Abstract
Metreleptin [Myalepta® (EU); Myalept® (USA)] is a recombinant analogue of human leptin and currently the only drug available for the specific treatment of lipodystrophy (LD). In the EU, metreleptin (administered once daily via subcutaneous injection) is indicated as replacement therapy to treat the complications of leptin deficiency in patients aged ≥ 2 years with generalized LD and in patients aged ≥ 12 years with partial LD who have failed to achieve adequate metabolic control with standard treatments. Its use in these rare settings is supported by data from open-label clinical studies and clinical practice, with the totality of evidence indicating that metreleptin improves metabolic abnormalities associated with generalized or partial LD, including in paediatric patients. Other potential benefits include improved hepatic parameters/disease, nephropathy and survival, although the impact of the drug on these outcomes would benefit from further analysis. Metreleptin is generally well tolerated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Recombinant analogue of human leptin |
Administered once daily via subcutaneous injection |
Improves metabolic abnormalities of generalized or partial lipodystrophy, including in paediatric patients |
May potentially improve hepatic parameters/disease, nephropathy and survival |
Hypoglycaemia and decreased bodyweight are the most common adverse effects |
What is the rationale for using metreleptin in lipodystrophy?
Lipodystrophies (LDs) are a group of rare, acquired or genetic, medical conditions characterized by adipose (fat) tissue deficiency in the absence of a catabolic state or nutritional deprivation [1, 2]. The deficiency of fat may be throughout the entire body (i.e. generalized LD) or only in specific areas (i.e. partial LD) [1], with abnormal accumulation of fat in unaffected regions often evident [3]. Fat tissue plays a key role in lipid metabolism and glucose homeostasis [4], and its loss in LD interferes with hunger/satiety signals (commonly leading to hyperphagia), resulting in inappropriate lipid storage in muscle, the liver and other organs [1, 2]. Consequently, patients with LD often have extreme insulin resistance, leading to hypertriglyceridaemia, diabetes, non-alcoholic fatty liver disease (NAFLD), non-alcoholic steatohepatitis (NASH), pancreatitis and other metabolic/endocrine abnormalities [1, 5].
Given the lack of curative therapies for LD, the standard-of-care is to manage the metabolic complications of the disorder, with options including lifestyle modification (i.e. diet and exercise) and pharmacotherapy for each specific complication (e.g. statins for hyperlipidaemia; glucose-lowering agents for diabetes) [2, 6]. However, these conventional therapies do not address the underlying causes of LD and often provide insufficient metabolic control [6]. These limitations and the knowledge that serum levels of leptin (an adipose tissue-secreted hormone with effects such as appetite suppression, promotion of hepatic lipolysis and glucose usage in skeletal muscle, and inhibition of insulin secretion [5, 7]) tend to be low in patients with LD [2], prompted investigation of leptin replacement therapy as a potential treatment option [6]. Metreleptin [Myalepta® (EU); Myalept® (USA)] is a recombinant analogue of human leptin [3, 5] and is currently the only drug available for the specific treatment of LD.
For whom is metreleptin indicated?
In the EU, metreleptin is indicated as a replacement therapy to treat the complications of leptin deficiency in patients aged ≥ 2 years with generalized LD, and in patients aged ≥ 12 years with partial LD who have failed to achieve adequate metabolic control with standard treatment [8]. A summary of how metreleptin should be administered in these patient populations is shown in Table 1.
How does metreleptin work?
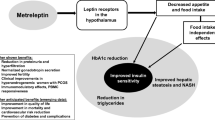
Metreleptin is a protein of ≈ 16 kDa that differs from endogenous human leptin by having an amino-terminal methionine residue [5]. Being an analogue of human leptin [3, 5], metreleptin binds to and activates the leptin receptor, thus mimicking the physiological effects of endogenous leptin [8]. Metreleptin improves metabolic abnormalities associated with LD, including glycaemic control, hypertriglyceridaemia and insulin sensitivity [5, 9]. How metreleptin ameliorates these metabolic abnormalities is not entirely clear, but is likely to involve a variety of factors [9]. One such factor is regulation of appetite/eating behaviour, with metreleptin reducing hunger [10, 11], eating importance/frequency [10], caloric intake [12] and time to satiety [12], as well as increasing satiety [11, 12]; however, some effects of metreleptin (including insulin sensitivity improvements) may be independent of food intake [13]. Changes in appetite/eating with metreleptin can lead to weight loss [5], although, even in lean hypoleptinaemic women, the drug reduced body fat without impacting lean body mass [14]. Reductions in facial soft tissue volume (in areas with adipose tissue compartments) have occurred with metreleptin in patients with LD, with the changes being of varying magnitude and generally not visible to the naked eye [15]. However, metreleptin did not increase patient energy expenditure in a recent non-randomized crossover study [13].
Metreleptin may disrupt events that lead to lipotoxicity, as improvements in hepatic and intramyocellular lipid content, liver volume and steatohepatitis have occurred with the drug in LD patients [5, 9]. Elevated levels of apolipoprotein CII and ANGPTL3 (proteins that inhibit lipoprotein lipase) in patients with LD may contribute to hypertriglyceridaemia, with levels of ANGPTL3 (but not apolipoprotein CII) being reduced by metreleptin [16, 17]. Reductions in plasma levels of PCSK9 (a protein that promotes hypercholesterolaemia) have also been seen with metreleptin in LD patients, with this change correlating with a reduction in low-density lipoprotein cholesterol [18].
What is the efficacy of metreleptin?
Generalized lipodystrophy
Metreleptin improved glycaemic control and hypertriglyceridaemia in patients aged ≥ 6 months (median age 15 years) with generalized LD in an integrated analysis of an open-label pilot study + its long-term extension [19], representing the largest available data set. In this analysis, metreleptin significantly reduced levels of glycosylated haemoglobin (HbA1C) and triglycerides from baseline over 12 months of treatment (co-primary endpoints; Table 2) and sustained these benefits through to month 48. Most metreleptin recipients (79.7%) achieved a metabolic response (i.e. a ≥ 1% reduction in HbA1C or a ≥ 30% reduction in triglycerides) by month 12. After starting metreleptin, some patients were able to discontinue treatment with insulin (16 of 39; 41%), oral anti-diabetic agents (7 of 32; 22%) and lipid-lowering medications (8 of 34; 24%) [19].
Having worse metabolic characteristics at baseline appeared to enhance these benefits of metreleptin, as indicated by co-primary endpoint analyses in patients with HbA1C ≥ 7% or triglycerides ≥ 5.65 mmol/L at baseline (Table 2) [8] and the proportion of patients who achieved a metabolic response at 12 months despite a baseline HbA1C level ≥ 6.5 or ≥ 8.0% (89 and 79 vs 11% of patients with baseline level < 6.5%) or a baseline triglyceride level ≥ 2.26 or ≥ 5.65 mmol/L (83 and 49 vs 15% of patients with baseline level < 2.26 mmol/L) [19].
At 12 months, metreleptin also improved some hepatic and renal outcomes from baseline. Metreleptin was associated with significant reductions from baseline in mean liver volume in 12 evaluable patients (33.8% reduction; p < 0.001), as well as ALT levels (44%; p = 0.01), but not AST levels (32%; p = 0.29), in the full analysis set [19]. The mean rate of protein excretion (a renal impairment measure) decreased from baseline by 54% (decrease of 906 mg/24 h from a baseline of 1676 mg/24 h) in 12 patients with renal data [8].
The findings of this analysis generally support those of earlier analyses of the pilot/extension study [20,21,22], as well as a trial in seven Japanese patients with generalized LD [23], the latter of which also reported improvements from baseline in insulin secretion and insulin resistance with metreleptin [23]. Moreover, a significant (p < 0.001) reduction from baseline in proteinuria was observed after 4 months of leptin therapy in some patients with generalized LD in an open-label study (11 of 15; 73%), with this benefit maintained for up to 36 months and being associated with a decline in creatinine clearance (likely indicating reduced glomerular hyperfiltration) [24].
Metreleptin also generally reversed metabolic complications in paediatric patients with generalized LD [8, 25]. At 12 months in the integrated analysis of the pilot/extension study, 5 patients aged < 6 years, 12 aged ≥ 6 to < 12 years and 28 aged ≥ 12 to < 18 years had mean changes from baseline in triglycerides of − 10.5, − 14.1 and − 42.9%, respectively, and mean changes from baseline in HbA1C of + 0.2, − 1.1 and − 2.6%, with the between-age-group differences in the latter parameter likely being due to their mean HbA1C levels at baseline (5.7, 6.4 and 9.7%) [8]. Similarly, among seven children aged 2.4–13.6 years with Berardinelli-Seip congenital lipoatrophy who received metreleptin for 4 months in an open-label trial, fasting triglyceride levels significantly (p = 0.017) declined from baseline and, although fasting plasma glucose levels did not change significantly, there was a significant (p = 0.04) improvement in the fasting glucose to insulin ratio and a significant (p = 0.002) reduction in liver volume [25].
The findings of clinical trials are generally supported by real-world experience in European countries [26, 27] and Brazil [28] (n = 9–28). For instance, in the largest analysis (a retrospective collection of data from 28 patients in the metreleptin early access programme in Italy, Spain, France and the UK; treatment duration ≤ 14.7 years) [26], metreleptin significantly (p ≤ 0.001 vs baseline) reduced levels of triglyceride by a median of 61% and HbA1C by a mean of 1.9% after 12 months of therapy. Moreover, at this timepoint versus baseline, an increased proportion of metreleptin recipients had a triglyceride level of < 2.3 mmol/L (50 vs 5%) or a HbA1C level of < 8% (70 vs 26%) [26].
Partial lipodystrophy
Metreleptin improved glycaemic control and hypertriglyceridaemia in patients aged ≥ 6 months (overall median age 34 years) with partial LD in the integrated analysis of the pilot/extension study, significantly reducing levels of HbA1C and triglycerides over 12 months of treatment (co-primary endpoints) in the overall patient population and each of the patient subgroups assessed (Table 2) [8, 29]. Similar to patients with generalized LD, these benefits were more notable in the partial LD patients with higher baseline HbA1C or triglyceride levels (Table 2) [8, 29]. Consistent with these findings, some partial LD metreleptin recipients achieved a metabolic response, including 51.4% of the overall population and 67.9% of the patient subgroup with baseline HbA1C ≥ 6.5% or triglyceride ≥ 5.65 mmol/L [29]. None of the 19 patients receiving insulin at baseline were able to discontinue it once receiving metreleptin, although 1 of 28 patients receiving oral glucose-lowering medications at baseline, and 1 of 34 patients receiving lipid-lowering medications at baseline, discontinued these therapies once receiving metreleptin [29].
Whether the improvements in metabolic complications seen with metreleptin in this setting could be impacted by the degree of hypoleptinaemia was evaluated in an open-label observational study in 24 patients with familial partial LD of the Dunnigan variety (mean age ≈ 40 years) [30]. In this study, median fasting serum triglyceride levels significantly (p < 0.05) decreased from baseline to a similar degree after 6 months of treatment regardless of whether patients were moderately hypoleptinaemic (serum leptin 4–7 ng/mL; n = 10) [from 4.8 to 3.8 mmol/L] or severely hypoleptinaemic (serum leptin < 4 ng/mL; n = 14) [from 2.6 to 2.1 mmol/L]. Neither patient group experienced significant improvements in glycaemic control (i.e. HbA1C levels, fasting plasma glucose levels, fasting serum insulin levels or glucose tolerance), although each population had a significant (p < 0.005) decrease from baseline in median intrahepatic lipid content (23.7 to 9.2% in moderately hypoleptinaemic group; 8.8 to 4.9% in severely hypoleptinaemic group) [30].
Metreleptin therapy for 12 months was associated with significant reductions in liver volume in the integrated analysis [− 13.4% in overall population (n = 9); − 12.4% in subgroup with baseline HbA1C ≥ 6.5% or triglyceride ≥ 5.65 mmol/L (n = 8)], although liver enzyme levels were not significantly altered [29]. Notably, in an analysis of data from two clinical trials in patients with partial LD and NAFLD, 9 of the 18 patients who completed 12 months’ treatment with metreleptin achieved a clinical hepatic response (i.e. a 2-point reduction in total NASH score without an increase in fibrosis) [31]. Among pre-selected parameters, baseline carbohydrate consumption was the best predictor of response, with others including the baseline level of free fatty acid, leptin or insulin [31].
Data for metreleptin in paediatric patients with partial LD are more limited, with only four patients aged ≥ 12 to < 18 years available in the subgroup of partial LD patients evaluated in the integrated analysis [8]. The mean change from baseline to month 12 in this paediatric subgroup was − 0.7% for HbA1C and -55.1% for triglycerides [8].
Real-world data from a long-term open-label expanded-access study of metreleptin in 23 patients with partial LD [32] generally support the findings of clinical trial analyses, although the observed mean improvements from baseline in HbA1C (− 0.88% from a mean baseline of 7.9%) and triglyceride (− 1.35 mmol/L from a mean baseline of 4.54 mmol/L) levels after 12 months’ treatment with metreleptin, did not reach statistical significance.
Mixed lipodystrophy populations
Clinical study [33,34,35] and real-world [36] data from mixed populations of patients with generalized or partial LD, including paediatric patients (aged 6 months to < 18 years) [35], support the efficacy of metreleptin in improving metabolic parameters associated with LD. In addition to these benefits, one of the clinical trials (of open-label, prospective design) assessed the long-term effect of metreleptin on LD-associated liver disease and found that the drug reversed NASH in some patients [significantly (p = 0.0002) fewer patients had NASH after 25.8 months’ metreleptin therapy than at baseline; 33 vs 86% of 27 patients] and stabilized liver fibrosis [34]. The potential clinical benefit of metreleptin on liver disease and other LD-related complications (including hyperphagia and heart/kidney damage) in patients with generalized or partial LD was also observed in a combined analysis of 290 retrospective chart reviews [37]. Leptin replacement therapy may also improve survival in patients with LD, according to a matched analysis of 114 treated and 178 untreated patients, with the risk of death being significantly (p < 0.05) reduced by 66% (79% when adjusted for covariates) [38].
What is the tolerability profile of metreleptin?
Metreleptin is generally well tolerated in patients with LD, with an overall tolerability profile that is similar in adults and children [8]. Among patients with generalized LD and a subgroup of patients with partial LD (aged ≥ 12 years, with HbA1C ≥ 8%, triglyceride ≥ 5.65 mmol/L and/or leptin < 12 ng/mL) who received metreleptin in clinical studies, the most common adverse reactions were decreased body weight (17%) and hypoglycaemia (14%), with the latter possibly requiring insulin recipients to reduce anti-diabetic agent dosages and monitor blood glucose levels (Table 3) [8]. Other commonly occurring (incidence ≥ 1 to < 10%) adverse reactions with metreleptin in this analysis included nausea, abdominal pain, decreased appetite, headache, alopecia, menorrhagia, fatigue, injection-site reactions, including bruising and erythema (all mild/moderate and none resulting in metreleptin discontinuation) and neutralizing antibodies [8]. There are various warnings and precautions pertaining to metreleptin use (Table 3), including the potential for immunogenicity, pancreatitis and T-cell lymphoma [8].
Antibodies against metreleptin have been detected in most patients with LD treated with the drug [8, 40] (e.g. 65 of 74 patients across the pilot/extension and expanded-access studies; 88% [8]), with titres usually peaking in 4–6 months and declining thereafter [40]. In an extended data set, 98 of 102 recipients (96%) had blood that blocked metreleptin reacting with a recombinant leptin receptor in vitro [8]. Whether such activity may impact metreleptin efficacy is not clear [8], although all four patients with generalized LD in the extension study who developed in vitro neutralizing activity on metreleptin therapy had concurrent poor or worsening metabolic control [40]. The development of neutralizing activity has also been temporally associated with serious and/or severe infections in some patients (five generalized LD patients with > 80% neutralizing activity and one partial LD patient; all responded to standard treatment) [8].
Six patients (four with generalized LD and two with partial LD, all of whom had had pancreatitis and hypertriglyceridaemia previously) experienced pancreatitis in clinical trials of metreleptin; potential contributing factors included not complying with, or abruptly interrupting, metreleptin treatment [8].
T-cell lymphoma occurred in three patients with generalized LD receiving metreleptin in clinical trials. Two of these patients developed peripheral T-cell lymphoma and had immunodeficiency and significant haematological abnormalities before receiving metreleptin, whereas the third was a paediatric patient without pre-treatment haematological abnormalities who developed anaplastic large cell lymphoma [8]. However, a causal relationship between metreleptin and lymphoma development/progression has not been established [8].
In paediatric patients
Among 48 paediatric patients with generalized LD who received metreleptin in two clinical trials (pilot/extension and expanded-access studies), adverse reactions occurred with similar incidence irrespective of patient age and were serious in two patients (anaplastic large cell lymphoma and worsening hypertension) [8]. No adverse reactions were reported in the four paediatric patients in the partial LD subgroup assessed in the integrated analysis of the pilot/extension study [8].
What is the current clinical role of metreleptin in lipodystrophy?
Treating lipodystrophy-related metabolic abnormalities is a challenge, with many patients (particularly those with severe insulin resistance and hypertriglyceridaemia) unable to achieve adequate metabolic control with standard therapies [3, 6]. Metreleptin is the first drug available for the specific treatment of lipodystrophy and has been a welcome addition to the conventional treatment options available. Although the rarity of the disease limits the number of patients in whom treatment can be evaluated, overall, the totality of evidence from clinical trials and real-world experience indicate that metreleptin improves glycaemic control and triglyceride levels in patients with generalized or partial LD, with data from the largest clinical trial set indicating that these benefits are generally more notable in patients with worse metabolic parameters at baseline in each of these populations. There is also evidence (albeit more limited) that metreleptin has metabolic benefit in paediatric patients with generalized or partial LD. Patients with partial LD can have leptin levels ranging from low to normal with varying degrees of fat loss [3], although data from one study suggest that metreleptin may lower triglyceride levels regardless of whether a partial LD patient is moderately or severely hypoleptinaemic; however, no glycaemic control improvements were evident in either group. Whether metreleptin may be of benefit in partial LD patients without hypoleptinaemia is of interest.
Various other benefits have occurred with leptin therapy in patients with generalized or partial LD, including improvements in hepatic parameters/disease, such as liver volume, intrahepatic lipid content and NASH (a major complication of LD that can progress despite adequate metabolic control [3]), nephropathy and survival. However, the impact of metreleptin on these outcomes, particularly life expectancy, would benefit from further analysis.
Metreleptin is generally well tolerated in patients with LD, with a similar tolerability profile in adults and children. As with other therapeutic proteins, antibodies against leptin may develop in metreleptin recipients, and whether such antibodies may impact the efficacy of the drug long-term remains to be determined.
Although formal guidance for the treatment of lipodystrophy is limited, a 2016 multi-society practice guideline recommends metreleptin (in conjunction with diet) as a first-line option for the treatment of metabolic and endocrine abnormalities in patients with generalized LD and as an option to consider for preventing these abnormalities in children with generalized LD [1]. The guideline also recommends considering metreleptin for the treatment of hypoleptinaemic patients with partial LD who have severe metabolic abnormalities (HbA1C > 8% and/or triglycerides > 5.6 mmol/L) [1].
Change history
13 August 2019
The article Metreleptin in lipodystrophy: a profile of its use, written by Emma Deeks.
References
Brown RJ, Araujo-Vilar D, Cheung PT, et al. The diagnosis and management of lipodystrophy syndromes: a multi-society practice guideline. J Clin Endocrinol Metab. 2016;101(12):4500–11.
Araujo-Vilar D, Santini F. Diagnosis and treatment of lipodystrophy: a step-by-step approach. J Endocrinol Invest. 2019;42(1):61–73.
Simha V. Metreleptin for metabolic disorders associated with generalized or partial lipodystrophy. Expert Rev Endocrinol Metabol. 2014;9(3):205–12.
King MW. Adipose tissue: not just fat. 2017. https://themedicalbiochemistrypage.org/adipose-tissue.php. Accessed 25 Feb 2019.
Meehan CA, Cochran E, Kassai A, et al. Metreleptin for injection to treat the complications of leptin deficiency in patients with congenital or acquired generalized lipodystrophy. Expert Rev Clin Pharmacol. 2016;9(1):59–68.
Akinci B, Meral R, Oral EA. Update on therapeutic options in lipodystrophy. Curr Diabetes Rep. 2018;18(12):139.
Fiorenza CG, Chou SH, Mantzoros CS. Lipodystrophy: pathophysiology and advances in treatment. Nat Rev Endocrinol. 2011;7(3):137–50.
Myalepta (metreleptin) 3, 5.8 and 11.3 mg powder for solution for injection: EU summary of product characteristics. Windsor: Aegerion Pharmaceuticals Ltd; 2018.
Oral EA, Chan JL. Rationale for leptin-replacement therapy for severe lipodystrophy. Endocr Pract. 2010;16(2):324–33.
Schlogl H, Muller K, Horstmann A, et al. Leptin substitution in patients with lipodystrophy: neural correlates for long-term success in the normalization of eating behavior. Diabetes. 2016;65(8):2179–86.
Puschel J, Miehle K, Muller K, et al. Beneficial effects of leptin substitution on impaired eating behavior in lipodystrophy are sustained beyond 150 weeks of treatment. Cytokine. 2019;113:400–4.
McDuffie JR, Riggs PA, Calis KA, et al. Effects of exogenous leptin on satiety and satiation in patients with lipodystrophy and leptin insufficiency. J Clin Endocrinol Metab. 2004;89(9):4258–63.
Brown RJ, Valencia A, Startzell M, et al. Metreleptin-mediated improvements in insulin sensitivity are independent of food intake in humans with lipodystrophy. J Clin Invest. 2018;128(8):3504–16.
Brinkoetter M, Magkos F, Vamvini M, et al. Leptin treatment reduces body fat but does not affect lean body mass or the myostatin-follistatin-activin axis in lean hypoleptinemic women. Endocrinol Metab. 2011;301(1):E99–104.
Miehle K, Stumvoll M, Fasshauer M, et al. Facial soft tissue volume decreases during metreleptin treatment in patients with partial and generalized lipodystrophy. Endocrine. 2017;58(2):262–6.
Muniyappa R, Abel BS, Asthana A, et al. Metreleptin therapy lowers plasma angiopoietin-like protein 3 in patients with generalized lipodystrophy. J Clin Lipidol. 2017;11(2):543–50.
Kassai A, Muniyappa R, Levenson AE, et al. Effect of leptin administration on circulating apolipoprotein CIII levels in patients with lipodystrophy. J Clin Endocrinol Metab. 2016;101(4):1790–7.
Levenson AE, Haas ME, Miao J, et al. Effect of leptin replacement on PCSK9 in ob/ob mice and female lipodystrophic patients. Endocrinol. 2016;157(4):1421–9.
Brown RJ, Oral EA, Cochran E, et al. Long-term effectiveness and safety of metreleptin in the treatment of patients with generalized lipodystrophy. Endocrine. 2018;60(3):479–89.
Oral EA, Simha V, Ruiz E, et al. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346(8):570–8.
Javor ED, Cochran EK, Musso C, et al. Long-term efficacy of leptin replacement in patients with generalized lipodystrophy. Diabetes. 2005;54(Suppl 1):12.
Oral EA, Chiquette E, Lewis JH, et al. Impact of metreleptin on hepatomegaly in patients with generalised lipodystrophy [abstract no. 856]. Diabetologia. 2017;60 (Suppl 1):S396-S7.
Ebihara K, Kusakabe T, Hirata M, et al. Efficacy and safety of leptin-replacement therapy and possible mechanisms of leptin actions in patients with generalized lipodystrophy. J Clin Endocrinol Metab. 2007;92(2):532–41.
Javor ED, Moran SA, Young JR, et al. Proteinuric nephropathy in acquired and congenital generalized lipodystrophy: baseline characteristics and course during recombinant leptin therapy. J Clin Endocrinol Metab. 2004;89(7):3199–207.
Beltrand J, Beregszaszi M, Chevenne D, et al. Metabolic correction induced by leptin replacement treatment in young children with Berardinelli-Seip congenital lipoatrophy. Pediatrics. 2007;120(2):e291–6.
Cook K, Stears A, Araujo-Vilar D, et al. Real-world experience of generalized lipodystrophy patients enrolled in the metreleptin early access program: initial results [abstract no. PSY11 + poster]. Value Health. 2018;21 (Suppl 3):S437.
Araujo-Vilar D, Sanchez-Iglesias S, Guillin-Amarelle C, et al. Recombinant human leptin treatment in genetic lipodystrophic syndromes: the long-term Spanish experience. Endocrine. 2015;49(1):139–47.
Lima JG, Lima NN, Santos MCF, et al. Early results of the first Brazilian patients with generalised congenital lipodystrophy on treatment with metreleptin [abstract no. 855]. Diabetologia. 2017;60(Suppl 1):S396.
Oral EA, Gorden P, Cochran E, et al. Long-term effectiveness and safety of metreleptin in the treatment of patients with partial lipodystrophy. Endocrine. 2019. https://doi.org/10.1007/s12020-019-01862-8.
Simha V, Subramanyam L, Szczepaniak L, et al. Comparison of efficacy and safety of leptin replacement therapy in moderately and severely hypoleptinemic patients with familial partial lipodystrophy of the Dunnigan variety. J Clin Endocrinol Metab. 2012;97(3):785–92.
Meral R, Ajluni N, Koksal A, et al. Clinical predictors of leptin response for improvement in liver histopathology in a cohort of patients with partial lipodystrophy [abstract no. SUN-055]. Endocr Rev. 2018;39(2 Suppl 1).
Ajluni N, Dar M, Xu J, et al. Efficacy and safety of metreleptin in patients with partial lipodystrophy: lessons from an expanded access program. J Diabetes Metab. 2016;7(3).
Chan JL, Lutz K, Cochran E, et al. Clinical effects of long-term metreleptin treatment in patients with lipodystrophy. Endocr Pract. 2011;17(6):922–32.
Safar Zadeh E, Lungu AO, Cochran EK, et al. The liver diseases of lipodystrophy: the long-term effect of leptin treatment. J Hepatol. 2013;59(1):131–7.
Brown RJ, Meehan CA, Cochran E, et al. Effects of metreleptin in pediatric patients with lipodystrophy. J Clin Endocrinol Metab. 2017;102(5):1511–9.
Ceccarini G, Magno S, Ferrari F, et al. Recombinant leptin treatment for metabolic complications associated with lipodystrophy: results from the first Italian experience [abstract no. P027]. Eat Weight Disord. 2018;23 (5):720–1.
Akinci B, Oral EA, Neidert A, et al. Liver damage in lipodystrophy and impact of metreleptin: experience from a large retrospective review [abstract no. LB-19]. Hepatology. 2017;66(6):1266A.
Ali OA, Cook K, Gupta D, et al. Effect of leptin replacement therapy (LRT) on survival and disease progression in generalized and partial lipodystrophy (GL, PL) [abstract no. 106-LB]. Diabetes. 2018;67(Suppl 1).
Data on file, Aegerion Pharmaceuticals Inc., 2016.
Chan JL, Koda J, Heilig JS, et al. Immunogenicity associated with metreleptin treatment in patients with obesity or lipodystrophy. Clin Endocrinol (Oxf). 2016;85(1):137–49.
Acknowledgements
The manuscript was reviewed by: L. Roever, Department of Clinical Research, Federal University of Uberlândia, Uberlândia, Brazil; F. Santini, Endocrinology Unit, Obesity and Lipodystrophy Center, University Hospital of Pisa, Pisa, Italy. During the peer review process, Aegerion Pharmaceuticals B.V., the marketing-authorization holder of metreleptin, was also offered an opportunity to provide a scientific accuracy review of their data. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
E. D. Deeks is an employee of Adis/Springer, is responsible for the article content and declares no conflicts of interest.
Additional information
The original version of this article was revised due to a retrospective Open Access request.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
About this article
Cite this article
Deeks, E. Metreleptin in lipodystrophy: a profile of its use. Drugs Ther Perspect 35, 201–208 (2019). https://doi.org/10.1007/s40267-019-00622-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-019-00622-y