Abstract
A number of pharmacological options for the treatment of obesity are available, but their efficacy is limited to losses of 5–10% of patient weight and their use should be secondary to lifestyle changes. The safety and efficacy of anti-obesity drugs, as well as the characteristics and co-morbidities of the patient, should be taken into consideration when treating obesity, as not all treatments are appropriate for all patients. A variety of new anti-obesity drugs are currently undergoing clinical investigation, but none appears to be the ideal agent to treat obesity.

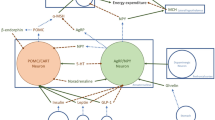
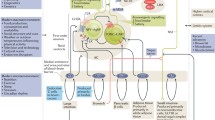
Adapted from Rosa-Gonçalves and Majerowicz [2]
Similar content being viewed by others
References
Christensen RM, Juhl CR, Torekov SS. Benefit-risk assessment of obesity drugs: focus on glucagon-like peptide-1 receptor agonists. Drug Saf. 2019;42(8):957–71.
Rosa-Gonçalves P, Majerowicz D. Pharmacotherapy of obesity: limits and perspectives. Am J Cardiovasc Drugs. 2019;19(4):349–64.
Kelly T, Yang W, Chen CS, et al. Global burden of obesity in 2005 and projections to 2030. Int J Obes. 2008;32(9):1431–7.
Lee PC, Dixon J. Pharmacotherapy for obesity. Aust Fam Physician. 2017;46(7):472–7.
Igel LI, Kumar RB, Saunders KH, et al. Practical use of pharmacotherapy for obesity. Gastroenterology. 2017;152(7):1765–79.
Zhang F, Tong Y, Su N, et al. Weight loss effect of glucagon-like peptide-1 mimetics on obese/overweight adults without diabetes: a systematic review and meta-analysis of randomized controlled trials. J Diabetes. 2015;7(3):329–39.
Siebenhofer A, Jeitler K, Horvath K, et al. Long-term effects of weight-reducing drugs in people with hypertension. Cochrane Database System Rev. 2016;(3):CD007654.
Halseth A, Shan K, Walsh B, et al. Method-of-use study of naltrexone sustained release (SR)/bupropion SR on body weight in individuals with obesity. Obesity. 2017;25(2):338–45.
Wadden TA, Foreyt JP, Foster GD, et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity. 2010;19(1):110–20.
Acosta A, Camilleri M, Shin A, et al. Quantitative gastrointestinal and psychological traits associated with obesity and response to weight-loss therapy. Gastroenterology. 2015;148(3):537–546.e4.
Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377(9774):1341–52.
Chan EW, He Y, Chui CS, et al. Efficacy and safety of lorcaserin in obese adults: a meta-analysis of 1-year randomized controlled trials (RCTs) and narrative review on short-term RCTs. Obes Rev. 2013;14(5):383–92.
Lucchetta RC, Riveros BS, Pontarolo R, et al. Systematic review and meta-analysis of the efficacy and safety of amfepramone and mazindol as a monotherapy for the treatment of obese or overweight patients. Clinics (Sao Paulo). 2017;72(5):317–24.
Suplicy H, Boguszewski CL, dos Santos CM, et al. A comparative study of five centrally acting drugs on the pharmacological treatment of obesity. Int J Obes. 2014;38(8):1097–103.
Astrup A, Meier DH, Mikkelsen BO, et al. Weight loss produced by tesofensine in patients with Parkinson’s or Alzheimer’s disease. Obesity. 2008;16(6):1363–9.
O’Neil PM, Birkenfeld AL, McGowan B, et al. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet. 2018;392(10148):637–49.
Ziauddeen H, Chamberlain SR, Nathan PJ, et al. Effects of the mu-opioid receptor antagonist GSK1521498 on hedonic and consummatory eating behaviour: a proof of mechanism study in binge-eating obese subjects. Mol Psychiatry. 2013;18(12):1287–93.
Allas S, Delale T, Ngo N, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics of AZP-531, a first-in-class analogue of unacylated ghrelin, in healthy and overweight/obese subjects and subjects with type 2 diabetes. Diabetes Obes Metab. 2016;18(9):868–74.
Kopelman P, Groot Gde H, Rissanen A, et al. Weight loss, HbA1c reduction, and tolerability of cetilistat in a randomized, placebo-controlled phase 2 trial in obese diabetics: comparison with orlistat (Xenical). Obesity. 2010;18(1):108–15.
Johnston R, Uthman O, Cummins E, et al. Canagliflozin, dapagliflozin and empagliflozin monotherapy for treating type 2 diabetes: systematic review and economic evaluation. Health Technol Assess. 2017;21(2):1–218.
Author information
Authors and Affiliations
Consortia
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
The article was adapted from Drug Safety 2019;42(8):957–71 [1] and the American Journal of Cardiovascular Drugs 2019;19(4):349–64 [2] by employees of Adis International Ltd./Springer Nature, who are responsible for the article content and declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Adis Medical Writers. Current and investigational anti-obesity drugs help reduce weight and offer additional benefits, but more effective options are needed. Drugs Ther Perspect 36, 12–16 (2020). https://doi.org/10.1007/s40267-019-00679-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-019-00679-9