Abstract
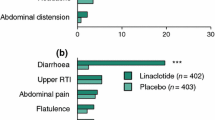
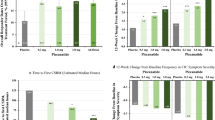
Plecanatide (Trulance®), a guanylate cyclase C agonist, is an effective and generally well-tolerated treatment option for adults with chronic idiopathic constipation or irritable bowel syndrome with constipation. It can be administered with or without food, at any time of the day. In phase 3 clinical trials, once-daily plecanatide 3 mg significantly improved stool frequency, stool consistency, clinical symptoms (e.g. straining, abdominal symptoms including pain), health-related quality of life, and treatment satisfaction. Plecanatide is generally well tolerated, with most adverse events being of mild or moderate severity. Consistent with its therapeutic action, the most common adverse event is diarrhea.
Similar content being viewed by others
References
Chey WD. Symposium report: an evidence-based approach to IBS and CIC: applying new advances to daily practice: a review of an adjunct clinical symposium of the American College of Gastroenterology meeting October 16, 2016, Las Vegas, Nevada. Gastroenterol Hepatol. 2017;13(2 Suppl 1):1–16.
Shailubhai K, Comiskey S, Foss JA, et al. Plecanatide, an oral guanylate cyclase C agonist acting locally in the gastrointestinal tract, is safe and well-tolerated in single doses. Dig Dis Sci. 2013;58(9):2580–6.
Waldman SA, Camilleri M. Guanylate cyclase-C as a therapeutic target in gastrointestinal disorders. Gut. 2018;67(8):1543–52.
Trulance (plecanatide) tablets: US prescribing information. New York: Synergy Pharmaceuticals Inc. 2018.
Shailubhai K, Palejwala V, Arjunan KP, et al. Plecanatide and dolcanatide, novel guanylate cyclase-C agonists, ameliorate gastrointestinal inflammation in experimental models of murine colitis. World J Gastrointest Pharmacol Ther. 2015;6(4):213–22.
Shailubhai K, Beaufrand C, Palejwala V, et al. Oral treatment with plecanatide and SP-333, agonists of guanylate cyclase-C, attenuates visceral hypersensitivity in rat models (abstract no. PP095). Neurogastroenterol Motil. 2014;26:Suppl 1–41.
Miner PB Jr, Koltun WD, Wiener GJ, et al. A randomized phase III clinical trial of plecanatide, a uroguanylin analog, in patients with chronic idiopathic constipation. Am J Gastroenterol. 2017;112:613–21.
DeMicco M, Barrow L, Hickey B, et al. Randomized clinical trial: efficacy and safety of plecanatide in the treatment of chronic idiopathic constipation. Ther Adv Gastroenterol. 2017;10(11):837–51.
Brenner DM, Fogel R, Dorn SD, et al. Efficacy, safety, and tolerability of plecanatide in patients with irritable bowel syndrome with constipation: results of two phase 3 randomized clinical trials. Am J Gastroenterol. 2018;113(5):735–45.
Rao S, Barrow L, Layton MB. Efficacy and safety of plecanatide in the treatment of chronic idiopathic constipation (CIC): pooled results from two phase 3 studies (abstract no. 523). Am J Gastroenterol. 2016;111(Suppl 1):S237.
Rao SS, Hixson MA, Mikhelashvili T. The impact of plecanatide on quality of life for patients with chronic idiopathic constipation (CIC): results from two phase 3 clinical studies (abstract no. PGI39 + poster). Value Health. 2017;20(5):A186–7.
Rao SS, Griffin P, Magnus L. Efficacy and safety of plecanatide in patients with chronic idiopathic constipation: an analysis of patients with moderate to very severe bloating (abstract no. Su1517 + poster). Gastroenterology. 2017;152((5 Suppl 1)):S507.
Moshiree B, Magnus L, Rezaie A. Acid suppression therapy does not affect the efficacy of plecanatide: a patient-level pooled analysis of two large randomized controlled trials (abstract no. P1222). In: American College of Gastroenterology Scientific Meeting & Postgraduate Course. 2018.
Menees SB, Chey WD, Crozier R. Evaluation of the safety and tolerability of plecanatide in CIC and IBS-C patients aged 65 and older (abstract no. P0335). In: American College of Gastroenterology Annual Scientific Meeting & Postgraduate Course. 2018.
Barish CF, Griffin P. Safety and tolerability of plecanatide in patients with chronic idiopathic constipation: long-term evidence from an open-label study. Curr Med Res Opin. 2018;34(4):751–5.
Barish CF, Crozier RA, Griffin PH. Long-term treatment with plecanatide was safe and tolerable in patients with irritable bowel syndrome with constipation. Curr Med Res Opin. 2018. https://doi.org/10.1080/03007995.2018.1527303 (e-pub).
Harris LA, Horn J, Kissous-Hunt M, et al. The better understanding and recognition of the disconnects, experiences, and needs of patients with chronic idiopathic constipation (BURDEN-CIC) study: results of an online questionnaire. Adv Ther. 2017;34(12):2661–73.
Quigley EMM, Horn J, Kissous-Hunt M, et al. Better understanding and recognition of the disconnects, experiences, and needs of patients with irritable bowel syndrome with constipation (BURDEN IBS-C) study: results of an online questionnaire. Adv Ther. 2018;35(7):967–80.
Linzess (linaclotide) capsules: US prescribing information. Irvine (CA): Allergan USA, Inc.; 2017.
Amitiza (lubiprostone) capsules: US prescribing information. Deerfield (IL): Takeda Pharmaceuticals America, Inc.; 2018.
Black CJ, Burr NE, Quigley EMM, et al. Efficacy of secretagogues in patients with irritable bowel syndrome with constipation: systematic review and network meta-analysis. Gastroenterology. 2018. https://doi.org/10.1053/j.gastro.2018.08.021 (e-pub).
Lasa JS, Altamirano MJ, Bracho LF, et al. Efficacy and safety of intestinal secretagogues for chronic constipation: a systematic review and meta-analysis. Arq Gastroenterol. 2018. https://doi.org/10.1590/s0004-2803.201800000-41 (e-pub).
Shah ED, Kim HM, Schoenfeld P. Efficacy and tolerability of guanylate cyclase-C agonists for irritable bowel syndrome with constipation and chronic idiopathic constipation: a systematic review and meta-analysis. Am J Gastroenterol. 2018;113(3):329–38.
Acknowledgements
The manuscript was reviewed by: C. F. Barish, Gastroenterology, University of North Carolina School of Medicine, Chapel Hill, NC, USA; L. A. Harris, Division of Gastroenterology & Hepatology, Mayo Clinic, Scottsdale, AZ, USA; Y-M. Huang, Division of Social and Administrative Sciences, School of Pharmacy, University of Wisconsin-Madison, Madison, WI, USA; I. Zaghloul, Massachusetts College of Pharmacy and Health Sciences University, Boston, MA, USA. During the peer review process, Synergy Pharmaceuticals, the marketing-authorization holder of plecanatide, was offered an opportunity to provide a scientific accuracy review of the data. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
H A. Blair is an employee of Adis/Springer, is responsible for the article content and declares no conflicts of interest.
Rights and permissions
About this article
Cite this article
Blair, H.A. Plecanatide in chronic idiopathic constipation and irritable bowel syndrome with constipation: a profile of its use in the USA. Drugs Ther Perspect 34, 554–559 (2018). https://doi.org/10.1007/s40267-018-0579-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-018-0579-y