Abstract
Background
Many patients with chronic idiopathic constipation (CIC) remain unsatisfied with their treatment options. Plecanatide is a pH-sensitive uroguanylin analog that increases fluid and ion movement into the gastrointestinal lumen, softening stools and encouraging motility, while limiting the risk of diarrhea.
Aims
The objective of this phase 2 study is to evaluate the safety and efficacy of once-daily oral plecanatide in patients with CIC and identify the most effective dose.
Methods
A 12-week, multicenter, randomized, double-blind, placebo-controlled, dose-ranging study was conducted in patients aged 18–75 years and diagnosed with CIC based on modified Rome III criteria (< 3 complete spontaneous bowel movements [CSBMs] per week and infrequent loose stools without the use of laxatives). Participants were randomized to placebo or plecanatide 0.3, 1.0, or 3.0 mg. The primary efficacy endpoint was the proportion of overall CSBM responders. Key secondary endpoints included time to first CSBM, change in CSBM and spontaneous bowel movement (SBM) frequency rates, patient-reported outcomes, safety, and tolerability.
Results
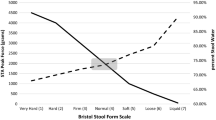
Of 951 randomized participants, 946 were included in the modified intent-to-treat population. Plecanatide 0.3 and 3.0 mg significantly increased overall CSBM responder rates compared with placebo (0.3 mg, P = 0.016; 3.0 mg, P = 0.009). Plecanatide was associated with decreased time to first CSBM, significant increases in CSBM and SBM frequency, and decreased patient-reported constipation severity compared with placebo. Diarrhea was the most frequently reported treatment-emergent adverse event.
Conclusions
Plecanatide is a well-tolerated treatment that relieved the symptoms of CIC with a relatively low incidence of diarrhea.

Similar content being viewed by others
Abbreviations
- CSBM:
-
Complete spontaneous bowel movement
- CIC:
-
Chronic idiopathic constipation
- PAC-QoL:
-
Patient Assessment of Constipation Quality of Life
- PAC-SYM:
-
Patient Assessment of Constipation Symptoms
- PGA:
-
Patient Global Assessment
- SBM:
-
Spontaneous bowel movement
- TEAE:
-
Treatment-emergent adverse event
References
American College of Gastroenterology Chronic Constipation Task Force. An evidence-based approach to the management of chronic constipation in North America. Am J Gastroenterol. 2005;100:S1–S4.
Heidelbaugh JJ, Stelwagon M, Miller SA, et al. The spectrum of constipation-predominant irritable bowel syndrome and chronic idiopathic constipation: US survey assessing symptoms, care seeking, and disease burden. Am J Gastroenterol. 2015;110:580–587.
Harris LA, Horn J, Kissous-Hunt M, et al. The better understanding and recognition of the disconnects, experiences, and needs of patients with chronic idiopathic constipation (BURDEN-CIC) study: results of an online questionnaire. Adv Ther. 2017;34:2661–2673.
Rao SSC. Plecanatide: a new guanylate cyclase agonist for the treatment of chronic idiopathic constipation. Ther Adv Gastroenterol. 2018;11:1756284818777945.
Miner PB Jr, Koltun WD, Wiener GJ, et al. A randomized phase III clinical trial of plecanatide, a uroguanylin analog, in patients with chronic idiopathic constipation. Am J Gastroenterol. 2017;112:613–621.
DeMicco M, Barrow L, Hickey B, et al. Randomized clinical trial: efficacy and safety of plecanatide in the treatment of chronic idiopathic constipation. Ther Adv Gastroenterol. 2017;10:837–851.
Miner PB, Surowitz R, Fogel R, et al. 925 g plecanatide, a novel guanylate cyclase-C (GC-C) receptor agonist, is efficacious and safe in patients with chronic idiopathic constipation (CIC): results from a 951 patient, 12 week, multi-center trial [abstract]. Gastroenterology. 2013;144:S-163.
Waldman SA, Camilleri M. Guanylate cyclase-C as a therapeutic target in gastrointestinal disorders. Gut. 2018;67:1543–1552.
Funding
This study was funded by Salix Pharmaceuticals, Inc. (Bridgewater, NJ, USA).
Author information
Authors and Affiliations
Contributions
CB contributed to conceptualization, data curation, formal analysis, investigation, methodology, writing—original draft, writing—review and editing. SD contributed to conceptualization, formal analysis, investigation, methodology, writing—original draft, writing—review and editing. RPF was involved in conceptualization, data curation, formal analysis, investigation, methodology, writing—original draft, writing—review and editing. RP was involved in conceptualization, data curation, formal analysis, investigation, methodology, writing—original draft, writing—review and editing. JR supported conceptualization, data curation, formal analysis, investigation, methodology, writing—original draft, writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
Charles Barish: nothing to disclose. Spencer Dorn: consultant/advisory board member to Synergy Pharmaceuticals regarding Plecanatide. Ronald P. Fogel: nothing to disclose. Reema Patel: Bausch Health Employee. Jonathan Rosenberg: Speakers Bureaus for Allergan, Salix, Takeda.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Barish, C., Dorn, S., Fogel, R.P. et al. Plecanatide Is Effective and Safe in the Treatment for Chronic Idiopathic Constipation: Results of a Phase II Trial. Dig Dis Sci 66, 537–540 (2021). https://doi.org/10.1007/s10620-020-06187-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-020-06187-5