Abstract
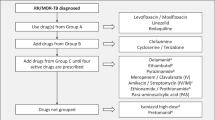
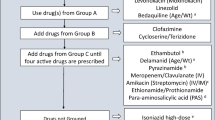
The first-line treatment of paediatric tuberculosis (TB) involves long-term treatment with regimens containing multiple anti-TB agents (i.e. isoniazid, rifampicin, pyrazinamide and/or ethambutol). The choice of treatment regimen is based on the clinical manifestation of TB, disease severity and the risk of antibacterial resistance. Paediatric patients should be carefully monitored for treatment compliance, response to treatment and drug-related adverse events, of which hepatotoxicity is the most common.

Similar content being viewed by others
References
Principi N, Galli L, Lancella L, et al. Recommendations concerning the first-line treatment of children with tuberculosis. Paediatr Drugs. 2016;18(1):13–23.
World Health Organization. Tuberculosis: fact sheet no. 104. 2016. http://www.who.int/mediacentre. Accessed 27 Apr 2016.
Piccini P, Chiappini E, Tortoli E, et al. Clinical peculiarities of tuberculosis. BMC Infect Dis. 2014;14(Suppl 1):S4.
World Health Organization. Guidance for national tuberculosis programmes on the management of tuberculosis in children, 2nd ed. 2014. http://www.who.int/tb. Accessed 28 Apr 2016.
TB Alliance. Child-friendly medicines. http://www.tballiance.org/child-friendly-medicines. Accessed 9 May 2016.
Donald PR. Antituberculosis drug-induced hepatotoxicity in children. Pediatr Rep. 2011;3:e16.
Huang YS, Chern HD, Su WJ, et al. Polymorphism of the N-acetyltransferase 2 gene as a susceptibility risk factor for antituberculosis drug-induced hepatitis. Hepatology. 2002;35:883–9.
Wang PY, Xie SY, Hao Q, et al. NAT2 polymorphisms and susceptibility to anti-tuberculosis drug-induced liver injury: a meta-analysis. Int J Tuberc Lung Dis. 2012;16:589–95.
Thee S, Detjen A, Quarcoo D, et al. Ethambutol in paediatric tuberculosis: aspects of ethambutol serum concentration, efficacy and toxicity in children. Int J Tuberc Lung Dis. 2007;11:965–71.
Author information
Authors and Affiliations
Consortia
Ethics declarations
The article was adapted from Pediatric Drugs 2016;18(1):13–23 [1] by salaried employees of Adis/Springer and was not supported by any external funding.
Rights and permissions
About this article
Cite this article
Adis Medical Writers. First-line treatment of paediatric tuberculosis requires consideration of disease type and severity followed by careful monitoring. Drugs Ther Perspect 32, 330–334 (2016). https://doi.org/10.1007/s40267-016-0317-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40267-016-0317-2