Abstract
Sarcopenia, the age-related loss of muscle strength and mass or quality, is a common condition with major adverse consequences. Although the pathophysiology is incompletely understood, there are common mechanisms between sarcopenia and the phenomenon of accelerated ageing seen in diabetes mellitus. Drugs currently used to treat type 2 diabetes mellitus may have mechanisms of action that are relevant to the prevention and treatment of sarcopenia, for those with type 2 diabetes and those without diabetes. This review summarises shared pathophysiology between sarcopenia and diabetes mellitus, including the effects of advanced glycation end products, mitochondrial dysfunction, chronic inflammation and changes to the insulin signalling pathway. Cellular and animal models have generated intriguing, albeit mixed, evidence that supports possible beneficial effects on skeletal muscle function for some classes of drugs used to treat diabetes, including metformin and SGLT2 inhibitors. Most human observational and intervention evidence for the effects of these drugs has been derived from populations with type 2 diabetes mellitus, and there is a need for intervention studies for older people with, and at risk of, sarcopenia to further investigate the balance of benefit and risk in these target populations. Not all diabetes treatments will be safe to use in those without diabetes because of variable side effects across classes. However, some agents [including glucagon-like peptide (GLP)-1 receptor agonists and SGLT2 inhibitors] have already demonstrated benefits in populations without diabetes, and it is these agents, along with metformin, that hold out the most promise for further investigation in sarcopenia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Type 2 diabetes mellitus and sarcopenia share multiple pathophysiological mechanisms. |
Preclinical, observational and interventional data suggest that some drugs used to treat diabetes may have beneficial effects on sarcopenia even in patients without diabetes. |
Clinical trials of these agents have not focussed on enrolling people with sarcopenia, and such trials are needed to evaluate whether diabetes drugs could improve or prevent sarcopenia in patients with or without diabetes. |
1 Introduction
Sarcopenia is the loss of muscle strength and muscle mass or quality commonly seen with advancing age [1]. It is now clear that sarcopenia is common; meta-analysis of community-based studies show a prevalence of 10% of the general population aged 60 years and over [2], albeit with wide differences (2–36%) between different populations and diagnostic methods. A higher prevalence is found in certain subgroups, for instance those living with multiple long-term conditions (MLTC) [3, 4]. Sarcopenia increases the risk of a series of adverse outcomes, including falls, fractures and immobility, as well as the need for health and social care, and is a key contributor to the syndrome of physical frailty [5,6,7]. It is also associated with earlier death [8]. Sarcopenia has been estimated to cost the UK health service £2 billion per year [9], and the direct costs of sarcopenia in the USA in 2000 were estimated at $18.5 billion per year [10].
Sarcopenia is therefore a key ageing syndrome, and preventive and treatment therapies are needed to help maintain health and wellbeing in later life. Resistance exercise, with or without adjunctive protein supplementation, is the only intervention with good evidence of efficacy for the prevention and treatment of sarcopenia [11, 12], but not all individuals with sarcopenia are willing or able to participate in such training. The place of vitamin D as a treatment for sarcopenia remains controversial, with trial results ranging from modest benefit to possible harm [13, 14]. Pharmacological therapies are therefore required [15], both as an alternative to resistance exercise but also to augment the effects of resistance training.
Type 2 diabetes mellitus (T2DM) has long been recognised as a condition associated with accelerated ageing [16]; it is also a condition characterised by impaired skeletal muscle function [17, 18]. A growing body of preclinical and clinical data suggest that medications used to treat T2DM may have important effects on muscle function, both by their effects on glucose metabolism and via effects not directly related to glucose metabolism [19, 20]. Understanding the mechanisms of action and clinical effects of these medications on skeletal muscle will enable more nuanced and informed choices of agent for those with T2DM and sarcopenia, but importantly may also suggest novel therapeutic avenues to prevent and treat sarcopenia in patients who do not have T2DM.
Integrating mechanistic and clinical research on the effects of these drugs for people with and without T2DM is needed to guide progress in research and practice by giving a broad, clinically applicable overview of current knowledge. The purpose of this review is therefore to examine the current evidence for mechanisms by which drugs for T2DM might improve skeletal muscle function, and to examine evidence of beneficial effects of these treatments on skeletal muscle function in individuals with and without diabetes.
1.1 The Biology of Sarcopenia
The pathophysiology of sarcopenia is incompletely understood, but a range of fundamental biological processes have been implicated. Histological studies suggest a preferential loss of type 2 (fast twitch) muscle fibres and a fast-to-slow fibre shift, although both type 1 (slow twitch) and type 2 fibres show evidence of atrophy in patients with sarcopenia [21, 22]. Histological studies also show evidence of denervation and neuromuscular junction dysfunction with each motor neuron innervating a larger number of motor units [23]. Infiltration of fat into muscle, and replacement of muscle tissue by fat, are commonly seen in sarcopenia; the combination of sarcopenia and obesity (sarcopenic obesity) predicts worse outcomes than sarcopenia alone in epidemiological studies [24, 25]. Chronic inflammation, mitochondrial dysfunction, oxidative stress, changes in the extracellular matrix such as increased collagen deposition [26], vascular dysfunction and neurohormonal changes have all been linked to the development of sarcopenia [1, 17, 27, 28].
1.2 Diabetes as a Syndrome of Accelerated Ageing
Accelerated ageing is a hallmark of T2DM. Life expectancy in type 1 diabetes is still 12 years shorter than for individuals without diabetes [29], and cardiometabolic conditions (e.g. renal disease, cardiovascular disease) typically manifest years earlier than in individuals without diabetes [15]. Both overt T2DM and other insulin-resistant states are associated with chronic inflammation, enhanced reactive oxygen species generation, telomere shortening and cellular senescence – processes that are driven in part by the obesity that commonly underlies T2DM [30]. Importantly, these are also fundamental biological processes that underpin ageing and sarcopenia [31].
2 The Relationship between Hyperglycaemic States and Skeletal Muscle Function
2.1 Biology
-
a.
Advanced glycation end products (AGEs)
Glycation of proteins and other macromolecules to form advanced glycation end products (AGEs) is a hallmark of T2DM and has been postulated to underpin many of the long-term adverse consequences of the condition, including both macrovascular and microvascular disease [32]. However, it is now clear that glycation is also an important contributor to the ageing phenotype even in individuals who do not have T2DM [33]. Skeletal muscle holds a significant proportion of total body protein [34], and glycation of skeletal muscle collagen occurs with advancing age in humans [35]. A range of other skeletal muscle proteins, including actin and creatine kinase, also show increased labelling with AGE-specific antibodies in older versus younger rats [36].
The adverse consequences of AGEs are not confined to the direct effect on macromolecular structure and function. These adducts, acting via AGE receptors [37], are a major driver of chronic inflammation [31]; inflammation in turn drives cellular senescence, oxidative stress and mitochondrial dysfunction [38] – key pathophysiological mechanisms also implicated in sarcopenia. Importantly, AGEs in in vitro myotube systems also directly impair myogenesis and induce muscle atrophy via AGE-receptor-mediated protein kinase B (Akt) pathway signalling [39].
-
b.
Insulin resistance and skeletal muscle glucose uptake
Raised concentrations of insulin are found in individuals with insulin resistance, usually thought to be a response to impaired insulin-mediated skeletal muscle glucose uptake and hence higher circulating glucose concentrations [40]. The loss of muscle mass that occurs in sarcopenia would be expected to reduce the amount of metabolically active muscle available to absorb glucose from the circulation under the influence of insulin, and this may explain the association between insulin resistance and sarcopenia [41]. Conversely, increasing muscle mass through pharmacological intervention in animal models leads to improvements in insulin sensitivity [42]. In addition, there are several health states or risk factors that are common to both sarcopenia and insulin resistance (for instance, physical inactivity and chronic inflammation [43, 44]) that may also explain the association even without accounting for the loss of muscle mass seen in sarcopenia.
Insulin has important effects not only on glucose uptake but also on protein turnover in skeletal muscle, reducing muscle protein breakdown and (in some studies where sufficient amino acids are present) increasing muscle protein synthesis [45]. There is some evidence from isotope tracer studies that skeletal muscle in older people is less sensitive to the anabolic effects of insulin than in younger individuals, and that this difference is greater than can be explained by differences in skeletal muscle glucose uptake in response to insulin [46, 47]. It is less clear whether impaired skeletal muscle glucose uptake is itself a limiting factor in skeletal muscle metabolism, or whether insulin resistance has direct effects on mitochondrial function [48] or other aspects of skeletal muscle metabolism.
-
c.
Direct toxic effects of hyperglycaemia
There is evidence that hyperglycaemia prevents satellite cell proliferation and differentiation in individuals with T2DM [49]. However, the significance of this finding for the development of sarcopenia in individuals without diabetes, or with well-controlled diabetes, is unclear, as satellite cell numbers and function have not been found to be consistently impaired in sarcopenia [50]. Glucose may also have directly toxic effects on mitochondrial function, either via osmotic effects or by driving metabolism via alternative pathways [51]. Although such phenomena are likely to be most relevant in patients with uncontrolled diabetes, recent work suggests that high-glucose diets in animal models impair mitochondrial function [52].
-
d.
Non-glycaemic effects
T2DM and sarcopenia share a number of pathophysiological processes, and current evidence suggests that they may all be reciprocally causal. Intramyocellular lipid deposition, common in obesity but also a feature of sarcopenia, may result in part from mitochondrial dysfunction with resultant accumulation of unmetabolised free fatty acids [53]. Lipid deposition in skeletal muscle or in adipose tissue is in turn is a driver of chronic inflammation [54, 55], which is known to be a cause of mitochondrial dysfunction [44]. The increased generation of reactive oxygen species resulting from mitochondrial dysfunction is in turn a driver of further mitochondrial dysfunction [53], creating a vicious cycle of pro-oxidative and pro-inflammatory activity.
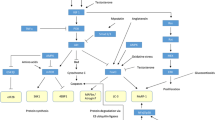
Microvascular dysfunction is another shared pathophysiological process. The microvascular dysfunction typically seen in patients with DM [56] may have direct adverse effects on skeletal muscle. Patients with T2DM have lower capillarisation of skeletal muscle [57] and ultrastructural changes in capillaries, including thickened basement membranes [58]. Sarcopenia is also associated with reduced skeletal muscle capillarisation [59], and low capillarisation has been proposed as a limiting factor in the ability of skeletal muscle to respond to resistance training [60]. This microvascular dysfunction may be driven in part by vascular insulin resistance; both conduit arteries and microvascular beds have been found to be less responsive to insulin in states of obesity and of T2DM [61, 62]. A selection of mechanisms that may drive sarcopenia, together with drugs used to treat diabetes that may ameliorate the adverse effects of these mechanisms, is shown in Fig. 1.
2.2 Epidemiology
Sarcopenia is more common in individuals with T2DM than in those without diabetes; estimates suggest a prevalence 50% higher in individuals with T2DM, after accounting for age and sex, and these associations are found across a range of different definitions of sarcopenia and ways of adjusting skeletal muscle mass [63, 64]. Longer duration of T2DM is associated with an increased risk of quadriceps weakness [65]. Interestingly, the link between T2DM and muscle strength measures appears much stronger than the association between T2DM and muscle mass. A Mendelian randomisation study also gave some limited support for the above findings, with genetically predicted diabetes risk in men (but not women) being associated with lower handgrip strength and lower whole-body lean mass measured using bioimpedance analysis in the UK Biobank cohort. This Mendelian randomisation study suggest a bidirectional relationship: low genetically predicted whole-body lean mass was a risk factor for future development of T2DM for women (but not for men) in this analysis [66].
Skeletal muscle plays an important role in taking up circulating glucose under the influence of insulin [40], and thus lower muscle mass can be expected to contribute to the syndrome of insulin resistance and consequent hyperglycaemia. Studies of insulin resistance suggest that this metabolic state is also associated with lower quadriceps strength in cross-sectional studies [41] and with an accelerated loss of lean mass measured by dual-energy X ray absorptiometry (DXA) over time [67]. In keeping with this finding, elevated circulating glucose concentrations are associated with a higher incidence of mobility limitation (self-reported difficulty walking a quarter of a mile, short physical performance battery <9 points) and physical frailty measured using the Fried frailty criteria in older people [68] – key outcomes driven by reduced muscle mass and strength. In addition, an inverse dose–response relationship is evident between blood glucose concentrations and handgrip strength [18] and between higher hemoglobin A1c (HbA1c) within the normal range and lower knee extensor strength [69]. However, no difference was seen in the rate of decline in muscle strength over 2 years between participants in the highest and lowest quartiles of HbA1c in this analysis.
The relationship between skeletal muscle mass and dysregulation of metabolism is made more complex by the role of fat. Studies that adjust skeletal muscle mass for height squared find weaker relationships with cardiometabolic syndromes than those that adjust for body weight or body mass index (BMI) [70]; for a given body weight, less muscle means more fat, and obesity is well-known to be a driver of insulin resistance and diabetes [71]. Both low skeletal muscle mass and high fat mass may play independent roles; individuals with the combination of these states (sarcopenic obesity) are at higher risk of death and mobility limitation than those with sarcopenia alone or obesity alone [24, 25], and the degree of sarcopenia is strongly associated with the degree of insulin resistance seen in individuals with obesity [72].
Concentrations of AGEs either in the skin or the circulation are higher in individuals with lower muscle mass and strength [73], both for the general population [74, 75] and for individuals with T2DM [76]. Some, but not all, of these analyses suggest a dose-response between higher AGEs and lower muscle mass or strength. Finally, there is some evidence that the association between glycaemia and muscle function may be causal. An observational study of Japanese patients with T2DM [77] found that a reduction in HbA1c during treatment with oral diabetes medications or with insulin was associated with increased walk speed, but not grip strength. This difference between upper and lower limb function may suggest that other aspects of walking control (e.g. central or peripheral nerve function [78]) could be important in explaining the improvement in walk speed.
3 Diabetes Drugs and Skeletal Muscle
3.1 Metformin
Metformin is a biguanide molecule, in use for the treatment of T2DM since the 1950s [79]. Despite initial concerns over the ability of biguanides to induce lactic acidosis [80], metformin has proven to be a safe and effective member of the therapeutic armamentarium in the management of T2DM. It effectively lowers plasma glucose concentrations when concentrations are high, but it is rare for metformin to be associated with hypoglycaemia when used as a single agent [81]. Unlike insulin and some other medications used for treating diabetes, it does not cause weight gain. Lactic acidosis is very rare side effect (and usually precipitated by intercurrent illness); gastrointestinal disturbance limits tolerability for 5–10% of users [82].
Despite nearly 70 years of use, the mechanisms of action of metformin continue to be debated. Metformin inhibits gluconeogenesis in the liver [83] in patients with T2DM and elevated blood glucose concentrations, but in patients without T2DM, new evidence suggests that metformin may increase endogenous glucose production [84]. Multiple other pathways have been identified by which metformin might exert beneficial effects on age-related pathophysiology – particularly on mechanisms relevant to energy utilisation. Many of these mechanisms may be relevant to skeletal muscle function. Firstly, metformin inhibits mitochondrial complex 1, and this in turn may reduce the production of reactive oxygen species [85, 86], thought to be involved in cellular damage and mitochondrial dysfunction. Secondly, metformin activates adenosine monophosphate (AMP)-activated kinase (AMPK), the ‘master regulator’ of energy utilisation by cells. AMPK has effects on autophagy and stress resistance, and in stimulating mitochondrial biogenesis [87]. A further AMPK-driven action of metformin is to inhibit the mammalian target of rapamycin complex (mTORC). The mTORC pathway is critical to multiple ageing processes, a key example of which is regulation of autophagy. Constitutive mTORC upregulation inhibits autophagy, including in skeletal muscle cells; in animal models the loss of autophagy leads to a skeletal myopathy that is reversible with the mTORC inhibitor rapamycin [88, 89]. Although high mTORC activity is associated with shorter lifespan in multiple species, mTORC also plays important roles in the maintenance of skeletal muscle mass and prevention of atrophy [90] suggesting that mTORC inhibition could have deleterious effects on skeletal muscle. The net effect of mTORC inhibition may depend on both the starting activity and the degree of inhibition – skeletal muscle from sarcopenic rats was found to have highly active mTORC, and partial inhibition of mTORC was able to increase muscle mass in this animal model [91].
Metformin may not act directly on myocytes in a clinically important way however. Human skeletal muscle uptake of metformin appears low in comparison to hepatic, renal and intestinal uptake in radioactive tracer studies using 11C-labelled metformin [92]. It is therefore not certain that sufficient metformin reaches skeletal muscle to mediate the aforementioned effects directly within myocytes.
Thirdly, metformin inhibits pro-inflammatory cytokine production [including interleukin (IL)-1 and tumour necrosis factor (TNF)-alpha], and intracellular pathways activated by inflammation [via inhibition of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)] [93,94,95]; it has also been shown to increase circulating levels of irisin, a key myokine [96]. Fourthly, metformin suppresses cellular senescence in multiple tissues, including skeletal muscle, which is a major driver of ageing in mammals via its impact on mitochondrial complex I, AMPK, mTOR and NF-κB. Metformin’s senostatic action has been shown to improve frailty (including improvements in skeletal muscle mass and function in a mouse model of premature ageing [97]) and led to improvements in muscle strength and endurance in a sarcopenic mouse model, along with reduction in a suite of pro-inflammatory cytokines [98].
Finally, metformin also precipitates changes to the gut microbiome [99]. This has been proposed as an indirect mechanism of action for some of the effects of metformin, and although the precise biological pathways involved remain unclear, a range of actions have been postulated, including changes to bacterial-derived pro-inflammatory compounds, changes to production of circulating bacterial metabolites with actions on AMPK and indirect effects to stimulate release of GLP-1 by the gut [100]. Not all effects of metformin on skeletal muscle may be beneficial however; metformin has been shown to reduce gastrocnemius mass in mice in one study, an effect likely driven by induction of myostatin transcription [101].
3.2 Evidence from Observational and Intervention Studies
Few studies have examined the relationship between metformin use and skeletal muscle health – such studies are challenging because metformin is currently indicated for use in T2DM – a condition which is itself implicated in accelerated ageing and muscle dysfunction. Observational data from the Osteoporotic Fracture in Men (MrOS) study suggested that men with T2DM treated with metformin showed slower loss of muscle mass measured by DXA over time than those not treated with insulin-sensitising agents [102]; similar results were found among women with T2DM in the Study of Osteoporotic Fractures (SOF) [103].
One randomised controlled trial suggests promising effects of metformin on physical function [104]. A total of 120 participants aged 60 years and over with prefrailty (measured and without diabetes were randomised to receive 16 weeks of metformin 500 mg three times a day or matching placebo. Walk speed increased significantly in the treatment group compared with placebo (by 0.13 m/s, which exceeds the minimum clinically important difference for this measure [105]). No differences were seen for quality of life as measured by the EQ5D tool, handgrip strength or myostatin level; weight change was not reported, and dropout rates were high (≥ 20%) in both arms. The MASTERS trial [106] also found no augmentation of muscle strength when metformin 850 mg twice daily was added to a 14-week resistance training programme for older men and women, and indeed the muscle hypertrophic response measured by both computed tomography and by DXA to exercise was blunted by metformin when compared with placebo. Similar findings were seen in a small but detailed trial of metformin (up to 1 g twice daily versus placebo) given to healthy older people undertaking 12 weeks of aerobic exercise training. In this trial, metformin prevented the expected improvement in whole-body insulin sensitivity measured by oral glucose tolerance test, and attenuated the expected improvement in maximal oxygen uptake and skeletal muscle mitochondrial respiration from aerobic training [107]. The focus of these trials was on augmenting the effect of training rather than standalone effects of metformin (the effect of metformin alone was not studied), and although the trial included people with a range of physical capabilities, it did not specifically target those with sarcopenia. These results suggest however that careful targeting may be needed to avoid deleterious effects of metformin by targeting only those populations most likely be benefit (perhaps those with over-active mTOR as discussed above, or those not willing or able to undertake exercise training).
A number of other trials are under way that seek to test the effects of metformin on skeletal muscle function. Of particular note are a randomised, placebo-controlled trial enrolling participants with impaired glucose tolerance [108], testing metformin up to 1 g twice daily versus placebo for 2 years, and a trial (MET-PREVENT) [109] enrolling older people with probable sarcopenia, testing metformin 500 mg three times a day versus placebo for 4 months. The results of these trials are necessary to test whether the potential advantages of metformin outweigh any adverse effects of metformin on appetite, food intake, weight and exercise benefits.
3.3 Sulphonylureas and Insulin Secretagogues
Sulphonylureas are another class of drug that have been used for decades to treat DM [110]. Glinides are a more-recently introduced, molecularly distinct class of compounds with faster onset and offset of action [111]. Both work by closing adenosine triphosphate (ATP)-sensitive potassium channels (KATP channels), a mechanism that parallels the effect of ATP on channel closure. The closure of the KATP channel triggers membrane depolarization, calcium influx and insulin release from pancreatic beta cells [112]. A number of different subtypes of the KATP channel exist, with differential expression across a wide range of tissues, including skeletal muscle [113]. Individual members of the sulphonylurea and glinide drug classes bind with differential potency to these subtypes of KATP channel [114], which may lead to effects outwith the primary mechanism of action based on insulin release.
Although many of the actions of sulphonylureas and glinides are therefore attributable to the actions of endogenous insulin, additional actions may also be mediated by direct effects on skeletal muscle KATP channels. Not all sulphonylureas bind to skeletal muscle KATP channels, but for those that do (e.g. glibenclamide), this may represent a further mechanism of action. Open KATP channels are postulated to protect skeletal muscle against energy depletion in fatigue [115]; closure of these channels has also been shown to precipitate muscle atrophy in animal models [116]. Conversely, sulphonylureas may also enhance glucose uptake by enhancing glucose transporter type 4 (GLUT-4) expression at the cell membrane [117].
Because sulphonylureas and glinide medications lower blood glucose and precipitate hypoglycaemia, studies examining the effect of these medications on muscle function in patients without T2DM have not been performed. Observational studies in patients with T2DM are difficult to interpret; in many studies, sulphonylureas are the comparator class of medication, and it is difficult to dissect out whether sulphonylureas have deleterious effects on muscle function or whether comparator agents have beneficial effects [101,102,103]. For example, falls were more frequent in patients with T2DM taking sulphonylureas than other drugs for T2DM in a recent observational study [118]. Skeletal muscle weakness is known to be an important risk factor for falls [119], but this study was not able to ascertain how much of the effect was attributable to skeletal muscle dysfunction and how much to hypoglycaemic episodes. The current evidence does not support a beneficial effect of these drug classes on muscle function, and their hypoglycaemic effects in people without diabetes mean that they are unlikely to be practical to test further.
3.4 Thiazolidinediones (Glitazones)
Thiazolidinediones (glitazones) activate the peroxisome proliferator-activated receptor (PPAR) gamma, triggering a complex set of metabolic changes centred around lipid metabolism, primarily in adipocytes but also in a wide range of other tissues, including skeletal muscle [120]. Theoretically, these changes in lipid metabolism, including reductions in free fatty acid concentrations, could reduce intramyocellular lipid deposition, leading to improvements in mitochondrial function and insulin sensitivity [53, 54] that could be beneficial to skeletal muscle function. Human studies show a reduction in intramyocellular lipid deposition in patients with T2DM treated with glitazones [121]. However, data from laboratory studies on glitazones is less encouraging; in a young non-diabetic mouse model, pioglitazone did not improve mitochondrial function or grip strength alone or as an adjunct to exercise training [122].
Observational data from the MrOS study suggested that men with T2DM treated with glitazones showed slower loss of total and appendicular lean mass over time as measured by DXA than those not treated with insulin-sensitising agents [102]. Uncontrolled muscle biopsy studies enrolling patients with T2DM show that 12 weeks of pioglitazone 30 mg once daily improved skeletal muscle mitochondrial respiration measured by high-resolution respirometry of muscle biopsies, whereas 12 weeks of rosiglitazone 4 mg once daily worsened mitochondrial respiration [123]; this was despite both agents improving insulin resistance. Beneficial effects were also seen in a randomised trial of pioglitazone 45 mg once daily versus diet for 6 months in patients with T2DM. Both groups showed similar improvements in HbA1c and insulin sensitivity, but the pioglitazone group additionally showed increased levels of mitochondrial gene expression and AMPK phosphorylation [124]. Similar increases in mitochondrial proteins involved in oxidative phosphorylation were observed in a placebo-controlled trial of pioglitazone 15 mg once daily given for 6 months [125]. However, another placebo-controlled randomised controlled trial (RCT) using a higher dose of pioglitazone (30–45 mg once a day for 12 weeks) in T2DM failed to find any improvement in maximal ATP synthesis measured using 31P magnetic resonance spectroscopy [126] despite a decrease in intramyocellular lipids in the pioglitazone arm relative to placebo.
Although no clinical trial has yet evaluated the effect of glitazone therapy on skeletal muscle mass or strength in humans as a standalone therapy, pioglitazone has been tested as an adjunctive therapy in older people with impaired physical function undergoing a weight loss programme. In this trial, 30 mg per day of pioglitazone was associated with less loss of muscle mass measured by computed tomography (CT) and DXA over the 16 week follow-up [127], although the difference compared with the control group did not reach statistical significance. In women, the pioglitazone group showed a greater increase in leg press power with resistance training than did the placebo group, but this effect was not seen in men [128]. Glitazones may have additional beneficial effects on skeletal muscle beyond effects on lipid metabolism; for example, troglitazone up to 600 mg per day improved skeletal muscle capillarisation and glucose disposal to a greater extent than metformin up to 2550 mg per day in patients with T2DM [57]. The broader balance of benefits and risks from glitazone therapy may not support use of these agents to prevent or treat sarcopenia however. Although glitazone therapy can reduce cardiovascular events in selected populations [129], these agents also increase the risk of clinically apparent heart failure and of fractures [129, 130], which in turn may reduce physical function and worsen sarcopenia.
3.5 Glucagon-Like Peptide 1 (GLP-1) Receptor Agonists
GLP-1 is released by cells in the gut in response to a meal and enhances the release of insulin from pancreatic beta cells and inhibits release of glucagon by pancreatic alpha cells in response to circulating glucose [131]. GLP-1 receptors are also present on a range of other cell types, including adipocytes and endothelial cells [132, 133]. As well as effects mediated by insulin release, GLP-1 receptor agonists improve endothelial function in healthy individuals and stimulate angiogenesis and endothelial cell proliferation [134]. These effects are also seen in patients with T2DM and in other insulin-resistant states such as obesity [135, 136] and may be mediated in part by restoration of vascular insulin sensitivity [137]. In addition, GLP-1 receptor agonists reduce expression of myostatin and pro-inflammatory cytokines in animal models [138].
In patients with T2DM, GLP-1 receptor agonists cause significant weight loss [139] – an action often viewed as beneficial, as many patients with T2DM have obesity. However, a recent review of studies of body composition suggests that up to 50% of the weight loss seen from GLP-1 receptor agonist use in patients with T2DM is attributable to a loss of lean body mass [140]. It is not known at present whether GLP-1 receptor agonist use in patients with or without T2DM causes a clinically significant change in skeletal muscle strength; the loss in total body weight might be expected to facilitate improved mobility, but any loss of lean body mass might attenuate or even negate this benefit. The most relevant evidence comes from three recent randomised controlled trials of liraglutide. The FIGHT trial enrolled 300 patients with chronic heart failure; T2DM was not an inclusion criterion in this trial. Participants had been recently hospitalised and had poor endurance (the mean 6-min walk distance at baseline was only 220 m) [141]. No difference was seen in 6-min walk distance between the group receiving liraglutide 1.8 mg once a day and the placebo group after 6 months of treatment (between-group difference 5 m; p = 0.79) but skeletal muscle strength was not tested in this trial. A small trial comparing liraglutide 1.8 mg once a day with placebo in 24 participants with T2DM measured maximal and submaximal measures of endurance but not measures of skeletal muscle strength [142]. Six months of treatment did not lead to a clinically or statistically significant improvement in maximal oxygen uptake (VO2 max) in the liraglutide group (liraglutide versus placebo: 18.0 versus 17.0 ml/min/kg; p = 0.31). Additionally, no significant between-group differences were seen in the improvement in cycle ergometry time or 6-min walk distance during the study. Finally, the large (n = 3731) SCALE randomised trial of liraglutide 3.0 mg once a day versus placebo to facilitate weight loss in patients with obesity showed a significant improvement in self-reported physical function measured using the SF-36 health status questionnaire over the 1-year follow-up, but objective measures of physical performance were not obtained in this trial [143]. It is possible that adjuvant interventions to preserve muscle mass and strength (e.g. resistance exercise and provision of adequate protein intake [144]) may need to be given alongside GLP-1 receptor agonists if they are to be used effectively for sarcopenia, and research on this class of treatments may be best targeted to patients with sarcopenic obesity rather than those of normal or low body mass index. To date, there have been no trials reporting the effect of GLP-1 receptor agonists on skeletal muscle mass or strength in older people with sarcopenia; no such trials are currently registered at either ISRCTN.com or Clinicaltrials.gov
3.6 Dipeptidyl Peptidase 4 (DPP4) Inhibitors
DPP4 inhibitors (‘gliptins’) exert their effects by preventing the inactivation of GLP-1 by the Dipeptidyl peptidase 4 (DPP4) enzyme [145]. There is therefore considerable overlap between the potential actions of DPP4 on skeletal muscle and the actions of GLP-1 receptor agonists, and the data presented above in relation to GLP-1 receptor agonists are likely to be relevant. Animal models suggest that DPP4 inhibitors increase circulating concentrations of the myokine irisin as well as peroxisome proliferator-activated receptor γ coactivator-1-alpha [146], a key component of the muscle response to exercise.
Two small observational studies suggest an association between DPP4 inhibitor use and better indices of muscle mass and strength in older people with T2DM. One study showed faster walk speed, greater muscle strength measured by handgrip dynamometry and higher fat free mass measured by bioimpedance (adjusted for height squared) in DPP4 users than those on sulphonylureas [147], although it is unclear whether this was due to beneficial effects of DPP4 inhibitors or deleterious effects of sulphonylureas. Another study found that patients with T2DM taking DPP4 inhibitors had a slower decline in skeletal muscle index measured by DXA than those not taking DPP4 inhibitors [148]. In contrast, pharmacovigilance studies have found a higher rate of arthralgia and myalgia (including rare reports of rhabdomyolysis in conjunction with statin medications) in users of DPP4 inhibitors [149]; the pathophysiological mechanisms underpinning this observation are unclear.
3.7 Sodium–Glucose Co-transporter 2 (SGLT2) Inhibitors
Sodium–glucose co-transporter 2 (SGLT2) inhibitors work by partly blocking reabsorption of filtered glucose in the proximal convoluted tubule of the kidney [150]. Although the SGLT2 transporter is localised to the kidney, a series of knock-on metabolic effects in patients with T2DM have been described. These include a degree of weight loss (thought to be due to glucose excretion and fluid depletion), but also a reduction in blood pressure as a result of volume depletion, sodium excretion, weight loss and reduction in renin–angiotensin–aldosterone system (RAAS) activity [151, 152]. Intriguingly, in other organ systems, SLGT2 inhibitors also appear to drive changes that are potentially relevant to skeletal muscle dysfunction, for example, increasing lipolysis and ketogenesis, reducing inflammatory cytokines in liver [153], the aforementioned reduction in RAAS activity [154] and upregulation of AMPK via the sestrin pathway with consequent inhibition of mTOR in cardiac myocytes [153]. Off-target effects via inhibition of the related SGLT1 transport protein may also mediate reductions in oxidative stress seen in cardiac tissue [155]. Although SGLT2 does not appear to be expressed in skeletal muscle, the related transporter SGLT3 is expressed near the neuromuscular junction [156]. Its physiological function is unclear, but it may play the role of a glucose sensor [157]; furthermore, phlorizin (the parent compound from which currently marketed SGLT2 inhibitors are derived) is able to inhibit SGLT3 [158]
Although few studies have assessed the effects of SGLT2 inhibitors in preclinical models, one recent study has shown that canagliflozin reduces inflammatory cytokine concentrations in skeletal muscle and improves skeletal muscle contractile force in an obese mouse model [159]. Similar improvements in skeletal muscle size and strength were seen with administration of luseoglifozin in another mouse model [160]. However, there is currently a lack of evidence from studies in humans. SGLT2 inhibitor use is associated with a reduction in both fat mass and lean body mass (measured using a range of techniques) similar to that caused by GLP-1 receptor agonists; between 20% and 50% of the weight loss seen with these agents may be due to loss of lean mass [140]. Preferential loss of fat mass may however improve the ratio of lean to fat mass, which would be expected to improve strength:weight ratio. Data on the effect of SGLT2 inhibitors on muscle strength in humans are lacking, but there is at least one trial underway testing the impact of SGLT2 inhibitors (empagliflozin 10 mg once daily versus placebo for 52 weeks) on both fat free mass (measured by bioimpedance) and muscle strength (handgrip strength and five times sit to stand test) in older patients with T2DM [161]. In patients with heart failure, it is also possible that SGLT2-inhibitor-mediated improvements in cardiac function could translate into improved exercise capacity (a recent meta-analysis suggests a modest 20 m improvement in 6-min walk distance with SGLT2 inhibitors in patients with heart failure [162], and hence into increased physical activity with consequent benefit on deconditiong. This hypothesis requires testing however. To date, no trials have been conducted using SGLT2 inhibitors as a treatment for sarcopenia in patients without T2DM.
3.8 Insulin
Insulin is a key anabolic hormone, and acts both by the classical insulin receptor and indirectly by stimulating release and availability of insulin-like growth factor 1 (IGF-1) [162,164,165]. Insulin stimulates glucose uptake into skeletal muscle [166], thus lowering circulating glucose concentrations. In addition, it inhibits glucose production via effects on glycogenolysis and gluconeogenesis, but also inhibits lipolysis and promotes fat synthesis [167, 168]. Perhaps most importantly for skeletal muscle health, insulin stimulates muscle protein synthesis and reduces protein degradation and autophagy [169]. Insulin also exerts important direct effects on the microvasculature, causing vasodilatation and reversing the endothelial dysfunction commonly seen in T2DM [45].
In patients with T2DM, insulin concentrations are often already supranormal, and treatment with exogenous insulin does not appear to increase protein synthesis, reduce muscle protein breakdown or improve mitochondrial function [170,171,172]. Nevertheless, in a cohort of Japanese patients with T2DM, decreases in HbA1c resulting from insulin treatment were associated with increases in skeletal muscle mass (measured by bioimpedance) and gait speed and, after adjusting for confounders, were associated with a 1.4% increase in skeletal muscle index over the 1-year follow-up period [77]. These findings suggest that, in patients with type 2 diabetes mellitus, it may be the reduction in hyperglycaemia, rather than direct anabolic signalling of exogenous insulin, that is of benefit. A similar association between insulin use and a slower decline in skeletal muscle mass measured by bioimpedance over a 3-year follow-up period was found in a cohort of older people in Augsburg, Germany [173], although measures of muscle function (grip strength and timed up and go) did not show comparable benefit. One small study of 40 patients found no change in handgrip over the first 6 months after starting insulin therapy [174].
The benefits of insulin treatment on skeletal muscle may be more apparent in patients with type 1 diabetes mellitus (T1DM) [16]; in patients with T1DM, muscle protein breakdown is inhibited by insulin, and several studies suggest an increase in muscle mass over the first few months after starting insulin therapy [175, 176], although it is unclear whether this is due to direct anabolic effects of insulin or to the reduction in hyperglycaemia. The effect on muscle strength of starting insulin therapy has not been studied in humans. The profound hypoglycaemia caused by administration of insulin to patients without DM means that the effects of insulin on muscle strength or muscle mass in patients without DM have not been able to be studied to date in humans.
4 Conclusions and Future Directions
This review considers the current evidence on whether drugs used to treat diabetes mellitus could have potential as therapies to prevent or reverse the loss of muscle strength and mass that characterises sarcopenia. Although there is a considerable body of preclinical and clinical research on the effects of these agents in patients with diabetes mellitus, research on this topic in patients without diabetes is much more limited and is not sufficient to make clinical recommendations about the use of any agent as a therapy for sarcopenia in clinical practice.
Considering the strength of current evidence alongside the risk profile for different agents is key to informing the direction of future research. Whilst all drugs used to treat diabetes can potentially be used by patients with T2DM, some cannot be safely used in patients without diabetes due to their ability to induce hypoglycaemia. Insulin and sulphonylureas are the two classes of agent most likely to result in hypoglycaemia, but glinides are also associated with this complication. GLP-1 receptor agonists can cause hypoglycaemia, but rates of symptomatic or spontaneously reported hypoglycaemia in clinical trials enrolling patients without diabetes (and thus not taking other hypoglycaemic medications) are low, typically between 1% and 5% [145]. Other classes of agents (particularly metformin, but also incretins and SGLT2 inhibitors), do not cause hypoglycaemia in individuals who are normoglycaemic at baseline in the absence of other hypoglycemic agents; in the case of SGLT2 inhibitors, considerable clinical trial experience has now accrued using these agents in patients without T2DM (only 22 instances of hypoglycaemia were noted in 5233 trial participants in a recent meta-analysis [177]).
All classes of agent used to treat T2DM have potential adverse effects other than hypoglycaemia. These include weight gain for insulin [178]; weight loss, gastrointestinal side effects and lactic acidosis for metformin [80, 82]; genital and urinary tract infections, weight loss and euglycaemic ketosis with SGLT2 inhibitors [179]; a possible association with bladder cancer for pioglitazone [180, 181] and with osteoporotic fracture [130]; and heart failure for glitazones as a class [129]. Balancing the risks of using these agents with any potential benefits on muscle function is therefore essential in selecting agents both for future study and for the design of trials examining their effects on skeletal muscle function in older people.
Of the agents discussed here, metformin, GLP-1 receptor agonists, DPP4 inhibitors and SGLT2 inhibitors appear worthy of further evaluation in sarcopenia clinical studies; Table 1 summarises the strength of current evidence and suitability for each class of agent. Some care may be required in selecting which populations to target with which interventions – metformin, for example, might be a good choice to test for people with established sarcopenia but could worsen muscle function in healthy older people without sarcopenia. Studies are needed for older people with sarcopenia but also for those at risk of sarcopenia. Although most studies to date have targeted patients with DM, there is also a need to target those without DM – either with impaired glucose tolerance or with normal glucose metabolism. Fat infiltration into muscle and consequent lipotoxicity is a potentially important mechanism driving sarcopenia which may be amenable to reversal by several agents used to treat diabetes, and patients with sarcopenic obesity are a particular group that may benefit from inclusion in future studies. The use of measures of physical performance (for example, five times sit to stand) and muscle mass (for example, adjusting for weight rather than height squared) that take account of increased body mass should therefore be encouraged to facilitate inclusion of this patient group. The broad range of biological pathways affected by these agents holds the promise of therapeutic benefit across multiple disease states (as has been seen already for SGLT2 inhibitors [182, 183]) but also necessitates careful assessment of potential harms across multiple organ systems. Given the intimate association between skeletal muscle function, glucose and fat metabolism in health and disease, these agents already marketed for DM deserve more thorough scrutiny as a potential therapeutic avenue for sarcopenia.
References
Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. 2019;393:2636–46.
Shafiee G, Keshtkar A, Soltani A, Ahadi Z, Larijani B, Heshmat R. Prevalence of sarcopenia in the world: a systematic review and meta-analysis of general population studies. J Diabetes Metab Disord. 2017;16:21.
Cruz-Jentoft AJ, Landi F, Schneider SM, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing. 2014;43:748–59.
Dodds RM, Granic A, Robinson SM, Sayer AA. Sarcopenia, long-term conditions, and multimorbidity: findings from UK Biobank participants. J Cachexia Sarcopenia Muscle. 2020;11:62–8.
Lunt E, Ong T, Gordon AL, Greenhaff PL, Gladman JRF. The clinical usefulness of muscle mass and strength measures in older people: a systematic review. Age Ageing. 2021;50:88–95.
Beaudart C, Zaaria M, Pasleau F, Reginster JY, Bruyère O. Health outcomes of sarcopenia: a systematic review and meta-analysis. PLoS ONE. 2017;12: e0169548.
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56.
Liu P, Hao Q, Hai S, Wang H, Cao L, Dong B. Sarcopenia as a predictor of all-cause mortality among community-dwelling older people: a systematic review and meta-analysis. Maturitas. 2017;103:16–22.
Pinedo-Villanueva R, Westbury LD, Syddall HE, Sanchez-Santos MT, Dennison EM, Robinson SM, et al. Health care costs associated with muscle weakness: a UK population-based estimate. Calcif Tissue Int. 2019;104:137–44.
Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52:80–5.
Moore SA, Hrisos N, Errington L, Rochester L, Rodgers H, Witham M, et al. Exercise as a treatment for sarcopenia: an umbrella review of systematic review evidence. Physiotherapy. 2019;107:189–201.
Kirwan RP, Mazidi M, Rodríguez García C, Lane KE, Jafari A, Butler T, et al. Protein interventions augment the effect of resistance exercise on appendicular lean mass and handgrip strength in older adults: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2022;115:897–913.
De Spiegeleer A, Beckwée D, Bautmans I, Petrovic M. Pharmacological interventions to improve muscle mass, muscle strength and physical performance in older people: an umbrella review of systematic reviews and meta-analyses. Drugs Aging. 2018;35:719–34.
Prokopidis K, Giannos P, Katsikas Triantafyllidis K, Kechagias KS, Mesinovic J, Witard OC, et al. Effect of vitamin D monotherapy on indices of sarcopenia in community-dwelling older adults: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2022;13:1642–52.
Kilsby AJ, Sayer AA, Witham MD. Selecting potential pharmacological interventions in sarcopenia. Drugs Aging. 2017;34:233–40.
Morley JE. Diabetes and aging: epidemiologic overview. Clin Geriatr Med. 2008;24:395–405.
Kalyani RR, Corriere M, Ferrucci L. Age-related and disease-related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014;2:819–29.
Sayer AA, Dennison EM, Syddall HE, Gilbody HJ, Phillips DI, Cooper C. Type 2 diabetes, muscle strength, and impaired physical function: the tip of the iceberg? Diabetes Care. 2005;28:2541–2.
Massimino E, Izzo A, Riccardi G, Della PG. The impact of glucose-lowering drugs on sarcopenia in type 2 diabetes: current evidence and underlying mechanisms. Cells. 2021;10:1958.
Kalaitzoglou E, Fowlkes JL, Popescu I, Thrailkill KM. Diabetes pharmacotherapy and effects on the musculoskeletal system. Diabetes Metab Res Rev. 2019;35: e3100.
Ciciliot S, Rossi AC, Dyar KA, Blaauw B, Schiaffino S. Muscle type and fiber type specificity in muscle wasting. Int J Biochem Cell Biol. 2013;45:2191–9.
Gannon J, Doran P, Kirwan A, Ohlendieck K. Drastic increase of myosin light chain MLC-2 in senescent skeletal muscle indicates fast-to-slow fibre transition in sarcopenia of old age. Eur J Cell Biol. 2009;88:685–700.
Larsson L, Degens H, Li M, Salviati L, Lee Y, Thompson W, et al. Sarcopenia: aging-related loss of muscle mass and function. Physiol Rev. 2019;99:427–511.
Stenholm S, Alley D, Bandinelli S, Griswold ME, Koskinen S, Rantanen T, et al. The effect of obesity combined with low muscle strength on decline in mobility in older persons: results from the InCHIANTI study. Int J Obes. 2009;33:635–44.
Stenholm S, Mehta NK, Elo ET, Heliovaara M, Koskinen S, Aromaa A. Obesity and muscle strength as long-term determinants of all-cause mortality – a 33 year of follow-up of the mini-Finland health examination survey. Int J Obes. 2014;38:1126–32.
Chen WJ, Lin IH, Lee CW, Chen YF. Aged skeletal muscle retains the ability to remodel extracellular matrix for degradation of collagen deposition after muscle injury. Int J Mol Sci. 2021;22:2123.
Bellatini F, Lo Buglio A, Vendemiale G. Mitochondrial impairment in sarcopenia. Biology. 2021;10:31.
Dalle S, Rossmeislova L, Koppo K. The role of inflammation in age-related sarcopenia. Front Physiol. 2017;8:1045.
Livingstone SJ, Levin D, Looker HC, Lindsay RS, Wild SH, Joss N, et al. Estimated life expectancy in a Scottish cohort with type 1 diabetes, 2008–2010. JAMA. 2015;313:37–44.
Ahima RS. Connecting obesity, aging and diabetes. Nat Med. 2009;15:996–7.
DiLoreto R, Murphy CT. The cell biology of aging. Mol Biol Cell. 2015;26:4524–31.
Friedman E. Advanced glycosylated end products and hyperglycemia in the pathogenesis of diabetic complications. Diabetes Care. 1999;22(Suppl 2):B51–71.
Chaudhuri J, Bains Y, Guha S, Kahn A, Hall D, Bose N, et al. The role of advanced glycation end products in aging and metabolic diseases: bridging association and causality. Cell Metab. 2018;28:337–52.
Snyder WS, Cook MJ, Nasset ES, Karhausen LR, Howells GP, Tipton IH. Report of the task group on reference man. Oxford: Pergamon Press; 1975.
Haus JM, Carrithers JA, Trappe SW, Trappe TA. Collagen, cross-linking and advanced glycation endproducts in aging human skeletal muscle. J Appl Physiol. 2007;103:2068–76.
Snow LM, Fugere NA, Thompson LV. Advanced glycation end-product accumulation and associated protein modification in type II skeletal muscle with aging. J Gerontol A Biol Sci Med Sci. 2007;62:1204–10.
Daussin FN, Boulanger E, Lancel S. From mitochondria to sarcopenia: role of inflammaging and RAGE-ligand axis implication. Exp Gerontol. 2021;146: 111247.
Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15:505–22.
Chiu CY, Yang RS, Sheu ML, Chan DC, Yang TH, Tsai KS, et al. Advanced glycation end-products induce skeletal muscle atrophy and dysfunction in diabetic mice via a RAGE-mediated, AMPK-down-regulated, Akt pathway. J Pathol. 2016;238:470–82.
DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. 2009;32(Suppl 2):S157–63.
Barzilay JI, Cotsonis GA, Walston J, et al. Health ABC Study. Insulin resistance is associated with decreased quadriceps muscle strength in nondiabetic adults aged ≥ 70 years. Diabetes Care. 2009;32:736–8.
Camporez JPG, Petersen MC, Abudukadier A, Shulman GI. Anti-myostatin antibody increases muscle mass and strength and improves insulin sensitivity in old mice. Proc Natl Acad Sci. 2016;113:2212–7.
Steffl M, Bohannon RW, Sontakova L, Tufano JJ, Shiells K, Holmerova I. Relationship between sarcopenia and physical activity in older people: a systematic review and meta-analysis. Clin Interv Aging. 2017;12:835–45.
Seijkens T, Kusters P, Chatzigeorgiou A, Chavakis T, Lutgens E. Immune cell crosstalk in obesity: a key role for costimulation? Diabetes. 2014;63:3982–91.
Abdulla H, Smith K, Atherton PJ, Idris I. Role of insulin in the regulation of human skeletal muscle protein synthesis and breakdown: a systematic review and meta-analysis. Diabetalogia. 2015;59:44–55.
Rasmussen BB, Fujita S, Wolfe RR, Mittendorfer B, Roy M, Rowe VL, et al. Insulin resistance of muscle protein metabolism in aging. FASEB J. 2006;20:768–9.
Fujita S, Rasmussen BB, Cadenas JG, Grady JJ, Volpi E. Effect of insulin on human skeletal muscle protein synthesis is modulated by insulin-induced changes in muscle blood flow and amino acid availability. Am J Physiol Endocrinol Metab. 2006;291:E745-754.
Dela F, Helge JW. Insulin resistance and mitochondrial function in skeletal muscle. Int J Biochem Cell Biol. 2013;45:11–5.
D’Souza DM, Al-Sajee D, Hawke TJ. Diabetic myopathy: impact of diabetes mellitus on skeletal muscle progenitor cells. Front Physiol. 2013;4:379.
Snijders T, Parise G. Role of muscle stem cells in sarcopenia. Curr Opin Nutr Metabol Care. 2017;20:186–90.
Rolo AP, Palmeira CM. Diabetes and mitochondrial function: role of hyperglycemia and oxidative stress. Toxicol Appl Pharmacol. 2006;212:167–78.
Alcantar-Fernandez J, Gonzalez-Maciel A, Reynoso-Robles R, Perez Andrade ME, Hernandez-Vazquez ADJ, Velazquez-Arellano A, et al. High-glucose diets induce mitochondrial dysfunction in Caenorhabditis elegans. PLoS ONE. 2019;14: e0226652.
Di Meo S, Iossa S, Venditti P. Skeletal muscle insulin resistance: role of mitochondria and other ROS sources. J Endocrinol. 2017;233:R15–42.
Meex RC, Blaak EE, van Loon LJC. Lipotoxicity plays a key role in the development of both insulin resistance and muscle atrophy in patients with type 2 diabetes. Obes Rev. 2019;20:1205–17.
Rivas DA, McDonald DJ, Rice NP, Haran PH, Dolnikowski GG, Fielding RA. Diminished anabolic signaling response to insulin induced by intramuscular lipid accumulation is associated with inflammation in aging but not obesity. Am J Physiol Regul Integr Comp Physiol. 2016;310:R561–9.
Schalkwijk CG, Stehouwer CD. Vascular complications in diabetes mellitus: the role of endothelial dysfunction. Clin Sci (Lond). 2005;109:143–59.
Mathieu-Costello O, Kong A, Ciaraldi TP, Cui L, Ju Y, Chu N, et al. Regulation of skeletal muscle morphology in type 2 diabetic subjects by troglitazone and metformin: relationship to glucose disposal. Metabolism. 2003;52:540–6.
Baum O, Bigler M. Pericapillary basement membrane thickening in human skeletal muscles. Am J Physiol Heart Circ Physiol. 2016;311:H654–66.
Prior SJ, Ryan AS, Blumenthal JB, Watson JM, Katzel LI, Goldberg AP. Sarcopenia is associated with lower skeletal muscle capillarization and exercise capacity in older adults. J Gerontol A Biol Sci Med Sci. 2016;71:1096–101.
Moro T, Brightwell CR, Phalen DE, McKenna CF, Lane SJ, Porter C, et al. Low skeletal muscle capillarization limits muscle adaptation to resistance exercise training in older adults. Exp Gerontol. 2019;127: 110723.
Clerk LH, Vincent MA, Jahn LA, Liu Z, Lindner JR, Barrett EJ. Obesity blunts insulin-mediated microvascular recruitment in human forearm muscle. Diabetes. 2006;55:1436–42.
Clerk LH, Vincent MA, Barrett EJ, Lankford MF, Lindner JR. Skeletal muscle capillary responses to insulin are abnormal in late-stage diabetes and are restored by angiotensin-converting enzyme inhibition. Am J Physiol Endocrinol Metab. 2007;293:E1804–9.
Anagnostis P, Gkekas NK, Achilla C, Pananastasiou G, Taouxidou P, Mitsiou M, et al. Type 2 diabetes mellitus is associated with increased risk of sarcopenia: a systematic review and meta-analysis. Calcif Tissue Int. 2020;107:453–63.
Chung SM, Moon JS, Chang MC. Prevalence of sarcopenia and its association with diabetes: a meta-analysis of community-dwelling Asian population. Front Med (Lausanne). 2021;8: 681232.
Kalyani RR, Tra Y, Yeh HC, Egan JM, Ferrucci L, Brancati FL. Quadriceps strength, quadriceps power, and gait speed in older U.S. adults with diabetes mellitus: results from the National Health and Nutrition Examination Survey, 1999–2002. J Am Geriatr Soc. 2013;61:769–75.
Yeung CHC, Au Yeung SL, Fong SSM, Schooling CM. Lean mass, grip strength and risk of type 2 diabetes: a bi-directional Mendelian randomisation study. Diabetologia. 2019;62:789–99.
Lee CG, Boyko EJ, Strotmeyer ES, Lewis CE, Cawthon PM, Hoffman AR, et al. Association between insulin resistance and lean mass loss and fat mass gain in older men without diabetes mellitus. J Am Geriatr Soc. 2011;59:1217–24.
Kalyani RR, Tian J, Xue QL, et al. Hyperglycemia and incidence of frailty and lower extremity mobility limitations in older women. J Am Geriatr Soc. 2012;60:1701–7.
Kalyani RR, Metter EJ, Egan J, Golden SH, Ferrucci L. Hyperglycemia predicts persistently lower muscle strength with aging. Diabetes Care. 2015;38:82–90.
Bahat G, Ilhan B. Sarcopenia and the cardiometabolic syndrome: a narrative review. Eur Geriatr Med. 2016;7:220–3.
Felber JP, Golay A. Pathways from obesity to diabetes. Int J Obesity. 2002;26:S39–45.
Srikanthan P, Hevener AL, Karlamangla AS. Sarcopenia exacerbates obesity-associated insulin resistance and dysglycemia: findings from the national health and nutrition examination survey III. PLoS ONE. 2010;5: e10805.
Granic A, Hurst C, Dismore L, Dodds RM, Witham MD, Robinson SM, et al. Advanced glycation end products in skeletal muscle health and sarcopenia: a systematic review of observational studies. Mech Aging Dev. 2022;209: 111744.
Dalal M, Ferrucci L, Sun K, Beck J, Fried LP, Semba RD. Elevated serum advanced glycation end products and poor grip strength in older community-dwelling women. J Gerontol A Biol Sci Med Sci. 2009;64:132–7.
Tabara Y, Ikezoe T, Yamanaka M, Setoh K, Segawa H, Kawaguchi T, et al. Advanced glycation end product accumulation is associated with low skeletal muscle mass, weak muscle strength, and reduced bone density: the Nagahama study. J Gerontol A Biol Sci Med Sci. 2019;74:1446–53.
Mori H, Kuroda A, Ishizu M, Ohishi M, Takashi Y, Otsuka Y, et al. Association of accumulated advanced glycation end-products with a high prevalence of sarcopenia and dynapenia in individuals with type 2 diabetes. J Diabetes Invest. 2019;10:1332–40.
Sugimoto K, Ikegami H, Takata Y, Katsuya T, Fukuda M, Akasaka H, et al. Glycemic control and insulin improve muscle mass and gait speed in type 2 diabetes: the MUSCLES-DM study. J Am Med Dir Assoc. 2021;22:834-838.e1.
Hamacher D, Liebl D, Hold C, Hessler V, Kniewasser CK, Thonnessen T, et al. Gait stability and its influencing factors in older adults. Front Physiol. 2019;9:1955.
Bailey CJ. Metformin: historical overview. Diabetologia. 2017;60:1566–76.
DrFronzo R, Fleming GA, Chen K, Bicsak TA. Metformin-associated lactic acidosis: current perspectives on causes and risk. Metabolism. 2016;65:20–9.
Palmer SC, Mavridis D, Nicolucci A, Johnson DW, Tonelli M, Craig JC, et al. Comparison of clinical outcomes and adverse events associated with glucose-lowering drugs in patients with type 2 diabetes: a meta-analysis. JAMA. 2016;316:313–24.
McCreight LJ, Bailey CJ, Pearson ER. Metformin and the gastrointestinal tract. Diabetologia. 2016;59:426–35.
Foretz M, Guigas B, Viollet B. Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nat Rev Endocrinol. 2019;15:569–89.
McCreight LJ, Mari A, Coppin L, Jackson N, Umpleby AM, Pearson ER. Metformin increases fasting glucose clearance and endogenous glucose production in non-diabetic individuals. Diabetologia. 2020;63:444–7.
Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13.
Kane DA, Anderson EJ, Price JW, Woodlief TL, Lin CT, Bikman BT, et al. Metformin selectively attenuates mitochondrial H2O2 emission without affecting respiratory capacity in skeletal muscle of obese rats. Free Radic Biol Med. 2010;49:1082–7.
Hardie DG. AMPK: positive and negative regulation, and its role in whole-body energy homeostasis. Curr Opin Cell Biol. 2015;33:1–7.
Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168:960–76.
Castets P, Lin S, Rion N, Di Fulvio S, Romanino K, Guridi M, et al. Sustained activation of mTORC1 in skeletal muscle inhibits constitutive and starvation-induced autophagy and causes a severe, late-onset myopathy. Cell Metabol. 2013;17:731–44.
Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3:1014–9.
Joseph GA, Wang SX, Jacobs CE, Zhou W, Kimble GC, Tse HW, et al. Partial inhibition of mTORC1 in aged rats counteracts the decline in muscle mass and reverses molecular signaling associated with sarcopenia. Mol Cell Biol. 2019;39(19):e00141-e219.
Gormsen LC, Sundelin EI, Jensen JB, Vendelbo MH, Jakobsen S, Munk OL, et al. In vivo imaging of human 11C-metformin in peripheral organs: dosimetry, biodistribution, and kinetic analyses. J Nucl Med. 2016;57:1920–6.
Kim SA, Choi HC. Metformin inhibits inflammatory response via AMPK-PTEN pathway in vascular smooth muscle cells. Biochem Biophys Res Commun. 2012;425:866–72.
Gu J, Ye S, Wang S, Sun W, Hu Y. Metformin inhibits nuclear factor-KB activation and inflammatory cytokines expression induced by high glucose via adenosine monophosphate-activated protein kinase activation in rat glomerular mesangial cells in vitro. Chin Med J. 2014;127:1755–60.
Andrews M, Soto N, Arredondo M. Effect of metformin on the expression of tumor necrosis factor-alpha, toll like receptors 2/4 and C reactive protein in obese type-2 diabetic patients. Rev Med Chil. 2012;140:1377–82.
Li DJ, Huang F, Lu WJ, Jiang GJ, Deng YP, Shen FM. Metformin promotes irisin release from murine skeletal muscle independently of AMP-activated protein kinase activation. Acta Physiol (Oxf). 2015;213:711–21.
Fielder E, Wan T, Alimohammadiha G, Ishaq A, Low E, Weigand M, et al. Short senolytic or senostatic interventions rescue progression of radiation-induced frailty and premature ageing in mice. Elife. 2022;11: e75492.
Lyu Q, Wen Y, He B, Zhang X, Chen J, Sun Y, et al. The ameliorating effects of metformin on disarrangement ongoing in gastrocnemius muscle of sarcopenic and obese sarcopenic mice. Biochim Biophys Acta. 2022;1868: 166508.
Wu H, Esteve E, Tremaroli V, Khan MT, Caesar R, Manneras-Holm L, et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. 2017;23:850–8.
Prattichizzo F, Giuliani A, Mensà E, Sabbatinelli J, De Nigris V, Rippo MR, et al. Pleiotropic effects of metformin: shaping the microbiome to manage type 2 diabetes and postpone ageing. Ageing Res Rev. 2018;48:87–98.
Kang MJ, Moon JW, Lee JO, Kim JH, Jung EJ, Kim SJ, et al. Metformin induces muscle atrophy by transcriptional regulation of myostatin via HDAC6 and FoxO3a. J Cachexia Sarcopenia Muscle. 2022;13:605–20.
Lee CG, Boyko EJ, Barrett-Connor E, Miljkovic I, Hoffman AR, Everson-Rose SA, et al. Insulin sensitizers may attenuate lean mass loss in older men with diabetes. Diabetes Care. 2011;34:2381–6.
Lee CG, Schwartz AV, Yaffe K, Hillier TA, LeBlanc ES, Cawthon PM. Changes in physical performance in older women according to presence and treatment of diabetes mellitus. J Am Geriatr Soc. 2013;61:1872–8.
Laksmi PW, Setiati S, Tamin TZ, Soewondo P, Rochmah W, Nafrialdi N, et al. Effect of metformin on handgrip strength, gait speed, myostatin serum level, and health-related quality of life: a double blind randomized controlled trial among non-diabetic pre-frail elderly patients. Acta Med Indones. 2017;49:118–27.
Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–9.
Walton RG, Dungan CM, Long DE, Tuggle SC, Kosmac K, Peck BD, et al. Metformin blunts muscle hypertrophy in response to progressive resistance exercise training in older adults: a randomized, double-blind, placebo-controlled, multicenter trial: the MASTERS trial. Aging Cell. 2019;18: e13039.
Konopka AR, Laurin JL, Schoenberg HM, Reid JJ, Castor WM, Wolff CA, et al. Metformin inhibits mitochondrial adaptations to aerobic exercise training in older adults. Aging Cell. 2019;18: e12880.
Espinoza SE, Musi N, Wang CP, Michalek J, Orsak B, Romo T, et al. Rationale and study design of a randomized clinical trial of metformin to prevent frailty in older adults with prediabetes. J Gerontol A Med Sci Biol Sci. 2020;75:102–9.
Rennie KJ, Witham M, Bradley P, Clegg A, Connolly S, Hancock HC, et al. MET-PREVENT: metformin to improve physical performance in older people with sarcopenia and physical prefrailty/frailty – protocol for a double-blind, randomised controlled proof-of-concept trial. BMJ Open. 2022;12: e061823.
Quianzon CCL, Cheikh IE. History of current non-insulin medications for diabetes mellitus. J Commun Hosp Intern Med Perspect. 2012;2:19081.
Seino S, Sugawara K, Yokoi N, Takahashi H. β-Cell signalling and insulin secretagogues: a path for improved diabetes therapy. Diabetes Obes Metab. 2017;19(Suppl 1):22–9.
Proks P, Reimann F, Green N, Gribble F, Ashcroft F. Sulfonylurea stimulation of insulin secretion. Diabetes. 2002;51(Suppl 3):S368–76.
Nichols CG. Personalised therapeutics for KATP-dependent pathologies. Ann Rev Pharmacol Toxicol. 2023;63:541–63.
Hu S, Wang S, Fanelli B, Bell PA, Dunning BE, Geisse S, et al. Pancreatic beta-cell K(ATP) channel activity and membrane-binding studies with nateglinide: a comparison with sulfonylureas and repaglinide. J Pharmacol Exp Therap. 2000;293:444–52.
Tricarico D, Selvaggi M, Passantino G, De Palo P, Dario C, Centoducati P, et al. ATP sensitive potassium channels in the skeletal muscle function: involvement of the KCNJ11(Kir6.2) gene in the determination of mechanical Warner Bratzer shear force. Front Physiol. 2016;7:167.
Cetrone M, Mele A, Tricarico D. Effects of the antidiabetic drugs on the age-related atrophy and sarcopenia associated with diabetes type II. Curr Diabetes Rev. 2014;10:231–7.
Pulido N, Romero R, Suarez AI, Rodriguez E, Casanova B, Rovira A. Sulfonylureas stimulate glucose uptake through GLUT4 transporter translocation in rat skeletal muscle. Biochem Biophys Res Commun. 1996;228:499–504.
Mesinovic J, Scott D, Seibel MJ, Cumming RG, Naganathan V, Blyth FM, et al. Risk factors for incident falls and fractures in older men with and without type 2 diabetes mellitus: the Concord Health and Ageing in Men Project. J Gerontol A Biol Sci Med Sci. 2021;76:1090–100.
Moreland JD, Richardson JA, Goldsmith CH, Clase CM. Muscle weakness and falls in older adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2004;52:1121–9.
Schoonjans K, Auwerx J. Thiazolidinediones: an update. Lancet. 2000;355:1008–10.
Bajaj M, Baig R, Suraamornkul S, Hardies LJ, Coletta DK, Cline GW, et al. Effects of pioglitazone on intramyocellular fat metabolism in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2010;95:1916–23.
Sanchis-Gomar F, Pareja-Galeano H, Martinez-Bello VE. PPAR-gamma agonist pioglitazone does not enhance performance in mice. Drug Test Anal. 2014;6:922–9.
Rabol R, Boushel R, Almdal T, Hansen CN, Ploug T, Haugaard SB, et al. Opposite effects of pioglitazone and rosiglitazone on mitochondrial respiration in skeletal muscle of patients with type 2 diabetes. Diabetes Obes Metab. 2010;12:806–14.
Coletta DK, Sriwijitkamol A, Wajcberg E, Tantiwong P, Li M, Prentki M, et al. Pioglitazone stimulates AMP-activated protein kinase signalling and increases the expression of genes involved in adiponectin signalling, mitochondrial function and fat oxidation in human skeletal muscle in vivo: a randomised trial. Diabetologia. 2009;52:723–32.
Fiorentino TV, Monroy A, Kamath S, Sotero R, Cas MD, Daniele G, et al. Pioglitazone corrects dysregulation of skeletal muscle mitochondrial proteins involved in ATP synthesis in type 2 diabetes. Metabolism. 2021;114: 154416.
Bajpeyi S, Pasarica M, Conley KE, Newcomer BR, Jubrais SA, Gamboa C, et al. Pioglitazone-induced improvements in insulin sensitivity occur without concomitant changes in muscle mitochondrial function. Metabolism. 2017;69:24–32.
Shea MK, Nicklas BJ, Marsh AP, Houston DK, Miller GD, Isom S, et al. The effect of pioglitazone and resistance training on body composition in older men and women undergoing hypocaloric weight loss. Obesity (Silver Spring). 2011;19:1636–46.
Marsh AP, Shea MK, Vance Locke RM, Miller ME, Isom S, Miller GD, et al. Resistance training and pioglitazone lead to improvements in muscle power during voluntary weight loss in older adults. J Gerontol A Biol Sci Med Sci. 2013;68:828–36.
Liao HW, Saver JL, Wu YL, Chen TH, Lee M, Ovbiagele B. Pioglitazone and cardiovascular outcomes in patients with insulin resistance, pre-diabetes and type 2 diabetes: a systematic review and meta-analysis. BMJ Open. 2017;7: e013927.
Viscoli CM, Inzucchi SE, Young LH, Insogna KL, Conwit R, Furie KL, et al. Pioglitazone and risk for bone fracture: safety data from a randomized clinical trial. J Clin Endocrinol Metab. 2017;102:914–22.
Nauck MA, Meier JJ. Incretin hormones: their role in health and disease. Diabetes Obes Metab. 2018;20(Suppl 1):5–21.
Erdogdu O, Nathanson D, Sjoholm A, Nystrom T, Zhang Q. Exendin-4 stimulates proliferation of human coronary artery endothelial cells through eNOS-, PKA- and PI3K/Akt-dependent pathways and requires GLP-1 receptor. Mol Cell Endocrinol. 2010;325:26–35.
Egan JM, Montrose-Rafizadeh C, Wang Y, Bernier M, Roth J. Glucagon-like peptide-1(7–36) amide (GLP-1) enhances insulin-stimulated glucose metabolism in 3T3-L1 adipocytes: one of several potential extrapancreatic sites of GLP-1 action. Endocrinology. 1994;135:2070–5.
Sjoberg KA, Holst JJ, Rattigan S, Richter EA, Kiens B. GLP-1 increases microvascular recruitment but not glucose uptake in human and rat skeletal muscle. Am J Physiol Endocrinol Metab. 2014;306:E355–62.
Wang N, Tan AWK, Jahn LA, et al. Vasodilatory actions of glucagon-like peptide 1 are preserved in skeletal and cardiac muscle microvasculature but not in conduit artery in obese humans with vascular insulin resistance. Diabetes Care. 2019;43:634–42.
Nystrom T, Gutniak MK, Zhang Q, et al. Effects of glucagonlike peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrinol Metab. 2004;287:E1209–15.
Chai W, Fu Z, Aylor KW, Barrett EJ, Liu Z. Liraglutide prevents microvascular insulin resistance and preserves muscle capillary density in high-fat diet-fed rats. Am J Physiol Endocrinol Metab. 2016;311:E640–8.
Khin PP, Hong Y, Yeon MH, Lee DH, Lee JH, Jun HS. Dulaglutide improves muscle function by attenuating inflammation through OPA-1-TLR-9 signaling in aged mice. Aging (Albany NY). 2021;13:21962–74.
Potts JE, Gray LJ, Brady EM, Khunti K, Davies MJ, Bodicoat DH. The effect of glucagon-like peptide 1 receptor agonists on weight loss in type 2 diabetes: a systematic review and mixed treatment comparison meta-analysis. PLoS ONE. 2015;10: e0126769.
Sargeant JA, Henson J, King JA, Yates T, Khunti K, Davies MJ. A review of the effects of glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter 2 inhibitors on lean body mass in humans. Endocrinol Metab. 2019;34:247–62.
Margulies KB, Hernandez AF, Redfield MM, Givertz MM, Oliveira GH, Cole R, et al. Effects of liraglutide on clinical stability among patients with advanced heart failure and reduced ejection fraction. JAMA. 2016;216:500–8.
Cava E, Yeat NC, Mittendorfer B. Preserving healthy muscle during weight loss. Adv Nutr. 2017;8:511–9.
Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 30 mg of liraglutide in weight management. N Engl J Med. 2015;373:11–22.
Wagner AM, Miranda-Calderin G, Ugarte-Lopetegui MA, Marrero-Santiago H, Suarez-Castellano L, Lopez-Madrazo MJ, et al. Effect of liraglutide on physical performance in type 2 diabetes: results of a randomized, double-blind, controlled trial (LIPER2). Diabetes Metab. 2019;45:268–75.
Holst JJ, Deacon CF. Inhibition of the activity of dipeptidyl-peptidase IV as a treatment for type 2 diabetes. Diabetes. 1998;47:1663–70.
Liu Y, Xu F, Jiang P. Effect of sitagliptin on expression of skeletal muscle peroxisome proliferator-activated receptor γ coactivator-1 α and irisin in a rat model of type 2 diabetes mellitus. J Int Med Res. 2020;48:300060519885569.
Rizzo MR, Barbieri M, Fava I, Desiderio M, Coppola C, Marfella R, Paolisso G. Sarcopenia in elderly diabetic patients: role of dipeptidyl peptidase 4 inhibitors. J Am Med Dir Assoc. 2016;17:896–901.
Bouchi R, Fukuda T, Takeuchi T, Nakano Y, Murakami M, Minami I, et al. Dipeptidyl peptidase 4 inhibitors attenuates the decline of skeletal muscle mass in patients with type 2 diabetes. Diabetes Metab Res Rev. 2018;34: e2957.
Tarapues M, Cereza G, Figueras A. Association of musculoskeletal complaints and gliptin use: review of spontaneous reports. Pharmacoepidemiol Drug Saf. 2013;22:1115–8.
Hummel CS, Lu C, Loo DDF, Hirayama BA, Voss AA, Wright EM. Glucose transport by human renal Na+/D-glucose cotransporters SGLT1 and SGLT2. Am J Physiol Cell Physiol. 2011;300:C14–21.
Hsia DS, Grove O, Cefalu WT. An update on SGLT2 inhibitors for the treatment of diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2017;24:73–9.
Shin SJ, Chung S, Kim SJ, Lee E-M, Yoo Y-H, Kim J-W, et al. Effect of sodium-glucose co-transporter 2 inhibitor, dapagliflozin, on renal renin-angiotensin system in an animal model of type 2 diabetes. PLoS ONE. 2016;11: e0165703.
El Mahdy MK, Helal MG, Ebrahim TM. Potential anti-inflammatory effect of dapagliflozin in HCHF diet-induced fatty liver degeneration through inhibition of TNF-α, IL-1β, and IL-18 in rat liver. Int Immunopharmacol. 2020;86: 106730.
Sun X, Han F, Lu Q, Li X, Ren D, Zhang J, et al. Empagliflozin ameliorates obesity-related cardiac dysfunction by regulating sestrin2-mediated AMPK-mTOR signaling and redox homeostasis in high-fat diet-induced obese mice. Diabetes. 2020;69:1292–305.
Kondo H, Akoumianakis I, Badi I, Akawi N, Kotanidis CP, Polkinghorne M, et al. Effects of canagliflozin on human myocardial redox signalling: clinical implications. Eur Heart J. 2021;42:4947–60.
Castaneda F, Layne JE, Castaneda C. Skeletal muscle sodium glucose co-transporters in older adults with type 2 diabetes undergoing resistance training. Int J Med Sci. 2006;3:84–91.
Sano R, Shinozaki Y, Ohta T. Sodium–glucose cotransporters: functional properties and pharmaceutical potential. J Diabetes Investig. 2020;11:770–82.
Diez-Sampedro A, Hirayama BA, Osswald C, Gorboulev V, Baumgarten K, Volk C, et al. A glucose sensor hiding in a family of transporters. Proc Natl Acad Sci U S A. 2003;100:11753–8.
Naznin F, Sakoda H, Okada T, Tsubouchi H, Waise TM, Arakawa K, et al. Canagliflozin, a sodium glucose cotransporter 2 inhibitor, attenuates obesity-induced inflammation in the nodose ganglion, hypothalamus, and skeletal muscle of mice. Eur J Pharmacol. 2017;794:37–44.
Bamba R, Okamura T, Hashimoto Y, Majima S, Senmaru T, Ushigome E, et al. Extracellular lipidome change by an SGLT2 inhibitor, luseogliflozin, contributes to prevent skeletal muscle atrophy in db/db mice. J Cachexia Sarcopenia Muscle. 2022;13:574–88.
Yabe D, Shiki K, Suzaki K, Meinicke T, Kotobuki Y, Nishida K, et al. Rationale and design of the EMPA-ELDERLY trial: a randomised, double-blind, placebo-controlled, 52-week clinical trial of the efficacy and safety of the sodium-glucose cotransporter-2 inhibitor empagliflozin in elderly Japanese patients with type 2 diabetes. BMJ Open. 2021;11: e045844.
Guo Z, Wang L, Yu J, Wang Y, Yang Z, Zhou C. The role of SGLT-2 inhibitors on health-related quality of life, exercise capacity, and volume depletion in patients with chronic heart failure: a meta-analysis of randomized controlled trials. Int J Clin Pharm. 2023. https://doi.org/10.1007/s11096-022-01504-6.
Clemmons D. Involvement of insulin-like growth factor-I in the control of glucose homeostasis. Curr Opin Pharmacol. 2006;6:620–5.
Böni-Schnetzler M, Schmid C, Meier PJ, Froesch ER. Insulin regulates insulin-like growth factor I mRNA in rat hepatocytes. Am J Physiol. 1991;260:E846–51.
Brismar K, Fernqvist-Forbes E, Wahren J, Hall K. Effect of insulin on the hepatic production of insulin-like growth factor-binding protein-1 (IGFBP-1), IGFBP-3, and IGF-I in insulin-dependent diabetes. J Clin Endocrinol Metab. 1994;79:872–8.
Roy D, Perreault M, Marette A. Insulin stimulation of glucose uptake in skeletal muscles and adipose tissues in vivo is NO dependent. Am J Physiol Endocrinol Metab. 1998;274:E692–9.
Hatting M, Tavares CDJ, Sharabi K, Rines AK, Puigserver P. Insulin regulation of gluconeogenesis. Ann N Y Acad Sci. 2018;1411:21–35.
Froesch ER. The physiology and pharmacology of adipose tissue lipolysis: its inhibition and implications for the treatment of diabetes. Diabetologia. 1967;3:475–87.
Keske MA, Premilovac D, Bradley EA, Dwyer RM, Richards SM, Rattigan S. Muscle microvascular blood flow responses in insulin resistance and ageing. J Physiol. 2016;594:2223–31.
Boirie Y. Insulin regulation of mitochondrial proteins and oxidative phosphorylation in human muscle. Trends Endocrinol Metab. 2003;14:393–4.
Pereira S, Marliss EB, Morais JA, Chevalier S, Gougeon R. Insulin resistance of protein metabolism in type 2 diabetes. Diabetes. 2008;57:56–63.
Denne SC, Brechtel G, Johnson A, Liechty EA, Baron AD. Skeletal muscle proteolysis is reduced in noninsulin-dependent diabetes mellitus and is unaltered by euglycemic hyperinsulinemia or intensive insulin therapy. J Clin Endocrinol Metab. 1995;80:2371–7.
Ferrari U, Then C, Rottenkolber M, Selte C, Seissler J, Conzade R, et al. Longitudinal association of type 2 diabetes and insulin therapy with muscle parameters in the KORA-Age study. Acta Diabetol. 2020;57:1057–63.
Gin H, Rigalleau V, Perlemoine C. Insulin therapy and body weight, body composition and muscular strength in patients with type 2 diabetes mellitus. J Nutr Metab. 2010;2010: 340570.
Rosenfalck AM, Almdal T, Hilsted J, Madsbad S. Body composition in adults with Type 1 diabetes at onset and during the first year of insulin therapy. Diabet Med. 2002;19:417–23.
Sinha A, Formica C, Tsalamandris C, Panagiotopoulos S, Hendrich E, DeLuise M, et al. Effects of insulin on body composition in patients with insulin-dependent and non-insulin-dependent diabetes. Diabet Med. 1996;13:40–6.
Teo YH, Teo YN, Syn NL, Kow CS, Yoong CSY, Tan BYQ, et al. Effects of sodium/glucose cotransporter2 (SGLT2) inhibitors on cardiovascular and metabolic outcomes in patients without diabetes mellitus: a systematic review and meta-analysis of randomized-controlled trials. J Am Heart Assoc. 2021;10: e019463.
Pontiroli AE, Miele L, Morabito A. Increase of body weight during the first year of intensive insulin treatment in type 2 diabetes: systematic review and meta-analysis. Diabetes Obes Metab. 2011;13:1008–19.
Pittampalli S, Upadyayula S, Mekala HM, Lippmann S. Risks vs benefits for SGLT2 inhibitor medications. Fed Pract. 2018;35:45–8.
Ripamonti E, Azoulay L, Abrahamowicz M, Platt RW, Suissa S. A systematic review of observational studies of the association between pioglitazone use and bladder cancer. Diabet Med. 2019;36:22–35.
Tang H, Shi W, Fu S, Wang T, Zhai S, Song Y, et al. Pioglitazone and bladder cancer risk: a systematic review and meta-analysis. Cancer Med. 2018;7:1070–80.
Brown E, Heerspink HJL, Cuthbertson DJ, Wilding JPH. SGLT2 inhibitors and GLP-1 receptor agonists: established and emerging indications. Lancet. 2021;398:262–76.
Baigent C, Emberson JR, Haynes R, Herrington WG, Judge P, Landray MJ, et al. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet. 2022;400:1788–801.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No specific funding was used to conduct this review. MDW, AG, SMR and AAS acknowledge support from the National Institute for Health and Care Research (NIHR) Newcastle Biomedical Research Centre.
Conflicts of Interest
MDW and AAS are investigators on the MET-PREVENT trial of metformin for sarcopenia.
Availability of Data and Material
Data sharing not applicable to this article, as no datasets were generated or analysed during the current study.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Code Availability
Not applicable.
Author Contributions
Conception: MDW and AAS. First draft: MDW. Critical revision and redrafting: AAS, AG, EP and SMR. All authors have read and approve the final version of the manuscript, and agree to be accountable for the work.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Witham, M.D., Granic, A., Pearson, E. et al. Repurposing Drugs for Diabetes Mellitus as Potential Pharmacological Treatments for Sarcopenia – A Narrative Review. Drugs Aging 40, 703–719 (2023). https://doi.org/10.1007/s40266-023-01042-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40266-023-01042-4