccines [35, 43,44,45]. Post-hoc analyses showed the Novavax NVX-CoV2373 vaccine was 86.3% effective against the ⍺ variant and 96.4% effective against other variants [35]. A Canadian study (preprint) in > 400,000 people found BNT162b2, mRNA-1273 and ChAdOx1 vaccines provided good protection against VOCs, especially after two doses [45]. Against all VOCs, one dose of mRNA-1273 vaccine provided 72–83% protection, versus 56–66% with BNT162b2 and 48–67% with ChAdOx1. Efficacy in preventing COVID-19 increased to 84–92% with two doses of BNT162b2 or mRNA-1273; there were insufficient data for ChAdOx1 [45].
The pattern of results was similar, albeit with slightly better efficacy, in two earlier Qatar studies of BNT162b2 [43] and mRNA-1273 [44] against ⍺ and β strains. The efficacy of BNT162b2 against the β variant was about 20% lower than that reported against other strains and both vaccines provided > 90% protection against severe COVID-19 [44, 46].
Vaccines in development may be more accessible
There are many COVID-19 vaccines in early-stage trials and Table 5 shows those registered at phase 3 level at 7 September [2, 5, 30]. These studies, if successful, may overcome some of the cost, VOC, logistical and other problems that will otherwise limit global access to effective COVID-19 vaccines [8]. Several companies have registered comparative trials, with placebo-controlled trials becoming less feasible as effective vaccines become more widely available, and some are targeting VOCs (Table 5). Among other areas of investigation are differing vaccines for doses one and two (which may be a reasonable and feasible strategy, although further research is needed [47]), and needle-free vaccines [2].
Take home messages
-
The global spread of SARS-CoV-2 and resultant COVID-19 pandemic has spawned the rapid development of effective vaccines.
-
The six vaccines with WHO EULs are effective, especially against severe COVID-19, but barriers to their global use, such as cost, formulation and storage, mean EULs for other vaccines with good, published phase 3 trial results are urgently needed.
-
SARS-CoV-2 mutates rapidly and vaccines must be effective against several highly transmissible VOCs; current indications are that vaccines still prevent severe COVID-19 when VOCs are prevalent.
-
All understanding of COVID-19 and vaccines, especially safety and immunogenicity, is short-term and incomplete, limiting the scope for vaccine optimisation.
References
McDonald I, Murray SM, Reynolds CJ, et al. Comparative systematic review and meta-analysis of reactogenicity, immunogenicity and efficacy of vaccines against SARS-CoV-2. npj Vaccines. 2021;6(1):74.
Carl Zimmer Jonathan Corum and Sui-Lee Wee for The New York Times. Coronavirus Vaccine Tracker. 2021. https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html. Accessed 7 Sep 2021.
Sadarangani M, Marchant A, Kollmann TR. Immunological mechanisms of vaccine- induced protection against COVID-19 in humans. Nat Rev Immunol. 2021. https://doi.org/10.1038/s41577-021-00578-z.
Kyriakidis NC, Lopez-Cortes A, Gonzalez EV, et al. SARS-CoV-2 vaccines strategies: a comprehensive review of phase 3 candidates. npj Vaccines. 2021. https://doi.org/10.1038/s41541-021-00292-w.
McGill University. Covid19 vaccine tracker. 2021. https://covid19.trackvaccines.org/. Accessed 7 Sep 2021.
World Health Organization. WHO top threats to global health—special article collection. 2021. https://www.annualreviews.org/. Accessed 7 Sep 2021.
Blasi F, Gramegna A, Sotgiu G, et al. SARS-CoV-2 vaccines: a critical perspective through efficacy data and barriers to herd immunity. Respir Med. 2021;180:106355.
Gavi The Vaccine Alliance. COVAX. 2021. https://www.gavi.org/covax-facility. Accessed 7 Sep 2021.
Dyer O. Covid-19: countries are learning what others paid for vaccines. BMJ. 2021. https://doi.org/10.1136/bmj.n281.
Duke Global Health Innovation Center. Launch & scale speedometer: vaccine manufacturing. 2021. https://launchandscalefaster.org/covid-19/vaccinemanufacturing. Accessed 7 Sep 2021.
European Medicines Agency. Comirnaty concentrate for dispersion for injection: summary of product characteristics. 2021. https://ec.europa.eu/. Accessed 7 Sep 2021.
Janssen Biotech. FDA fact sheet for healthcare providers administering vaccine (vaccination providers): Emergency Use Authorization (EUA) of the Janssen Covid-19 vaccine to prevent coronavirus disease 2019 (Covid-19). 2021. https://www.fda.gov/. Accessed 7 Sep 2021.
Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–11.
Strategic Advisory Group of Experts on Immunization (SAGE) fWHO. Covid-19 vaccines technical documents. 2021. https://www.who.int/. Accessed 7 Sep 2021.
P4H Social Health Protection Network. Thailand to procure an array of COVID-19 vaccines from multiple global suppliers to meet demand [media release]. 29 Apr 2021. https://p4h.world/
Rosenblum HG, Hadler SC, Moulia D, et al. Use of COVID-19 vaccines after reports of adverse events among adult recipients of Janssen (Johnson & Johnson) and mRNA COVID-19 vaccines (Pfizer-BioNTech and Moderna): update from the Advisory Committee on Immunization Practices — United States, July 2021. MMWR Morb Mortal Wkly Rep. 2021;70(32):1094–9.
Lai C-C, Ko W-C, Chen C-J, et al. COVID-19 vaccines and thrombosis with thrombocytopenia syndrome. Expert Rev Vaccines. 2021;20(8):1027–35.
U.S. Food & Drug Administration. FDA approves first COVID-19 vaccine [media release]. 23 Aug 2021. https://www.fda.gov/
Moderna. Moderna announces initiation of rolling submission of Biologics License Application (BLA) with U.S. FDA for the Moderna COVID-19 vaccine [media release]. 1 Jun 2021. https://investors.modernatx.com/
Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–16.
U.S. Food & Drug Administration. EUA fact sheet for providers. 2021. https://www.modernatx.com/. Accessed 7 Sep 2021.
Pfizer-BioNTEch. Pfizer-BioNTech Covid-19 vaccine—BNT162b2 injection, suspension: fact sheet for healthcare providers administering vaccine. 2021. https://dailymed.nlm.nih.gov/. Accessed 7 Sep 2021.
Moderna. FDA Emergency Use Authorization (EUA) of the Moderna Covid-19 vaccine to prevent coronavirus disease 2019 (COVID-19) in individuals 18 years of age and older [media release]. 2020. https://www.fda.gov/.
Pfizer-BioNTech. Pfizer-BioNTech Covid-19 vaccine (BNT162b2): FDA Emergency Use Authorization (EUA) for an unapproved product review memorandum. 2021. https://www.fda.gov/media/144416/download. Accessed 7 Sep 2021.
European Medicines Agency. COVID-19 vaccine AstraZeneca: EMA product information. 2021. https://www.ema.europa.eu/. Accessed 7 Sep 2021.
European Medicines Agency. COVID-19 vaccine Janssen suspension for injection (Ad26.COV2-S [recombinant]): summary of product characteristics. 2021. https://www.ema.europa.eu/. Accessed 7 Sep 2021.
Janssen Biotech. Janssen COVID-19 vaccine: Emergency Use Authorization (EUA) FDA review memorandum. 2021. https://www.fda.gov/. Accessed 7 Sep 2021.
Tanriover MD, Doganay HL, Akova M, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398(10296):213–22.
Moderna. Moderna announces TeenCOVE study of its COVID-19 vaccine in adolescents meets primary endpoint and plans to submit data to regulators in early June [media release]. 25 May 2021. https://investors.modernatx.com/
U.S. National Library of Medicine. Clinical Trials. 2021. https://clinicaltrials.gov/ct2/results?cond=COVID-19. Accessed 7 Sep 2021.
Shimabukuro T, Kim SY, Myers TR, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384:2273–82.
Sonani B, Aslam F, Goyal A, et al. COVID-19 vaccination in immunocompromised patients. Clin Rheumatol. 2021;40:797–8.
Duly K, Farraye FA, Bhat S. COVID-19 vaccine use in immunocompromised patients: a commentary on evidence and recommendations. Am J Health Syst Pharm. 2021. https://doi.org/10.1093/ajhp/zxab344.
U.S. Food & Drug Administration. Coronavirus (COVID-19) update: FDA authorizes additional vaccine dose for certain immunocompromised individuals [media release]. 12 Aug 2021. https://www.fda.gov/
Heath PT, Galiza EP, Baxter DN, et al. Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med. 2021. https://doi.org/10.1056/NEJMoa2107659
Zydus Cadila. Zydus applies to the DCGI for EUA to launch ZyCov-D, the world’s first plasmid DNA vaccine for COVID-19 [media release]. 1 Jul 2021. https://www.zyduscadila.com/
Bucci E, Andreev K, Bjorkman A, et al. Safety and efficacy of the Russian COVID-19 vaccine: more information needed. The Lancet. 2020. https://doi.org/10.1016/S0140-6736(20)31960-7.
Gorry C. SOBERANA, Cuba’s COVID-19 vaccine candidates: Dagmar Garcia-Rivera PhD. MEDICC Rev. 2020;22(4):10–5.
Al KN. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial. JAMA. 2021. https://doi.org/10.1001/jama.2021.8565.
Sewell HF, Agius RM, Stewart M, et al. Cellular immune responses to covid-19. BMJ. 2020;370:m3018.
U.S. Centers for Disease Control and Prevention. Covid-19. 2021. https://www.cdc.gov/. Accessed 7 Sep 2021.
Mlcochova P, Kemp SA, Shanka Dhar M, et al. SARS-CoV-2 B.1.617.2 delta variant emergence and breakthrough. Res Square. 2021. https://doi.org/10.21203/rs.3.rs-637724/v1.
Abu-Raddad LJ, Chemaitelly H, Butt AA, et al. Effectiveness of the BNT162b2 Covid-19 vaccine against the B.1.1.7 and B.1.351 variants. N Engl J Med. 2021;385(2):187–9.
Chemaitelly H, Yassine HM, Benslimane FM, et al. mRNA-1273 COVID-19 vaccine effectiveness against the B.1.1.7 and B.1.351 variants and severe COVID-19 disease in Qatar. Nat Med. 2021. https://doi.org/10.1038/s41591-021-01446-y.
Nasreen S, He S, Chung H, et al. Effectiveness of COVID-19 vaccines against variants of concern. medRxiv. 2021. https://doi.org/10.1101/2021.06.28.21259420.
Abu-Raddad LJ, Chemaitelly H, Butt AA, et al. Effectiveness of the BNT162b2 Covid-19 Vaccine against the B.1.1.7 and B.1.351 Variants. N Engl J Med. 2021;05:05.
Chiu N-C, Chi H, Tu Y-K, et al. To mix or not to mix? A rapid systematic review of heterologous prime–boost covid-19 vaccination. Expert Rev Vaccines. 2021. https://doi.org/10.1080/14760584.2021.1971522.
Acknowledgements
The manuscript was reviewed by: A. Al Hamid, Ministry of Health, Saudi Arabia; S. A. Antoniu, Department of Medicine II, Grigore T. Popa University of Medicine and Pharmacy, Iaşi, Romania; F. J. Araujo, Department of Pharmacy, Virgen del Rocio Hospital, Seville, Spain; M. Morgado, CICS-UBI Health Sciences Research Centre, University of Beira Interior, Covilhã, Portugal. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding. C Fenton, a contracted employee of Adis International Ltd/Springer Nature, declares no relevant conflicts of interest. Y Lamb, a salaried employee of Adis International Ltd/Springer Nature, declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent for publication, Availability of data and material, Code availability
Not applicable.
Rights and permissions
About this article
Cite this article
Fenton, C., Lamb, Y.N. COVID-19: State of the Vaccination. Drugs Ther Perspect 37, 508–518 (2021). https://doi.org/10.1007/s40267-021-00869-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-021-00869-4