Abstract
Background
Older people living with HIV (PLWH) are at increased risks of co-morbidities and polypharmacy. However, little is known about factors affecting their needs and concerns about medicines. This systematic review aims to describe these and to identify interventions to improve medicine optimisation outcomes in older PLWH.
Methods and Data Sources
Multiple databases and grey literature were searched from inception to February 2022 including MEDLINE, CINAHL, PsycInfo, PsychArticles, the Cochrane Database of Systematic Reviews and the Cochrane Controlled Register of Trials, Abstracts in Social Gerontology, and Academic Search Complete.
Eligibility Criteria
Studies reporting interventions/issues affecting older PLWH (sample populations with mean/median age ≥ 50 years; any aspect of medicine optimisation, or concerns). Quality assessments were completed by means of critical appraisal checklists for each study design. Title and abstract screening was led by one reviewer and a sample reviewed independently by two reviewers. Full-paper reviews were completed by one author and a 20% sample was reviewed independently by two reviewers.
Synthesis
Data were extracted by three independent reviewers using standardised data extraction forms and synthesised according to outcomes or interventions reported. Data were summarised to include key themes, outcomes or concerns, and summary of intervention.
Results
Seventy-nine (n = 79) studies met the eligibility criteria, most of which originated from the USA (n = 36). A few studies originated from Australia (n = 5), Canada (n = 5), Spain (n = 9), and the UK (n = 5). Ten studies originated from Sub-Saharan Africa (Kenya n = 1, South Africa n = 6, Tanzania n = 1, Uganda n = 1, Zimbabwe n = 1). The rest of the studies were from China (n = 1), France (n = 1), Germany (n = 1), Italy (n = 1), the Netherlands (n = 1), Pakistan (n = 1), Switzerland (n = 1), Saudi Arabia (n = 1) and Ukraine (n = 1). Publication dates ranged from 2002 to 2022. Sample sizes ranged from 10 to 15,602 across studies. The factors affecting older PLWH’s experience of and issues with medicines were co-morbidities, health-related quality of life, polypharmacy, drug interactions, adverse drug reactions, adherence, medicine burden, treatment burden, stigma, social support, and patient-healthcare provider relationships. Nine interventions were identified to target older persons, five aimed at improving medication adherence, two to reduce drug interactions, and two for medicine self-management initiatives.
Conclusion
Further in-depth research is needed to understand older PLWH’s experiences of medicines and their priority issues. Adherence-focused interventions are predominant, but there is a scarcity of interventions aimed at improving medicine experiences for this population. Multi-faceted interventions are needed to achieve medicine optimisation outcomes for PLWH.
Trial Registration
This study is registered with PROSPERO registration number: CRD42020188448.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Older people living with HIV have various factors that impact on their needs and concerns about medicines (e.g., polypharmacy, treatment burden, adherence support, stigma, and social support). |
Only a few interventions have been developed to improve medicines optimisation outcomes, but most of these are adherence-focussed. |
There is a need for further studies to better understand the needs and concerns of older people living with HIV in the UK about their medicines and to design multi-faceted interventions to reflect these. |
1 Introduction
Globally, about 38 million people continue to live with HIV [1]. Advances in treatment have transformed HIV into a complex chronic condition [2] and more people living with HIV (PLWH) have a near-normal life expectancy [3]. New diagnoses among PLWH over the age of 50 years are on the rise [4]. In the UK, nearly two-thirds (65%) of late HIV diagnoses were among those aged ≥ 65 years [5]. It is estimated that by 2030 nearly 75% of all PLWH will be 50 years or older, with the median age expected to increase gradually over the years [6]. A number of age cut-offs have been used to define older PLWH, ranging from 45 to 55 years old with 50 years and older used frequently across most literature [4, 7, 8]. For the purposes of this review, an ‘older person’ will include anyone aged 50 years or older.
Ageing within the context of HIV is associated with multimorbidity and polypharmacy [9, 10]. Polypharmacy has widely been defined as the use of five or more medicines, [11] and is linked to adverse health outcomes [12]. A recent multinational patient survey conducted in 24 countries including North America, Europe, Australia and China (n = 2112), reported a significantly higher level of polypharmacy among older PLWH (54.6%) compared with younger participants (36.5%, p < 0.001) [10]. The survey also found that people experiencing polypharmacy used an average of 6.5 pills per day, and willingness to change antiretroviral (ARV) regimens to those with a fewer number of medicines was significantly higher among older adults (79.9%) than those under 50 years old (70.1%, p < 0.001) [10]. An earlier study on older PLWH found that participants were taking a median of 13 (range 9–17) medicines, of which eight (range 4–14) were non-ARV medicines [13]. Polypharmacy is associated with regimen complexity, medicine burden, lower treatment satisfaction, potential drug–drug interactions (PDDIs), adverse drug reactions (ADRs), hospitalisation, non-adherence, and contributes to poor health outcomes [9, 10, 12, 14, 15]. A study investigating polypharmacy among PLWH found a correlation between the number of non-ARV medicines used and adverse health outcomes in older individuals [15]. There is a need to understand treatment experiences of older PLWH.
NICE guidelines define medicines optimisation, as “a person-centred approach to safe and effective medicines use, to ensure people obtain the best possible outcomes from their medicines”, which is fundamental in tackling the challenges presented by polypharmacy among older adults [16]. Medicine experiences are the summation of events involving drug therapy that one has encountered in their lives [17]. According to the UK’s Royal Pharmaceutical Society, medicines optimisation aims to understand patients’ experiences and to improve patient outcomes from a holistic perspective [18]. Across the literature, medicines optimisation is implemented through various interventions including medicine reviews [19], deprescribing [14], medicine reconciliations [11, 19], identifying potentially inappropriate prescribing (PIP) [19, 20], providing social support, and increasing antiretroviral therapy (ART) adherence [11]. A recent systematic review of interventions for frail older persons focused on medicines optimisation in secondary care settings [21], but was not specific to PLWH. Moreover, little is known about medicines optimisation interventions targeted at older PLWH. In some studies, older PLWH have reported concerns around stigma. Older PLWH may experience stigma twofold due to HIV-positive status and ageing [22]. It is therefore vital to understand the needs and concerns of older PLWH and to investigate interventions aimed at improving medicines optimisation outcomes for this population.
The aim of this review was to investigate medicines optimisation needs and interventions for older PLWH. The specific objectives were to determine: (a) the priority issues and concerns of older PLWH about their medicines, and (b) the types of medicines optimisation interventions developed for older PLWH, how they are implemented, and their effectiveness.
2 Methods
The systematic review was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. The review was registered with the international Prospective Register of Systematic Reviews PROSPERO prior to data abstraction (CRD42020188448) [23].
2.1 Study Eligibility—Inclusion and Exclusion Criteria
We included various study designs, including but not limited to randomised, controlled trials (RCTs), before and after experimental studies (controlled and non-controlled), observational studies (cohort studies, case-control, cross-sectional surveys), qualitative research studies, and retrospective and prospective reviews of prescription and/or dispensing records. Case reports and case series were excluded regardless of age composition. Service evaluations and audits conducted to improve medicine-related outcomes in a specific health facility or organisation were excluded. We included studies composed of HIV-positive older adults as the main participants or where the vast majority of participants were of mean/median age 50 years or older. Studies focusing on other age groups besides older persons were excluded (i.e., children, adolescents and younger adults under the age of 50 years). Abstracts did not always report participants’ age, and therefore extra screening of full texts was done to determine if studies met the age eligibility criterion. For studies not reporting the mean/median age of participants within the abstract, the full text was reviewed to ascertain age composition of participants [see Online Supplementary Material (OSM): Appendix 1]. Studies of HIV-negative older adults were excluded from the review. Studies relating to any aspect of medicines optimisation, medicine reviews, medicine reconciliation, deprescribing, or strategies being undertaken to support older PLWH with safe and effective use of ART and/or non-HIV medicines were included. Studies that did not discuss any aspect of medicines optimisation or issues relating to medicines experience or that concerned older persons’ needs in relation to their medicines were also excluded. The search was limited to studies published in English.
2.2 Information Sources and Search Strategy
A range of electronic databases were searched from date of inception to February 2022. We searched MEDLINE, CINAHL, PsycInfo, PsychArticles, the Cochrane Database of Systematic Reviews, the Cochrane Controlled Register of Trials, Abstracts in Social Gerontology, and Academic Search Complete. We also searched grey literature via OpenGreyTM, including doctoral theses, research reports and other publications. We searched reference lists of included studies and relevant systematic reviews to identify additional studies. A digital referencing manager, Zotero (5.0.89), was used to manage all searches and to remove duplicates. To answer the research question, our Population Intervention Comparison Outcome (PICO) search strategy [24] included key words to maximise our ability to find relevant articles (OSM: Appendix 2). Examples of search terms used include: HIV, AIDS, ageing/aging, older/elderly, medicines, antiretrovirals, HAART/ART, optimis*, intervention, pharmaceutical, medicine-related problems, concerns, needs, issues, outcome. A full list of search terms is provided in the OSM (Appendix 3). The same search strategy was adapted for all databases, with minor changes to the wildcard symbols and truncations for searching different words with similar prefixes.
2.3 Selection of Studies
Titles were screened for eligibility by one author (PS). Abstracts and full texts were then independently reviewed by three authors (PS, RC, BK) using pre-specified screening criteria (OSM: Appendix 4). Each study was then categorised into: ‘definitely include’, ‘possibly include’ and ‘definitely exclude’. Full texts for all studies in the ‘definitely include’ and ‘possibly include’ categories were retrieved for assessment against eligibility criteria by PS and then a sample (20%) independently reviewed (RC, SC, BK) [25]. Disagreements at any stage of screening were resolved through discussions among the research team.
2.4 Data Extraction, Synthesis Methods, and Risk of Bias Assessment
Data from eligible articles were extracted using a standardised data extraction form (OSM: Appendix 5). One reviewer (PS) led data extraction and a sample of the results (20%) were independently reviewed by two reviewers (RC, SC). Discrepancies in data extracted were resolved by discussion and consensus among the research team.
Synthesis- research papers were categorised thematically (e.g., polypharmacy, treatment burden, medicine burden, adherence) and by the interventions reported (OSM: Appendix 5, Part 2).
The Critical Appraisal Skills Programme (CASP) checklist and the Appraisal tool for Cross-Sectional Studies (AXIS) were used to assess the risk of bias in and quality of the studies included in the final pool. Specific checklists were used as appropriate for each study design. Any disagreements were resolved through discussion with the team. Each question in the appraisal tool was graded as 1 or 0 for meeting or not meeting predefined criteria, respectively; scores and percentages were then calculated to assess overall quality. Overall, studies achieving 0–49% were defined as poor quality, 50–69% were fair quality and 70–100% were of excellent quality.
3 Results
3.1 Study Selection
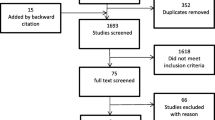
The search identified 26,154 articles from electronic databases and three articles from grey literature. After duplicate removal, 21,350 articles were title screened, of which 1305 were found to be eligible for abstract screening. Of the 1305 abstracts, 768 full texts were searched to determine whether they met the age criterion. 139 remaining articles were then assessed for other eligibility criteria, of which 60 articles were excluded due to either the study not having outcomes of interest (n = 17), the trial being discontinued (n = 1), incomplete study/no results (n = 30), and unable to access due to journal restrictions (n = 12). Overall, 79 (n = 79) studies were included in this review (Fig. 1).
3.2 Study Characteristics
The review included 46 cross-sectional studies, 20 qualitative studies, five cohort studies, four RCTs, and four mixed-methods studies. Overall, all articles included were of excellent quality (70–100%). The mean score for the cross-sectional studies was 84% (range 73–90%) based on the AXIS quality assessments. Mean quality scores of 91% (range 70–100%), 91% and 79% (range 71–93%) were obtained for qualitative studies, RCTs and cohort studies, respectively.
The 79 studies that met the inclusion criteria were largely from the USA (n = 36). A few studies originated from Australia (n = 5), Canada (n = 5), Spain (n = 9) and the UK (n = 5). Ten studies originated from Sub-Saharan Africa (Kenya n = 1, South Africa n = 6, Tanzania n = 1, Uganda n = 1 and Zimbabwe n = 1). The rest of the studies were from China (n = 1), France (n = 1), Germany (n = 1), Italy (n = 1), the Netherlands (n = 1), Pakistan (n = 1), Switzerland (n = 1), Saudi Arabia (n = 1) and Ukraine (n = 1). Publication dates ranged from 2002 to 2022. Sample sizes ranged from 10 to 15,602 across individual studies.
3.3 Issues Affecting Older People Living with HIV (PLWH)
The studies reviewed showed a wide range of issues affecting older PLWH that impacted on their needs for and experiences of using medicines including co-morbidities, polypharmacy, drug interactions, adverse drug reactions, adherence, stigma, medicine burden, treatment burden, health-related quality of life (HRQOL), and patient and healthcare provider relationships (Table 1).
3.3.1 Co-morbidities and Health-Related Quality of Life (HRQOL)
Most older PLWH are affected by multiple co-morbidities [14, 26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63]. One study reported 93% of participants having one or more co-morbidities, with a mean of 3.2 conditions per person [30]. Similarly, other studies have also shown the mean number of co-morbidities to range from 2.4 to 3.6 [39, 40, 42].
Older PLWH are more likely to be affected by anxiety, depression, cancer, chronic kidney disease (CKD), chronic pain, diabetes, hypertension, osteoporosis, hepatitis B/C infections than HIV-negative individuals [31, 32, 34, 36, 39, 42,43,44,45, 47, 51, 55, 58, 61]. A study comparing HIV-positive older males to their HIV-negative counterparts found that older participants were six times more likely to be diagnosed with osteoporosis [31]. Similarly, cardiovascular disease (CVD) and CKD are more common in older males living with HIV [31]. Other studies indicate a higher incidence of mental conditions in PLWH over the age of 50 years [30, 33, 36, 37, 43, 44, 64, 65]. One study [43] reported over half (58%) of participants experiencing depression, with a greater proportion having received treatment for depression in the year prior to the study. Poorer health outcomes have been associated with depression in ageing PLWH [66], and depression has also been linked non-adherence behaviour [66]. The duration since HIV diagnosis and time since ART initiation are predictors of co-morbidity, and both factors are associated with frailty [36, 40]. Frailty is linked to higher mortality rates among older PLWH, and its incidence increases with age, presence of co-morbidities, falls and disability [27, 28, 67]. The presence of one or more co-morbidities also increases the risk of falls in PLWH [50]. Among older PLWH, poor HRQOL is associated with co-morbidities, especially AIDS-defining illnesses, longer time since ART initiation, loneliness and a lower level of education [26, 54, 62, 65, 68,69,70]. A study focusing on women living with HIV showed that physical HRQOL was lower in older participants than in younger age groups [69]. Older PLWH are also more likely to have poor mental HRQOL than patients with other non-HIV conditions [26].
Concerns about co-morbidities and ageing have been reported by older PLWH in the qualitative studies reviewed [27, 41, 71]. One older participant expressed their frustration of having to manage HIV, ageing and co-morbidities, particularly when they do not know which is the cause of a health issue they are experiencing [41]. Another participant reported not knowing if they were tired due to getting older, their co-morbidities or side effects of their medicines [71]. Many PLWH who were diagnosed in the 1990s or just after the 2000s do not view HIV as their most pressing concern, but rather prioritise co-morbidities—their HIV is perceived to be under control due to adherence to ART [55].
3.3.2 Polypharmacy, Drug Interactions, and Adverse Drug Reactions
3.3.2.1 Polypharmacy
The average number of concomitant medicines used by older PLWH varies [13, 14, 32, 33, 38,39,40, 49, 52, 61, 70, 72,73,74]. Several studies have found that older PLWH experience more polypharmacy than HIV-negative participants [14, 29, 40, 72, 75]. One study reported 35% of participants received 16 or more medicines and 16% were using ≥ 20 long-term medicines. Two studies reported that 66% of participants experienced polypharmacy even when ARVs were excluded from the analysis [13, 20]. Co-morbidities are expectedly a precipitating factor for polypharmacy in older PLWH [38], and some studies have demonstrated a positive correlation between the number of conditions and the number of concomitant medicines used (p < 0.0001) [40]. Cardiovascular and gastrointestinal medicines were identified as the most common classes of concomitant medicines used by older PLWH in several studies [13, 14, 39, 40, 44, 52, 72]. Similarly, another study identified 667 potentially inappropriate medicines in 482 older PLWH with 60.8% (n = 293) involving benzodiazepines, 27.2% (n = 131) involving non-steroidal anti-inflammatory drugs, and 14.9% (n = 72) involving anticholinergic drugs [20].
3.3.2.2 Drug interactions
With higher prevalence of polypharmacy, drug interactions among older PLWH are common, including those occurring between ARV and non-ARV medicines, or between two or more non-ARV medicines [13, 14, 29, 31, 38, 40, 41, 44, 46, 48, 61, 64, 67, 71, 74,75,76,77]. A large number of drug–drug interactions have been identified in the ageing HIV population, as demonstrated in several studies reviewed, with up to 2552 potential drug–drug interactions (PDDIs) being identified in 1947 participants [13, 14, 29, 44, 61, 77]. A study comparing PDDIs between older and younger PLWH showed that a significantly higher proportion of older PLWH had at least one PDDI consisting of both an ARV and a non-ARV combination than those in the younger group (i.e., 913 compared to 201 potential interactions, respectively, p = 0.001) [14]. Moreover, older PLWH had a larger number of interactions that were classified as “Do not co-administer” than the older HIV-negative or younger HIV-positive groups [14].
In a study using the Beers criteria for PIP, the authors reported one or more potentially inappropriate medicines in half (52%) of all participants [13]. The most frequent drugs implicated were testosterone (n = 20), ibuprofen (n = 15), zolpidem (n = 9) and lorazepam (n = 5) [13]. Other studies measuring drug–drug interactions have listed the same drug groups, among others, as interacting with ART [13, 14, 29, 38, 40, 44, 49, 61, 73, 77]. PLWH were more likely to have a high anticholinergic burden, with 17% having a score of ≥ 3, in contrast to 4% seen among HIV-negative participants [13]. One study reported that statins have a high involvement in severe interactions (24%), followed by inhaled corticosteroids (15%) [77]. One study showed that 80% of participants were prescribed at least one sedating medicine, and the risk for a non-fatal overdose to occur in the past year was higher with each additional sedating medicine and non-ARV medicine, but this association was only marginally significant (p = 0.048) [33]. Another study assessing PIP and therapeutic complexity in older PLWH found at least one medication to deprescribe based on the STOPP criteria or the list of evidence-based deprescribing for chronic patients (LESS-CHRON) criteria in 70% of participants [49]. Meeting STOPP or LESS-CHRON criteria was significantly associated with the number of concomitant prescriptions (p = 0.008) [49].
Similarly, another study assessed the incidence and severity of drug interactions before and after ART simplification. In this study, the authors reported lower rates of PDDIs after regimen simplification, with median interaction scores being 3 (range 1–6) and 1 (range 0–2), respectively [29]. Moreover, this study showed that their participants were taking a median of three ARV pills a day, and discontinuing a protease inhibitor during ART simplification was a strong predictor for lowering the number of PDDIs [29]. Another study found that 70% of older participants had at least one drug–drug interaction that was categorised as ‘consider therapy modification’ and 11% had an interaction that was categorised as ‘avoid combination’ [13].
3.3.2.3 Adverse Drug Reactions
Adverse drug reactions such as cognitive impairment, delirium, dry mouth and constipation have all been reported among older PLWH [13, 20]. A study led by pharmacists in the USA to evaluate and reduce PIP identified an increase of 85% for the likelihood of an adverse drug event occurring with each inappropriate prescribing that occurs in older PLWH [38].
A mixed methods study with older African-American PLWH highlighted the effects caused by adverse drug reactions, with one participant explaining the stress caused by drug toxicity associated with her medicines on her body over time: “Even though I’m taking medicine to keep my immune system up and everything like that, it is still somewhat ‘wear and tear’ on my body” [64]. Long-term ART toxicity has been linked to early onset and/or a higher incidence of frailty among PWLH [67]. This uncertainty of adverse outcomes makes it difficult for older PLWH to predict their future care needs [27, 41]. In addition, physical changes experienced by PLWH relating to the condition, treatments and side effects can increase stigma levels and treatment burden, affect mental health and lower quality of life [26, 78].
3.3.3 Adherence
Several studies showed that older PLWH were more adherent to their ART than younger PLWH [32, 49, 54, 69, 76, 79,80,81,82]. Two studies found older women living with HIV were more likely to be adherent to ART, and subsequently be virally suppressed [69, 79]. McInnes et al. demonstrated that increasing age was associated with higher medicine adherence for PLWH. PLWH aged 55–64 years and those ≥ 65 years were twice or three times as likely to adhere than those under 45 years [81]. Two studies reported high adherence rates for ART among older PLWH but low adherence when taking their concomitant medicines [39, 44]. A study comparing adherence rates in older PLWH taking single-tablet or multiple-tablet ART regimens found that patients on a single-tablet regimen had significantly greater adherence than patients on a multiple-tablet regimen (p < 0.001) [83]. Simplifying medicine regimens can increase adherence in older PLWH [37, 48].
Several factors may explain higher rates of adherence to ART among older people, particularly longevity. It is likely that living longer allows older people to be better at taking their medicines throughout life and subsequently continue to live longer. Living longer with HIV may lead to many older PLWH experiencing HIV-related loss of friends/family, these experiences may have reinforced adherence behaviour [37], although newly diagnosed older people may not be affected by this concern. The association between social support and adherence to ART has been reported in studies [80, 84]. Older PLWH living with someone who knows their serostatus may increase their adherence rates due to increased social support (e.g., medicine reminders) [41, 45, 85, 86].
Nonetheless, barriers to achieving optimal adherence may affect older PLWH [66], including co-morbidities, regimen complexity and fatigue, mental health conditions and cognitive impairment, lack of social support and social isolation, and stigma [30, 36, 37, 54, 55, 61, 64, 66, 80, 82, 86, 87]. Practical difficulties (e.g., those associated with obtaining refills) can lead to medicine non-adherence among older PLWH [61, 81]. Some studies found that financial concerns, for instance costs of travel to clinic appointments, prescription costs or lacking basic necessities such as food, can lead to non-adherence in older PLWH [34, 57, 86, 88].
3.3.4 Treatment and Medicine Burden
Treatment burden is a broad concept and has been defined as the ‘work’ of being a patient, requiring significant investment of time, effort, attention and cognitive energy [89]. With a higher number of co-morbidities in older PLWH, polypharmacy can increase medicine burden [61]. Medicine burden is associated with regimen complexity, number, size and taste of daily pills, and side effects [13, 14, 17, 47, 74]. Side effects (e.g., diarrhoea, hyperlipidaemia) could result in additional prescribing cascades to manage symptoms, adding to the complexity of a treatment regimen [13, 33, 74].
Fragmentation of care can lead to missed diagnoses and inappropriate management of co-morbidities [27], and some patients may receive conflicting information from clinicians [57]. In another study, the desire for a comprehensive care programme for older PLWH including primary care, geriatric consultations and speciality care for co-morbidities was noted [27]. HIV-only clinics, which may not offer other services, may cause access challenges for older patients (e.g., poor co-ordination of appointments, travel difficulties, fatigue from prolonged waiting times at different facilities) [27, 35, 56, 57]. Many older PLWH may struggle with travel to and from clinic appointments due to physical difficulties, access to public transport or a personal vehicle/driving licence, or parking difficulties [27, 48, 58, 64]. A lack of transportation could lead to disengagement from care [57, 64]. Engagement in care is also related to everyday life demands and financial concerns [34, 45, 48, 57, 64, 88]. Older PLWH face significant burden with everyday tasks, such as shopping, managing medicines and keeping to daily treatment regimens, especially for those who require assistance with these tasks [32, 45].
One study measured treatment burden in older PLWH (mean age, 53 years) using a validated outcome measure (i.e., the Treatment Burden Questionnaire-13). Using a self-reported measure, Schreiner et al. found a mean treatment burden score of 22.8 (SD = 24.6) with overall findings indicating low level of treatment burden (58%, n = 60) in the sample population [42]. High levels of treatment burden were associated with remembering to take medicines throughout the day and associated limitations, and adhering to prescribed exercise regimens and administrative paperwork [42].
Medicine burden in older PLWH was measured using the Living with Medicines Questionnaire (Chinese version). Zheng et al. found that polypharmacy was associated with moderate-high levels of medicine burden [61]. Socioeconomic status, particularly lower income, was associated with higher medicine burden. Females were also reported to experience significantly more medicine burden than males (110.5 vs. 102.5, p = 0.002). Adherence levels were negatively associated with overall medicine burden (r = − 0.3, p = 0.001). Analyses of medicine burden domains showed similar correlations between adherence to ART and practical difficulties (r = − 0.3), communication/relationships with other HCPs (r = − 0.3) and interferences to day-to-day life (r = 0.2, p < 0.05). Nonetheless, the authors acknowledged challenges of self-reported data and small sample size (n = 185) recruited from two clinics within the Chinese Hunan province, limiting generalisability of findings from this study. Moreover, another study reported that 80% of caregivers strongly agreed/agreed that “My care recipient’s medicines are a burden to them” [90]. A study investigating women’s interest in long-acting injectable ART reported that the majority of participants preferred long-acting injectable ART (56%) over daily pills [91]. A participant in this study with a history of drug use indicated that injectable drugs users have unpredictable lifestyles that make daily pill taking challenging, whereas an appointment every 1 or 2 months would be more manageable [91].
3.3.5 Stigma and Need for Social Support
3.3.5.1 Stigma
Findings from the studies reviewed indicate that stigma is still prevalent among PLWH, and more so in older persons [27, 34, 37, 41, 43, 48, 50, 55, 64, 66, 71, 78, 84, 86, 92,93,94,95]. Several studies have reported that older PLWH experience more stigma than younger PLWH due to the HIV condition and age-related stigma [34, 41, 43, 64, 86, 96]. Many HIV-positive participants experience psychological burden arising from being stigmatised by partners, family members, friends, work colleagues, insurance companies, housing departments, law enforcement officials, and healthcare professionals [55, 92]. Studies have also suggested that poor mental wellbeing or anxiety among older PLWH may be linked to greater stigma levels [26, 95]. Older PLWH who are long-term survivors of HIV may also experience stigma from healthcare professionals, leading to instances of disengagement from care [55, 64]. HIV-related stigma can create barriers to accessing support and increase non-adherence rates [34, 37, 43, 55, 66, 86]. A study exploring stigma and engagement in HIV care among older PLWH found that there was a significant correlation between negative self-image stigma and engagement in care (r = − 0.38, p = 0.03) [64]. Similar to the general population, some older PLWH may worry about being recognised by a friend or family member while attending appointments at an HIV clinic [34, 64]. Stigma can affect an older person’s sense of self-worth and contributes to mental distress [92]. Moreover, many older PLWH want more support services to address mental health, stigma and isolation [27, 34]. Furthermore, studies exploring stigma in older African American PLWH found individuals experienced stigma due to ethnicity, gender identity, sexual orientation and socio-economic status [37, 64, 95, 96]. Another study comparing older and younger PLWH reported that participants over 55 years of age experienced less internalised stigma than the younger groups under the age of 40 years, possibly due to learning to cope and manage negative aspects of living with HIV and the associated stigma over time [64, 93].
3.3.5.2 Need for Social Support
Themes around social isolation, loneliness and a need for support among the ageing HIV population have been highlighted in the studies reviewed [27, 32, 34, 41, 48, 68, 69, 71, 78, 80, 84, 87, 93, 97]. A study focusing on geriatric assessments in older PLWH indicated that up to 50% had low social support and nearly 60% showed some form of loneliness [32]. One study reported lower levels of social support among older HIV-positive women than younger HIV-positive woman (p < 0.001) [69]. In another study, those who reported more instances of hostility, temper outbursts and arguments tended to alienate potential allies and therefore not receive vital sources of emotional support [69, 78]. Lower mean social support scores were reported among participants who had a high prevalence of mental conditions, psychosocial stressors and disrupted social systems [78, 80]. Older PLWH’s need for improved social support networks was evident in the studies reviewed, with one patient suggesting: “We should have. . .somewhere we can go and socialise. . .have lunch and have social workers there, if we need to get stuff done. For me, I live alone, I have friends but sometimes my friends work and I like to get out and be around other people socially during the day, I have groups but they are in the evening, but in the daytime I'm just stuck at home” [27, 32]. One study observed a statistically significant association between adherence and social support in older PLWH (p = 0.02) [80]. Similarly, other studies have shown that support from family members was highly rated as a facilitator for medicine adherence and engagement in care [34, 87].
A recent study on older PLWH and substance use disorders found that social support is critical to minimising treatment interruptions [97]. Studies have also suggested the usefulness of peer support groups, particularly to counteract the social isolation and stigma experienced by older HIV-positive people [34, 41]. One study showed that older adults accessed fewer HIV-related health information sources than the younger participants, including primary-care physicians, family/friends, the HIV clinic, support groups, and the Internet [96]. Moreover, one study found only 14.8% (n = 77) of participants reported being linked to one or more HIV support groups or communities [85]. Older adults living with HIV have suggested the need for age-specific peer support groups as some have felt out of place at support groups consisting of younger participants [34, 96]. One study suggested that social welfare programmes and primary health promotion should focus on educating older PLWH and their service providers on methods to cope with health and social difficulties of ageing with HIV [45].
A study conducted in Canada reported that all participants attended at least one mental health service, including local drop-in services, support and self-help groups, and visiting therapists [92]. Participants described achieving resilience by lowering the space that HIV occupies in their lives, creating lifestyle changes around the illness and engaging in social support [92]. Another study found that older and younger PLWH access a similar number of social and emotional supports, and the older group reported fewer barriers to accessing medical and social services than the younger groups, but this was not statistically significant [96].
3.3.6 Patient and Healthcare Provider Relationships
Older persons’ wants and desires for comprehensive and integrated care are noteworthy. Some older persons want healthcare professionals to consider different HIV- and age-related co-morbidities, with one participant describing concerns with their HIV doctor focusing on viral loads and not their co-morbidities as they aged [27]. Some older PLWH reported that provider perceptions may negatively impact HIV care and lead to late diagnoses. Perceptions about sexual activity among older people are associated with poorer care around sexually transmitted infections [79].
In addition, a study investigating the pharmacist’s role in HIV care in France reported that 21.6% (n = 233) of participants would agree to interviews with pharmacists during ARV dispensing to improve their knowledge and help manage their ART, and 18.9% (n = 204) would participate to improve their follow-up and compliance [59]. It was reported by 94% of pharmacists recruited in this study that more training would be required for them to manage and address the needs of PLWH [59]. One study using patient and provider focus groups found that healthcare providers need a deeper knowledge base to care for the ageing HIV population [27]. In another study, 83.8% of participants reported that they would be willing to stop one or more of their regular medicines if their doctor said it was possible [90].
Some older PLWH have reported concerns regarding fragmentation of HIV care services [14, 27, 30, 34, 35, 55, 57, 64]. A study evaluating the impact of integrated HIV care on patient health outcomes found that patients attending HIV clinics that also offered other services (e.g., hepatitis treatment, psychological and social services) were three times more likely to achieve better clinical outcomes (e.g., viral suppression) than patients attending HIV-only clinics (p < 0.001) [30].
3.4 Interventions Used in Studies of Older PLWH
A total of nine interventions were presented in the studies reviewed (Table 2) [37, 38, 53, 87, 94, 98,99,100,101,102]. Five of the interventions were designed to improve adherence to medicines, two utilised medicine reviews, and two interventions focussed on self-management apps.
3.4.1 Adherence Interventions
SMS reminders were used as ART adherence support in one study [37]. This involved a 6-week campaign, where reminder messages were sent to each participant’s mobile phone by the study co-ordinator. The campaign randomly assigned participants to two intervention groups and one control group. Two intervention groups received two-way SMS reminders three and two times per week, respectively. Intervention groups were required to reply immediately, with the first SMS being “Stop, drop and pop. Take your ART meds now. Have you taken ART drugs on time in the last 48 h?”, if the response was “Yes”, the next SMS would be “Perfect med adherence. Great job!”, if the response was “No” or “Not yet”, the following SMS would be “Please take your meds ASAP. If you need any help, please contact BGF.” The control group received one-way non-specific greeting messages once weekly and were not required to respond and received a general SMS, such as “G’day, BGF wishes you a nice day!” The author reported no statistically significant differences between the intervention and control arms at the end of the SMS campaign [37]. The intervention was useful for adherence support and reassurance for some participants, but others perceived limited effectiveness especially those on established long-term ART.
In another adherence intervention, a Smartphone app (SteadyRX) was tested through an RCT. The intervention group received the smartphone app and usual care, whilst the control group received usual care only [98]. Each participant was given login details to access three sections of the app: (a) the “PillWatch” section allowed users to upload video recordings within in a 6-h dosing window with SMS notifications sent daily 30 min before the start of the dosing window, (b) The “MyRewards” section allowed users to view their monetary incentives for adherence, for example, the number of consecutive days of adherence, countdown to bonus earnings, total earnings, and current balance, (c) The “InTouch” section provided additional resources e.g. adherence-related documents, a user guide/manual, and contact information. Study participants were followed up monthly for 6 months, and adherence was measured by Medicine Event Monitoring System (MEMS) caps. The percentage of adherent participants in the control group reduced across the study duration, with a significant difference between the control and intervention group being evident at 6 months (p = 0.037), with the latter being more adherent [98].
A mobile health intervention app, Rango, designed to support care engagement and treatment adherence for PLWH, was evaluated in a pre-/post-intervention study [53]. The app consisted of three primary components. Firstly, a social network interface to facilitate social support, secondly, personalised medication and appointment reminders, and thirdly, access to services within the app for social services referrals based on each patient’s needs [53]. Participants responded positively to the app and found it acceptable. Lower patient activation of the app was associated with reports of missed doses [53]. A statistically significant difference was evident in the number of participants with unsuppressed viral loads becoming suppressed post-intervention with the Rango app (p = 0.006) [53].
Acceptance-based behaviour therapy was used in another study to promote ART adherence. This intervention used direct cognitive and emotional control strategies, for example encouraging participants to accept HIV-related distress, and to focus their own values [87]. Intervention activities included experiential group exercises, role playing, and homework completed in weekly sessions lasting 60 min. Most participants (75%) found the groups to be helpful [87]. The study reported that qualitative observations suggested that the acceptance-based intervention strategies were well suited to the target population [87]. The sessions emphasised psychological acceptance of the condition and medicines and avoiding denial. The participants reflected on medicine effectiveness, side effects, and taste [87]. The study emphasised social support when living with HIV. This intervention was perceived as less judgemental than compared to traditional approaches for increasing adherence [87].
An RCT using cognitive behavioural therapy utilised psychoeducation to discuss HIV disparities, discrimination and treatment adherence. Adherence was measured electronically using Medication Event Monitoring System (MEMS) bottle caps and through a self-reported questionnaire. A significant improvement in self-reported adherence was seen among participants (p = 0.02) [94]. Electronically monitored adherence showed an increase in adherence; however, this was not significant (p = 0.06) [94]. The study reported high intervention acceptability by participants.
3.4.2 Pharmacist-Led Medicine Reviews
Medicine reviews/reconciliations were conducted by pharmacists in a study evaluating polypharmacy and PIP in older PLWH (n = 248) [38]. This study found 54% and 63% of older people had PIP when using the Screening Tool of Older Persons’ Potentially Inappropriate Prescriptions (STOPP) and Beers criteria, respectively [38]. Pharmacist-led reviews led to discontinuation of PIP in older PLWH with two medicines stopped, on average, per patient. At least one medicine was stopped in over two-thirds (69%) of older PLWH, while six medicines were stopped in ≥ 10% of older persons [38]. Pharmacists play a big role in optimising medicines for older PLWH, and this study found that nearly half of participants had one or more medicine-related problems (e.g., drug interactions, duplication of medicines, high dosage, use of medicines with higher anticholinergic burden) that needed urgent attention by the pharmacist. The study provides useful insight regarding how pharmacist involvement in HIV care can potentially improve pharmaceutical care outcomes. Regardless, the authors recognised limitations around sample selection and excluding potentially inappropriate prescriptions that required a multidisciplinary approach. The study lacked a control/comparator and did not appear to report effectiveness of this pharmacist-led intervention.
A Medicines Management Optimisation Review (MOR) toolkit aids the identification and reviewing of patients that are high risk for polypharmacy and PDDIs to improve patient safety [101]. It consists of a user guide, a patient-orientated questionnaire promoting self-report and adherence to medications named ‘My Clinic Companion’, and the ‘MOR consultation form’ that is used to record the information elicited in the patient consultations and primary-care/hospital records, and to identify beneficial interventions, such as adherence education and smoking cessation [101]. An RCT evaluating the toolkit reported 93 medication-related problems (MRPs) (e.g., PDDIs, dose adjustments, and potential adverse drug reactions) in the intervention group and two in the standard-care control group at baseline. Six to 8 months later, 33 new MRPs were identified in the intervention group compared to three in the control group (r = 0.4, p = 0.001) [101]. More participants in the intervention group had polypharmacy compared to the control group. A reduction in non-ART drugs was evident at 6 months in the intervention arm; however, this was not significant (p = 0.217) [101]. There were no significant changes in HRQOL in both groups throughout the study. Thirty-eight patients completed patient satisfaction questionnaires, and all agreed that the service provided was excellent or very good, most agreed that they had an increased confidence in managing their medicines post intervention [101].
3.4.3 Self-Management Interventions
A self-management app (mVIP) covers 143 self-care strategies for different symptoms including anxiety, depression, sleeping difficulties, cough/shortness of breath, diarrhoea, memory difficulties, fatigue, dizziness, fever, chills/sweats, neuropathy, nausea/vomiting, skin issues and weight loss/wasting. App users are guided through questions relating to symptoms and based on their responses, self-care strategies are recommended [99, 100]. In an RCT, weekly surveys were given through the app asking participants if they had experienced one of the symptoms in the past 7 days and how much they were bothered by the symptoms [99]. For bothersome symptoms, the intervention group was given self-care strategies and illustrative videos. After the RCT was complete, the follow-up study used convenience sampling to recruit participants to focus groups [100]. Common symptoms reported by participants included difficulty sleeping, anxiety, neuropathy and depression [99]. The intervention group showed improvements in 12 out of 13 symptoms compared to the control group [99]. The RCT trial study did not find a significant difference in HRQOL between the groups; improvement in ART adherence was found in the intervention group [99, 100]. The intervention group found the app useful for self-care strategies to manage their HIV-related symptoms [99]. Participants suggested the addition of more languages to the app [99, 100]. The ability of the intervention group to review symptoms previously logged was reported as being useful and could potentially aid interactions with healthcare providers [100].
ThE CARE (Technology Enhanced Competence, Autonomy, and Relatedness Intervention for Elderly women with HIV) uses a web-based app to provide users with interactive features and functionality within modules to enhance physical and psychosocial functions and increase self-determination of older PLWH [102]. A study conducted a pre-/post-test intervention using ThE CARE app [102]. Overall, participants found the app and information useful, despite some encountering challenges navigating the contents of the modules. Participants found the social support aspect of the intervention positive, with one participant stating “…support groups are so important because I would die to have somebody to share with daily…” [102]. During the post-intervention focus group, all seven participants agreed that they would recommend the app to other PLWH [102]. Although not statistically significant, slight improvements were seen post intervention for treatment confidence, seeking help, and internal motivation for treatment entry and adherence [102].
4 Discussion
This systematic review was designed to identify the main issues and concerns in relation to medicines in the ageing HIV population, the interventions available to target these concerns and for medicines optimisation, and how effective the interventions are. There were a variety of tools used among the studies to identify and quantify issues, such as stigma, polypharmacy, drug–drug interactions, and frailty. However, the studies reviewed were largely descriptive, with only nine intervention studies reported out of the 79 appraised. A majority of the studies included were conducted in the USA, whereas only five were conducted in the UK, highlighting the need for further research in this population.
The priority issues and concerns identified in the studies reviewed were co-morbidities, HRQOL, polypharmacy, drug interactions, adverse drug reactions, adherence, medicine burden, treatment burden, stigma, social support and patient-healthcare provider relationships. The results of the review showed that these issues are interrelated with complex impacts on each other, and so must be considered holistically when optimising the medicines of older PLWH. A higher number of co-morbidities have been reported among the ageing HIV population compared to younger PLWH and the HIV-negative population of the same age. The studies reviewed have shown that older PLWH have an average of three co-morbidities, with duration since HIV diagnosis and ART initiation being predictors of co-morbidity. As the number of concomitant medicines taken by older PLWH increases with the number of presenting co-morbidities, this leaves this population vulnerable to potential drug–drug interactions. There is evidence from the included studies that the conditions that affect this population the most are gastrointestinal, mental health, cancer, CKD, diabetes, CVD, chronic pain and osteoporosis. It has been reported that this population hold uncertainty about the physical, psychological and social impacts of ageing with HIV. Moreover, there are concerns that certain conditions are being overlooked in older PLWH, this could lead to late diagnosis and the potential addition of medicines that could have been avoided if earlier screening occurred. A poor physical HRQOL can be observed in older PLWH, and has been associated with a history of AIDS, a longer duration since ART initiation, multiple co-morbidities, and loneliness [26, 68, 69]. Lower mental HRQOL has been observed in HIV-positive adults compared to HIV-negative adults [26]. Moreover, depression has been linked to poorer health outcomes in this population [66]. Mental health conditions in older PLWH have been mentioned as a concern across several studies in this review, including depression, anxiety and, although not as common, one study showed post-traumatic stress disorder (PTSD) in 12.5% of long-term survivors of the disease [32, 92]. A participant from one study described his fallout and anger that he experienced when unexpectedly surviving HIV with the term “reactive depression” [92]. This suggests support for further research in this area and in HIV survivorship [103, 104]. Moreover, one study reported mental disorders as being the most prevalent co-morbidity in older PLWH, with other studies also reporting high incidences of mental illness [30, 33, 36, 37, 43, 44, 64].
The studies reviewed have shown that older PLWH experience higher rates of polypharmacy than younger PLWH and HIV-negative people [14, 29, 40, 72, 75]. Several studies in this review have identified a high number of drug–drug interactions in the ageing HIV population. Commonly prescribed concomitant medicines in older PLWH have been reported to interact with ART; examples include statins, proton pump inhibitors, antidepressants, phosphodiesterase-5 inhibitors and inhaled corticosteroids. These interactions can result in negative and unpleasant effects for the patient. Older PLWH have expressed drug toxicity of medicines causing stress on their body over time. Adverse drug reactions and physical changes relating to their HIV disease can increase the stigma and treatment burden, affect mental health, and lower the quality of life experienced by older individuals with HIV. Long-term ART toxicity and the presence of co-morbidities has been reported to increase the incidence of frailty [27, 28, 67]. Frailty is an emerging concern among the ageing HIV population and can have a significant impact on clinical care and health outcomes [105]. The STOPP and Beers criteria are validated tools that are useful in identifying PIP, drug–drug interactions, drug-disease interactions, and medicine to avoid in older adults [106]. Several studies in this review highlighted the usefulness of using such tools and medicine reconciliations in older PLWH [13, 20, 38, 49, 73], with the Beers criteria evident in over 50% of participants in two studies and over 60% of participants with the STOPP criteria in one study [13, 38]. Moreover, medicine reconciliation leading to an average of two medicines being stopped per patient in one study is incredibly important, particularly when each PIP can increase the likelihood of ADRs by 85% [38]. Simplified medicine regimens leading to increased adherence rates and less adverse drug reactions of medicines can potentially reduce the number of further medicines needed. It is important that the cause of ADRs is identified and treated to prevent further inappropriate prescribing. Pharmacist reviews are therefore effective in reducing both polypharmacy and potentially inappropriate prescribing [38, 39]. The findings from this review suggest that using medicine reviews to target individuals affected by polypharmacy would have the highest yield and greatest impact in reducing PIP [38]. Medicine reviews can lead to ART simplification, reducing polypharmacy, pill burden and PDDIs, and improve health outcomes [29].
Co-morbidities, polypharmacy, regimen complexity, medicine refill difficulties, stigma, mental health disorders, poor finances and low social support are all factors that lead to suboptimal adherence in the ageing HIV population. Older adults who have been living with HIV for a long time and have witnessed the loss of friends and family to the disease have reported that the importance of medicine adherence has been reinforced within them through these experiences [37]. This may be the reason for higher adherence rates of ART reported in some studies among older PLWH compared to their younger counterparts. A higher adherence rate for ARTs compared to concomitant medicines suggests that some patients prioritise medicine based on their beliefs and perceptions of their importance [39, 44]. Medicine regimen complexity may leave patients confused about how to take their medicines, and this in addition to the number of medicines taken, pill size/shape, and dosing instructions can increase medicine burden. Co-morbidities, polypharmacy and adverse drug reactions increase the medicine burden experienced by older HIV-positive adults. Further to medicine burden, the difficulties experienced by older PLWH adhering to their medicines and treatment regimens can lead to treatment burden. This includes the time and effort taken to manage medicines, attending clinic appointments, laboratory tests, lifestyle changes and self-monitoring. A higher number of co-morbidities can lead to higher requirements to maintain the treatment of each condition, thus augmenting the treatment burden.
Despite the advances in HIV care, older PLWH are still facing stigmatisation not only for their serostatus but also due to ageism. In addition, race, gender identity, and sexual orientation have also been reported to cause stigmatisation [37, 64, 95, 96]. This has led to an increase in mental burden, decrease in mental wellbeing, and lower engagement in care [26, 64]. Stigma can create barriers to accessing care services and subsequently lower adherence in older PLWH [34, 37, 43, 66]. Moreover, previous mistreatment and stigmatisation from healthcare professionals and the anticipation of future instances can cause disengagement from care [64]. Other examples of stigma experienced include stigma due to misinformation, physical appearance, discrimination, and compounded stigma [66, 71, 78, 92].
The need for social support was a common theme across the studies reviewed. Lower levels of social support have been seen in older PLWH compared to younger PLWH [69]. Several studies have reported an association between social support and adherence to medicines [41, 45, 80, 85]. Social support has also proven critical for PLWH with substance use disorders to help them avoid treatment interruption [97]. Low social support has been observed among older PLWH who have a higher prevalence of psychiatric illness and psychosocial stressors [80]. Older HIV-positive individuals have expressed a need for more support services focusing on mental health, stigma and isolation [27, 34]. Targeting vulnerable individuals and providing this support could help to prevent negative health outcomes in this population. Current peer support groups for PLWH of all ages have left older HIV-positive adults feeling out of place and wanting age-specific groups [34, 96]. Social support among older PLWH is of particular interest within the UK as in 2017 it was reported that 82% of this population experienced moderate to high levels of loneliness [107]. Understanding the role of stigma and resilience is vital, since these factors may contribute to mental health co-morbidities and the addition of further medicines in older PLWH.
The fragmentation of services for PLWH is particularly of concern, due to difficulties involved in travelling between care facilities, time taken for visits, and finances [27, 30, 34, 35, 48, 56]. Older PLWH have expressed concerns of missed diagnoses of co-morbidities as they age due to fragmentation of care [27]. They have also suggested a need for specific care programmes tailored to their requirements. Studies have shown the effectiveness of integrated HIV care, with patients being three times more likely to be virally suppressed than clinics with HIV services alone [30]. Therefore, providing integrated care for the management and treatment of co-morbid conditions is vital for long-term care in this population [30, 34, 55, 56].
4.1 Implications for Research and Practice
The medicines optimisation interventions in the studies reviewed targeted medicine reviews, adherence, and physical and psychological self-management methods. Five interventions were technology based, requiring a mobile phone and/or internet accessibility. The interventions were well received by most of the participants, showing the desire and usability of medicines optimisation interventions among this population. The reported interventions exhibited positive outcomes in adherence among participants. For instance, SteadyRX, a Smartphone app, utilising daily SMS reminders, monetary incentives for adherence, and adherence-related documents reported increases in participant adherence [98]. Similarly, another mobile intervention app, Rango, providing daily reminders, access to social services referrals, and facilitating social support, demonstrated increases in suppressed viral loads among participants post-intervention [53]. Participants in one study using acceptance-based behaviour therapy to increase adherence found it helpful and perceived it as less judgemental than traditional adherence interventions [87]. In addition, increases in adherence were reported in participants using mVIP, a self-management app [99, 100]. The web-based app ThE CARE showed small improvements in treatment confidence and internal motivation for adherence among participants through modules to increase physical and psychosocial functioning with a social support aspect; however, the difference was not significant. Medicine reviews/reconciliations and a MOR toolkit to reduce PIP and PDDIs were effective among intervention studies, with an average of two medicines being discontinued per patient in one study [38], and significantly more MRPs being identified in intervention groups compared to the control (p = 0.001) in another study [101]. However, the majority of the key issues of concern for older PLWH regarding their medicines, as identified in this review, were not reflected in the few studies with interventions. The need for social support and its importance was highlighted in the studies included as a fundamental medicines optimisation intervention. The lack of medicines optimisation interventions targeted specifically for older PLWH reinforces the need for further research exploring these key issues within this population, utilising a variety of qualitative and quantitative methods. This would then inform subsequent work for the design, implementation, and evaluation of medicines optimisation interventions in older PLWH with the intention to improve patient health outcomes.
4.2 Study Limitations
Many of the studies included in our review had a small sample size. The adherence interventions reported in the studies reviewed focus on adherence of ART and have not considered concomitant medicine, which is prevalent in the older HIV population. A majority of the studies include people who have been living with HIV for many years and therefore the data may not be generalisable to newly diagnosed PLWH 50 years of age and over. Although searches of multiple databases and grey literature were conducted, it may be possible for some relevant papers to have been missed in this review.
5 Conclusion
This systematic review synthesised available data on the priority issues and concerns of older PLWH about their medicines, and the type and effectiveness of interventions developed to tackle optimising their medicines. Our findings provide evidence that the ageing HIV population are at high risk for co-morbidity, polypharmacy, drug interactions, adverse drug reactions, medicine burden, treatment burden and stigma. Mental health and social support have been identified as important factors that need attention to improve patient health-related outcomes. Moreover, the fragmentation of care diminishes the relationship between the patient and healthcare providers, creating several barriers and issues for the ageing HIV population. The interventions identified in this review, although sparse, show the potential for medicines optimisation to improve patient health-related outcomes in the ageing HIV population. The use of acceptance-based therapy, SMS reminders and apps for medicines optimisation interventions has high potential; however, the usability and acceptability need to be investigated further in older PLWH. There is a need for further medicines optimisation interventions and the involvement of targeted support groups in the ageing HIV population.
References
Global HIV and AIDS statistics—2020 fact sheet [Internet]. [cited 2020 Aug 4]. Available from: https://www.unaids.org/en/resources/fact-sheet.
Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet. 2013;382:1525–33.
Trickey A, May MT, Vehreschild J-J, Obel N, Gill MJ, Crane HM, et al. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV. 2017;4:e349–56.
Date HL. Optimising the health and wellbeing of older people living with HIV in the United Kingdom. Clin Pharm. 2018;10:56–64.
Public Health England (PHE). Trends in new HIV diagnoses and in people receiving HIV-related care in the United Kingdom: data to the end of December 2018. 2019;13:8.
Smit M, Brinkman K, Geerlings S, Smit C, Thyagarajan K, van Sighem A, et al. Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infect Dis. 2015;15:810–8.
Angus B, Brook G, Awosusi E, et al. BHIVA guidelines for the routine investigation and monitoring of adult HIV-1 positive individuals (2019 interim update). BHIVA. 2019. Available from: https://www.bhiva.org/monitoring-guidelines.
American Academy of HIV Medicine. The HIV and aging consensus project: recommended treatment strategies for clinicians managing older patients with HIV. [Internet]. Available from: https://aahivm.org/wp-content/uploads/2017/02/Aging-report-working-document-FINAL-12.1.pdf.
Gleason LJ, Luque AE, Shah K. Polypharmacy in the HIV-infected older adult population. Clin Interv Aging. 2013;8:749–63.
Okoli C. Relationship between polypharmacy and quality of life among people in 24 countries living with HIV. Prev Chronic Dis [Internet]. 2020 [cited 2021 Jul 4];17. Available from: https://www.cdc.gov/pcd/issues/2020/19_0359.htm.
Edelman EJ, Gordon KS, Glover J, McNicholl IR, Fiellin DA, Justice AC. The next therapeutic challenge in HIV: polypharmacy. Drugs Aging. 2013;30:613–28.
Back D, Marzolini C. The challenge of HIV treatment in an era of polypharmacy. J Int AIDS Soc. 2020;23:1–12.
Greene M, Steinman MA, McNicholl IR, Valcour V. Polypharmacy, drug–drug interactions, and potentially inappropriate medications in older adults with human immunodeficiency virus infection. J Am Geriatr Soc. 2014;62:447–53.
Halloran MO, Boyle C, Kehoe B, Bagkeris E, Mallon P, Post FA, et al. Polypharmacy and drug–drug interactions in older and younger people living with HIV: the POPPY study. Antivir Ther. 2019;24:193–201.
Justice AC, Gordon KS, Skanderson M, Edelman EJ, Akgün KM, Gibert CL, et al. Nonantiretroviral polypharmacy and adverse health outcomes among HIV-infected and uninfected individuals. AIDS Lond Engl. 2018;32:739–49.
Introduction | Medicines optimisation | Quality standards | NICE [Internet]. NICE; [cited 2020 Aug 31]. Available from: https://www.nice.org.uk/guidance/qs120/chapter/Introduction.
Mohammed MA, Moles RJ, Chen TF. Medication-related burden and patients’ lived experience with medicine: a systematic review and metasynthesis of qualitative studies. BMJ Open. 2016;6:e010035.
Royal Pharmaceutical Society. Medicines optimisation: helping patients to make the most of medicines [Internet]. 2013 [cited 2021 Aug 3]. Available from: https://www.rpharms.com/Portals/0/RPS%20document%20library/Open%20access/Policy/helping-patients-make-the-most-of-their-medicines.pdf.
Marzolini C, Livio F. Prescribing issues in elderly individuals living with HIV. Expert Rev Clin Pharmacol. 2019;12:643–59.
López-Centeno B, Badenes-Olmedo C, Mataix-Sanjuan A, Bellón J, Pérez-Latorre L, López J, et al. Potentially inappropriate medications in older adults living with HIV. HIV Med. 2020;21:541–6.
Saeed D, Carter G, Parsons C. A systematic review of interventions to improve medicines optimisation in frail older patients in secondary and acute care settings. Int J Pharm Pract. 2021;29:i22–3.
Emlet CA. ‘You’re awfully old to have this disease’: experiences of stigma and ageism in adults 50 years and older living with HIV/AIDS. Gerontologist. 2006;46:781–90.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ [Internet]. British Medical Journal Publishing Group; 2009 [cited 2020 Jul 21];339. Available from: https://www.bmj.com/content/339/bmj.b2535.
da Costa Santos CM, de Mattos Pimenta CA, Nobre MRC. The PICO strategy for the research question construction and evidence search. Rev Lat Am Enfermagem. 2007;15:508–11.
Mahtani KR, Heneghan C, Aronson J. Single screening or double screening for study selection in systematic reviews? BMJ Evid-Based Med. 2020;25:149–50.
Engelhard EAN, Smit C, van Dijk PR, Kuijper TM, Wermeling PR, Weel AE, et al. Health-related quality of life of people with HIV: an assessment of patient related factors and comparison with other chronic diseases. AIDS Lond Engl. 2018;32:103–12.
Greene ML, Tan JY, Weiser SD, Christopoulos K, Shiels M, O’Hollaren A, et al. Patient and provider perceptions of a comprehensive care program for HIV-positive adults over 50 years of age: the formation of the Golden Compass HIV and aging care program in San Francisco. PLoS ONE. 2018;13:e0208486.
Guaraldi G, Malagoli A, Theou O, Brothers TD, Wallace L, Torelli R, et al. Correlates of frailty phenotype and frailty index and their associations with clinical outcomes. HIV Med. 2017;18:764–71.
Hastain NV, Santana A, Schafer JJ. The incidence and severity of drug interactions before and after antiretroviral therapy simplification in treatment-experienced patients with HIV infection. Ann Pharmacother. 2020;54:36–42.
Hoang T, Goetz MB, Yano EM, Rossman B, Anaya HD, Knapp H, et al. The impact of integrated HIV care on patient health outcomes. Med Care. 2009;47:560–7.
Heron JE, Norman SM, Yoo J, Lembke K, O’Connor CC, Weston CE, et al. The prevalence and risk of non-infectious comorbidities in HIV-infected and non-HIV infected men attending general practice in Australia. PLoS ONE. 2019;14:e0223224.
John MD, Greene M, Hessol NA, Zepf R, Parrott AH, Foreman C, et al. Geriatric assessments and association with VACS Index among HIV-infected older adults in San Francisco. J Acquir Immune Defic Syndr. 2016;72:534–41.
Kim TW, Walley AY, Heeren TC, Patts GJ, Ventura AS, Lerner GB, et al. Polypharmacy and risk of non-fatal overdose for patients with HIV infection and substance dependence. J Subst Abuse Treat. 2017;81:1–10.
Kiplagat J, Mwangi A, Chasela C, Huschke S. Challenges with seeking HIV care services: perspectives of older adults infected with HIV in western Kenya. BMC Public Health. 2019;19:929.
Knight L, Schatz E, Mukumbang FC. ‘I attend at Vanguard and I attend here as well’: barriers to accessing healthcare services among older South Africans with HIV and non-communicable diseases. Int J Equity Health. 2018;17:147.
Levy ME, Greenberg AE, Hart R, Powers Happ L, Hadigan C, Castel A. High burden of metabolic comorbidities in a citywide cohort of HIV outpatients: evolving health care needs of people aging with HIV in Washington, DC. HIV Med. 2017;18:724–35.
Mao L, Buchanan A, Wong HTH, Persson A. Beyond mere pill taking: SMS reminders for HIV treatment adherence delivered to mobile phones of clients in a community support network in Australia. Health Soc Care Community. 2018;26:486–94.
McNicholl IR, Gandhi M, Hare CB, Greene M, Pierluissi E. A Pharmacist-led program to evaluate and reduce polypharmacy and potentially inappropriate prescribing in older HIV-positive patients. Pharmacotherapy. 2017;37:1498–506.
Morillo-Verdugo R, Robustillo-Cortés MA, Abdel-Kader Martín L, ÁlvarezdeSotomayor Paz M, LozanodeLeón Naranjo F, Almeida González CV. Determination of a cutoff value for medication regimen complexity index to predict polypharmacy in HIV+ older patient. Rev Espanola Quimioter Publicacion Of Soc Espanola Quimioter. 2019;32:458–64.
Patel R, Moore T, Cooper V, McArdle C, Perry N, Cheek E, et al. An observational study of comorbidity and healthcare utilisation among HIV-positive patients aged 50 years and over. Int J STD AIDS. 2016;27:628–37.
Rosenfeld D, Anderson J. ‘The own’ and ‘the wise’ as social support for older people living with HIV in the United Kingdom. Ageing Soc. 2020;40:188–204.
Schreiner N, Perazzo J, Currie J, Daly B, Webel A. A descriptive, cross-sectional study examining treatment burden in people living with HIV. Appl Nurs Res. 2019;46:31–6.
Shippy RA, Karpiak SE. The aging HIV/AIDS population: fragile social networks. Aging Ment Health. 2005;9:246–54.
Siefried KJ, Mao L, Cysique LA, Rule J, Giles ML, Smith DE, et al. Concomitant medication polypharmacy, interactions and imperfect adherence are common in Australian adults on suppressive antiretroviral therapy. AIDS Lond Engl. 2018;32:35–48.
Singo VJ, Lebese RT, Maluleke TX, Nemathaga LH. The views of the elderly on the impact that HIV and AIDS has on their lives in the Thulamela Municipality, Vhembe District, Limpopo province. Curationis [Internet]. South Africa: AOSIS Publishing; 2015;38. Available from: http://search.ebscohost.com/login.aspx?direct=true&db=cmedm&AN=26244455&site=ehost-live.
Uphold CR, Maruenda J, Yarandi HN, Sleasman JW, Bender BS. HIV and older adults: clinical outcomes in the era of HAART. J Gerontol Nurs. 2004;30:16–24.
Farahat FM, Alghamdi YS, Farahat AF, Alqurashi AA, Alburayk AK, Alabbasi AA, et al. The prevalence of comorbidities among adult people diagnosed with HIV infection in a tertiary care hospital in western Saudi Arabia. J Infect Public Health. 2020;13:1699–704.
Schatz E, Knight L, Mukumbang FC, Teti M, Myroniuk TW. ‘You Have to Withstand That Because You Have Come for What You Have Come for’: barriers and facilitators to antiretroviral treatment access among older South Africans living with HIV. Sociol Health Illn. 2021;43:624–41.
Vinuesa-Hernando JM, Gimeno-Gracia M, Malo S, Sanjoaquin-Conde I, Crusells-Canales MJ, Letona-Carbajo S, et al. Potentially inappropriate prescriptions and therapeutic complexity in older HIV patients with comorbidities. Int J Clin Pharm. 2021;43:1245–50.
Drewes J, Ebert J, Langer PC, Kleiber D, Gusy B. Comorbidities and psychosocial factors as correlates of self-reported falls in a nationwide sample of community-dwelling people aging with HIV in Germany. BMC Public Health. 2021;21:1–9.
Fischetti B, Sorbera M, Michael R, Njeim N. Evaluation of rates of virologic suppression in HIV-positive patients with varying numbers of comorbidities. Am J Health Syst Pharm. 2022;79:72–7.
Shamu T, Chimbetete C, Egger M, Mudzviti T. Treatment outcomes in HIV infected patients older than 50 years attending an HIV clinic in Harare, Zimbabwe: a cohort study. PLoS ONE. 2021;16:e0253000.
Ventuneac A, Kaplan-Lewis E, Buck J, Roy R, Aberg CE, Duah BA, et al. A mobile health intervention in HIV primary care: supporting patients at risk for ART non-adherence. HIV Res Clin Pract. 2020;21:140–50.
Zepf R, Greene M, Hessol NA, Johnson MO, Santos GM, John MD, et al. Syndemic conditions and medication adherence in older men living with HIV who have sex with men. AIDS Care. 2020;32:1610–6.
Chayama KL, Ng C, Small W, Ivsins A, McNeil R. ‘It’s a burden, it’s a nuisance I wish I didn’t have these other ailments’: a qualitative exploration of comorbidities management among older people living with HIV who use drugs in Vancouver, British Columbia. J Int AIDS Soc. 2021;24:e25785.
Haruna T, Somba M, Siril H, Mahiti G, August F, Minja A, et al. Factors hindering integration of care for non-communicable diseases within HIV care services in Dar es Salaam, Tanzania: the perspectives of health workers and people living with HIV. PLoS ONE. 2021;16:e0254436.
Bosire EN. Patients’ experiences of comorbid HIV/AIDS and diabetes care and management in Soweto, South Africa. Qual Health Res. 2021;31:373–84.
McKetchnie SM, Beaugard C, Taylor SW, O’Cleirigh C. Perspectives on pain, engagement in HIV care, and behavioral interventions for chronic pain among older sexual minority men living with HIV and chronic pain: a qualitative analysis. Pain Med. 2021;22:577–84.
Jacomet C, Langlois J, Secher S, Coban D, Lambert C, Zucman D, et al. Pharmacist’s role in HIV care in France. Implication for clinical improvement of people living with HIV worldwide. Pharmacol Res Perspect. 2020;8:e00629.
Hartzler B, Dombrowski JC, Donovan DM. Contextual compatibility of three empirically supported behavior therapies for cART adherence among patients with substance use disorders. AIDS Care. 2019;31:19–24.
Zheng C, Meng J, Xiao X, Xie Y, Zhao D, Wang H. Polypharmacy, medication-related burden and antiretroviral therapy adherence in people living with HIV aged 50 and above: a cross-sectional study in Hunan, China. Patient Prefer Adherence. 2022;16:41–9.
Nguyen AL, McNeil CJ, Han SD, Rhodes SD. Risk and protective factors for health-related quality of life among persons aging with HIV. AIDS Care. 2018;30:518–22.
Townsend ML, Jackson GL, Smith R, Wilson KH. Association between pharmacy medication refill-based adherence rates and cd4 count and viral-load responses: a retrospective analysis in treatment-experienced adults with HIV. Clin Ther. 2007;29:711–6.
Harris LM, Crawford TN, Kerr JC, Thomas TA, Schmidt V. African American older adults living with HIV: exploring stress, stigma, and engagement in HIV care. J Health Care Poor Underserved. 2020;31:265–86.
Ahmed A, Saqlain M, Bashir N, Dujaili J, Hashmi F, Mazhar F, et al. Health-related quality of life and its predictors among adults living with HIV/AIDS and receiving antiretroviral therapy in Pakistan. Qual Life Res Int J Qual Life Asp Treat Care Rehabil. 2021;30:1653–64.
Halkitis PN, Perez-Figueroa RE, Carreiro T, Kingdon MJ, Kupprat SA, Eddy J. Psychosocial burdens negatively impact HIV antiretroviral adherence in gay, bisexual, and other men who have sex with men aged 50 and older. AIDS Care. 2014;26:1426–34.
McMillan JM, Krentz HB, Gill MJ, Hogan DB. An emerging concern—high rates of frailty among middle-aged and older individuals living with HIV. Can Geriatr J. 2019;22:190–8.
Greene M, Hessol NA, Perissinotto C, Zepf R, Hutton Parrott A, Foreman C, et al. Loneliness in older adults living with HIV. AIDS Behav. 2018;22:1475–84.
Kteily-Hawa R, Andany N, Wang Y, Logie CH, Tharao W, Conway T, et al. Quality of life of older women living with HIV: comparative assessment of physical and mental health-related markers using a large Canadian Sexual and Reproductive Health Cohort Study. HIV Res Clin Pract. 2019;20:35–47.
Contreras-Macías E, Gutiérrez-Pizarraya A, RobustilloCortés MA, Morillo-Verdugo R. High level of medication regimen complexity index correlate with worse quality of life in people living with HIV. Rev Espanola Quimioter Publicacion Of Soc Espanola Quimioter. 2021;34:93–9.
Owen G, Catalan J. ‘We never expected this to happen’: narratives of ageing with HIV among gay men living in London, UK. Cult Health Sex. 2012;14:59–72.
Gimeno-Gracia M, Crusells-Canales MJ, Armesto-Gómez FJ, Compaired-Turlán V, Rabanaque-Hernández MJ. Polypharmacy in older adults with human immunodeficiency virus infection compared with the general population. Clin Interv Aging. 2016;11:1149–57.
Contreras Macías E, Serrano Giménez R, Morillo Verdugo R. Prevalence of prescription of the Top-10 drug classes to avoid in elderly people living with HIV in a real practice cohort. Rev Espanola Quimioter Publicacion Of Soc Espanola Quimioter. 2021;34:28–32.
Jakeman B, Scherrer A, Battegay M, Gunthard HF, Hachfeld A, Calmy A, et al. Anticholinergic medication use in elderly people living with HIV and self-reported neurocognitive impairment: a prospective cohort study. J Antimicrob Chemother. 2022;77:492–9.
Katende-Kyenda NL, Lubbe MS, Serfontein JHP, Truter I. Prevalence of possible drug–drug interactions between antiretroviral agents in different age groups in a section of the private health care sector setting in South Africa. J Clin Pharm Ther. 2008;33:393–400.
Gimeno-Gracia M, Crusells-Canales MJ, Rabanaque-Hernández MJ. Clinical characteristics and antiretroviral treatment of older HIV-infected patients. Int J Clin Pharm. 2014;36:1190–5.
Jiménez-Guerrero L, Núñez-Núñez M, Castañeda-Macías I, Sandoval-Fernández Del Castillo S. Potential interactions in a cohort of elderly hiv-positive patients. Farm Hosp Organo Of Expresion Cient Soc Espanola Farm Hosp. 2018;42:163–7.
Heckman TG, Heckman BD, Kochman A, Sikkema KJ, Suhr J, Goodkin K. Psychological symptoms among persons 50 years of age and older living with HIV disease. Aging Ment Health. 2002;6:121–8.
Frazier EL, Sutton MY, Tie Y, Collison M, Do A. Clinical characteristics and outcomes among older women with HIV. J Womens Health. 2018;27:6–13.
Gardenier D, Andrews CM, Thomas DC, Bookhardt-Murray LJ, Fitzpatrick JJ. Social support and adherence: differences among clients in an AIDS day health care program. JANAC J Assoc Nurses AIDS Care. 2010;21:75–85.
Keith McInnes D, Shimada SL, Rao SR, Quill A, Duggal M, Gifford AL, et al. Personal health record use and its association with antiretroviral adherence: survey and medical record data from 1871 US veterans infected with HIV. AIDS Behav. 2013;17:3091–100.
Lee CJ. Executive functioning, cigarette smoking, and medication adherence in people living with HIV/AIDS [Internet]. ProQuest Information & Learning; 2019. Available from: http://search.ebscohost.com/login.aspx?direct=true&db=psyh&AN=2018-52510-087&site=ehost-live.
Sutton SS, Hardin JW, Bramley TJ, D’Souza AO, Bennett CL. Single- versus multiple-tablet HIV regimens: adherence and hospitalization risks. Am J Manag Care. 2016;22:242–8.
Schatz E, David I, Angotti N, Gómez-Olivé FX, Mojola SA. From ‘Secret’ to ‘Sensitive Issue’: shifting ideas about HIV disclosure among middle-aged and older rural South Africans in the era of antiretroviral treatment. J Aging Health. 2022;34:14–24.
Siefried KJ, Mao L, Kerr S, Cysique LA, Gates TM, McAllister J, et al. Socioeconomic factors explain suboptimal adherence to antiretroviral therapy among HIV-infected Australian adults with viral suppression. PLoS ONE. 2017;12:e0174613.
Kuteesa MO, Seeley J, Cumming RG, Negin J. Older people living with HIV in Uganda: understanding their experience and needs. Afr J AIDS Res. 2012;11:295–305.
Moitra E, Herbert JD, Forman EM. Acceptance-based behavior therapy to promote HIV medication adherence. AIDS Care. 2011;23:1660–7.
McAllister J, Beardsworth G, Lavie E, MacRae K, Carr A. Financial stress is associated with reduced treatment adherence in HIV-infected adults in a resource-rich setting. HIV Med. 2013;14:120–4.
Tran V-T, Harrington M, Montori VM, Barnes C, Wicks P, Ravaud P. Adaptation and validation of the Treatment Burden Questionnaire (TBQ) in English using an internet platform. BMC Med. 2014;12:109.
Serrano Giménez R, Gallardo Anciano J, Robustillo Cortés MA, Blanco Ramos JR, Gutiérrez Pizarraya A, Morillo Verdugo R. Beliefs and attitudes about deprescription in older HIV-infected patients: ICARD Project. Rev Espanola Quimioter Publicacion Of Soc Espanola Quimioter. 2021;34:18–27.
Philbin MM, Parish C, Bergen S, Kerrigan D, Kinnard EN, Reed SE, et al. A qualitative exploration of women’s interest in long-acting injectable antiretroviral therapy across six cities in the Women’s Interagency HIV study: intersections with current and past injectable medication and substance use. AIDS Patient Care STDs. 2021;35:23–30.
Furlotte C, Schwartz K. Mental health experiences of older adults living with HIV: uncertainty, stigma, and approaches to resilience. Can J Aging Rev Can Vieil. 2017;36:125–40.
Foster PP, Gaskins SW. Older African Americans’ management of HIV/AIDS stigma. AIDS Care. 2009;21:1306–12.
Bogart LM, Barreras JL, Gonzalez A, Klein DJ, Marsh T, Agniel D, et al. Pilot randomized controlled trial of an intervention to improve coping with intersectional stigma and medication adherence among HIV-positive Latinx sexual minority men. AIDS Behav. 2021;25:1647–60.
Hojilla JC, Santiago-Rodriguez EI, Sterling S, Williams EC, Leyden W, Hare CB, et al. HIV stigma and its associations with longitudinal health outcomes among persons living with HIV with a history of unhealthy alcohol use. AIDS Behav. 2021;25:215–24.
Fritsch T. HIV/AIDS and the older adult: an exploratory study of the age-related differences in access to medical and social services. J Appl Gerontol. 2005;24:35–54.
Rozanova J, Shenoi S, Zaviryukha I, Zeziulin O, Kiriazova T, Rich K, et al. Social support is key to retention in care during Covid-19 pandemic among older people with HIV and substance use disorders in Ukraine. Subst Use Misuse. 2020;55:1902–4.
DeFulio A, Devoto A, Traxler H, Cosottile D, Fingerhood M, Nuzzo P, et al. Smartphone-based incentives for promoting adherence to antiretroviral therapy: a randomized controlled trial. Prev Med Rep. 2021;21: 101318.
Schnall R, Cho H, Mangone A, Pichon A, Jia H. Mobile health technology for improving symptom management in low income persons living with HIV. AIDS Behav. 2018;22:3373–83.
Cho H, Porras T, Baik D, Beauchemin M, Schnall R. Understanding the predisposing, enabling, and reinforcing factors influencing the use of a mobile-based HIV management app: a real-world usability evaluation. Int J Med Inf. 2018;117:88–95.
Leake Date HA, Alford K, Hounsome N, Moore D, Ing K, Vera JH. Structured medicines reviews in HIV outpatients: a feasibility study (the MOR study). HIV Med. 2022;23:39–47.
Njie-Carr VPS, Zhu S, Williams GC, Corless IB, Himelhoch S. Evaluation of a technology-enhanced intervention for older women with HIV infection: a proof of concept study. AIDS Care. 2021;33:983–92.
Freeman R, Gwadz M, Wilton L, Collins LM, Dorsen C, Hawkins RL, et al. Understanding long-term HIV survivorship among African American/Black and Latinx persons living with HIV in the United States: a qualitative exploration through the lens of symbolic violence. Int J Equity Health. 2020;19:146.
Psaros C, Barinas J, Robbins GK, Bedoya CA, Park ER, Safren SA. Reflections on living with HIV over time: exploring the perspective of HIV-infected women over 50. Aging Ment Health. 2015;19:121–8.
Willig AL, Overton ET, Saag MS. The silent epidemic—frailty and aging with HIV. Total Patient Care HIV HCV. 2016;1:6–17.
Duerden M, Avery T, Payne R. Polypharmacy and medicines optimisation: making it safe and sound. London: The King’s Fund; 2013.
Terrence Higgins Trust. Uncharted Territory: a report into the first generation growing older with HIV. 2017.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open Access funding provided by The University of Kent.
Conflict of interest
Authors PS, RC, SC and BK declare that they have no conflicts of interest.
Ethics approval
This study did not require institutional ethics approval due to using secondary data (i.e., no direct involvement of human participants or their data).
Informed consent
Not applicable.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Author contributions
All authors contributed to study conception and design. PS conducted the literature search and data abstraction. RC, SC and BK contributed to all stages of the systematic review, including independent screening of titles, abstracts and/full articles. PS drafted preliminary versions of the manuscript. BK critically revised the manuscript and offered overall supervision of the project. All authors reviewed and agreed the final version of this paper.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Sarma, P., Cassidy, R., Corlett, S. et al. Ageing with HIV: Medicine Optimisation Challenges and Support Needs for Older People Living with HIV: A Systematic Review. Drugs Aging 40, 179–240 (2023). https://doi.org/10.1007/s40266-022-01003-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40266-022-01003-3