Abstract
Background
Patients taking medication with high anticholinergic and sedative properties are at increased risk of experiencing poor cognitive and physical outcomes. Therefore, precise quantification of the cumulative burden of their drug regimen is advisable. There is no agreement regarding which scale to use to simultaneously quantify the burden associated with medications.
Objectives
The objective of this review was to assess the strengths and limitations of available tools to quantify medication-related anticholinergic burden and sedative load in older adults. We discuss specific limitations and agreements between currently available scales and models and propose a comprehensive table combining drugs categorized as high, moderate, low, or no anticholinergic or sedative activity as excerpted from the selected studies.
Methods
A targeted search was carried out using the National Library of Medicine through PubMed using medical subject heading terms and text words around the following search terms: (anticholinergic OR sedative) AND (load OR burden OR scale) for studies published between 1 January 1945 and 5 June 2021. In addition, the following databases were searched using the same terms: MEDLINE-EBSCO, APA PsycInfo, CINAHL Plus, Cochrane Library, Scopus, OAIster, OVID-MEDLINE, Web of Science, and Google Scholar. Screening by titles was followed by an abstract and full-text review. After blind evaluation, agreement between reviewers was reached to establish drug characteristics and categories.
Results
After 3163 articles were identified, 13 were included: 11 assigned risk scores to anticholinergic drugs and two to sedative drugs. Considerable variability between anticholinergic scales was observed; scales included between 27 and 548 drugs. We generated a comprehensive table combining the anticholinergic and sedative activities of drugs evaluated and proposed a categorization of these drugs based on available scientific and clinical evidence. Our table combines information about 642 drugs and categorizes 44, 25, 99, and 474 drugs as high, moderate, low, or no anticholinergic and sedative activity, respectively.
Conclusions
Variability and inconsistency exists among scales used to categorize drugs with anticholinergic or sedative burden. In this review, we provide a comprehensive table that proposes a new categorization of these drugs. A longitudinal study will be required to validate the new proposed anticholinergic and sedative burden catalog in an evidence-based manner.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Older adults taking medications with anticholinergic and sedative properties are at a higher risk of experiencing poor cognitive and physical outcomes. |
We describe the strengths and limitations of 13 existing scales used to measure medication-related anticholinergic burden and sedative load in the elderly. |
A novel comprehensive catalog combining medications with anticholinergic and sedative potency excerpted from the selected studies is provided. |
This review highlights the importance of understanding drug-induced anticholinergic and sedative side effects in clinical practice, especially in patients with polypharmacy, as this can significantly improve their quality of life and reduce the risk of dementia and associated cognitive impairment. |
1 Introduction
About 35% of adults aged ≥ 60 years consume more than five prescription drugs at a time, a condition referred to as polypharmacy [1,2,3]. Polypharmacy, although needed to treat co-occurring chronic diseases, increase the risk of inappropriate drug use resulting from prescription cascade used to circumvent adverse drug reactions (ADRs) [4, 5]. Drugs with anticholinergic or sedative properties are among the most commonly prescribed medications in patients with polypharmacy [6,7,8,9,10]. These medications are associated with central effects (such as cognitive dysfunction, delirium, frailty, confusion, falls, and brain atrophy) and peripheral side effects, including dry mouth, urinary retention, visual disturbance, tachycardia, lower gastrointestinal motility, and increased risk of pulmonary infections [11, 12]. The cumulative anticholinergic burden and sedative load is usually unintentional and is aggravated by several medications with anticholinergic properties that also have sedative properties [13].
Anticholinergic burden is defined as the “accumulation of higher levels of exposure due to one or more anticholinergic medications and the attendant increased risk of medication-related adverse effects” [14]. Defining the sedative load is more complex, as sedation is commonly described through subjective feelings of sleepiness, lethargy, drowsiness, and reduced psychomotor processing [15].
A longitudinal analysis of data collected over 20 years showed that higher long-term cumulative exposure to anticholinergic and sedative medications was associated with poorer physical and cognitive functioning [16]. A cross-sectional analysis of a population-based cohort of 2087 participants aged ≥65 years showed that almost half of the population were using anticholinergics or sedatives (n=954 [45.7%]); the use of these drugs was associated with poorer grip strength, slower walking speed, poorer instrumental activities of daily living, and poorer appetite [9]. Hanlon et al. [17] conducted a longitudinal analysis to quantify anticholinergic burden in a large older and middle-aged cohort using ten different anticholinergic scales. Their results showed that anticholinergic medication use was common (8–18%) and that anticholinergic burden was associated with adverse outcomes in both age groups [17].
Given the associations between anticholinergic exposure, sedative exposure, and poor clinical outcomes in older adults, precise quantification of the cumulative anticholinergic burden and sedative load appears advisable to evaluate the risk–benefit ratio of prescribing or deprescribing some medications [18]. Several tools assess exposure to either anticholinergic or sedative medications, but few evaluate cumulative anticholinergic or sedative burden simultaneously [19, 20].
Examples of anticholinergic quantification scales that have been used for the past decade include the Anticholinergic Drug Scale (ADS), the Anticholinergic Cognitive Burden (ACB) scale, and the Anticholinergic Risk Scale (ARS) [14, 21, 22]. Newer scales, such as the ACB for German prescribers, the Brazilian scale, the Korean version of anticholinergic burden scale (KABS), and the modified ACB scale, are derived from one or more of the original scales with expert opinion sought for drugs not included in the original series [23,24,25,26]. These were all developed using varying methods of measuring the anticholinergic or sedative activity of drugs and varying methods of classifying drugs into distinct potency categories [14, 21,22,23,24,25,26]. To date, no international consensus has been reached regarding which scale or tool to use to quantify the simultaneous anticholinergic and/or sedative burden.
To collate information, Durán et al. [27] and Salahudeen et al. [28] published two comprehensive systematic reviews that compared the anticholinergic burden of drugs using anticholinergic risk scales to quantify drug effects. They also evaluated associations between anticholinergic activity and adverse outcomes in older people and provided a composite table and lists of drugs with their anticholinergic potencies. Although both reviews compiled cumulative tables of drug properties, the information presented was extracted directly from original scales for drugs listed with two or more anticholinergic potency scores [27, 28].
For drugs with sedative effects, fewer tools are available. In 2003, a detailed classification of drug sedation potency referred to as the “sedative load scale” was published, with an adaptation of this model published later in 2008 as part of a clinical trial [29, 30]. Therefore, an up-to-date systematic review of studies utilizing tools to quantify the anticholinergic burden and/or sedative burden of drugs with some examples of clinically relevant scale-validation studies is needed. Moreover, an evidence-based resource combining the anticholinergic and sedative burden scores of drugs in one table would be greatly useful for healthcare providers and pharmacists in community and clinical settings.
In this review, we summarized studies that used a published tool to quantify anticholinergic burden and/or sedative load in older adults to describe the potential advantages, disadvantages, or challenges of using these tools and to present a comprehensive table combining drugs evaluated in selected studies with categorization of drugs as high, moderate, low, or no anticholinergic and sedative activity based on available information. We have named this table the AntiCholinergic and Sedative Burden Catalog (ACSBC), and it will be validated in our future studies.
2 Methods
This systematic review was carried out in accordance with the procedures proposed by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) protocol guidelines and following the Cochrane Collaboration guidelines where possible [31]. The focus of this work was formulated considering the PICOS/PECOS (population, intervention/exposure, comparison, outcome, study design) approach, leading to the design of a search strategy intended to be as inclusive as possible to properly address the main purpose of this review [32,33,34,35]. No protocol registration was made for this review.
2.1 Search Strategy
A targeted literature search of the published peer-reviewed literature and gray literature was first conducted in October 2019. In June 2021, the search was updated to include any new publications in the field. The National Library of Medicine through PubMed was queried using medical subject headings terms and text words around the following search terms: (anticholinergic OR sedative) AND (load OR burden OR scale). The search was conducted for studies published between 1 January 1945 and 5 June 2021. In addition, the following databases were searched using the same terms: MEDLINE-EBSCO, APA PsycInfo, CINAHL Plus, Cochrane Library, Scopus, OAIster, OVID-MEDLINE, Web of Science, and Google Scholar. Finally, a citation analysis was conducted in Google Scholar and Web of Science to track the prospective citing of selected articles as it pertained to assessment of individual scales for utilization and validation.
2.2 Inclusion and Exclusion Criteria
Clinical trials, randomized controlled trials, reviews, systematic reviews, meta-analyses, and different retrospective, prospective, and cross-sectional observational studies evaluating the anticholinergic burden and/or the sedative load of drugs used in patients aged ≥ 65 years were subject to a thorough full-text evaluation to meet our predefined inclusion criteria. Studies were included in the final review if they included a list of drugs that were classified into categories based on the potency of their anticholinergic/sedative effects and if scores were assigned to each category to calculate a total anticholinergic and/or sedative burden. Case reports, letters, commentaries, editorials, articles in languages other than English, and anticholinergic rating scales based exclusively on in vitro studies using radioreceptor assays without extensive literature review and expert opinion were excluded from this systemic review.
Three important clinical tools and scoring systems (Drug Burden Index [DBI], Chew’s list of anticholinergic drugs, and the original Clinician-rated Anticholinergic score [CrAS]) were not considered in our analyses for the following reasons.
2.2.1 The Drug Burden Index
The DBI was developed and published in 2007 by Hilmer et al. [19] and is a great pharmacological tool that measures the effect of cumulative exposure to both anticholinergic and sedative medications on physical and cognitive function in older adults. Unfortunately, the DBI does not provide a quantitative grading score for drugs with anticholinergic or sedative properties. This system uses a specific drug monograph to determine whether a drug has an anticholinergic or sedative effect by considering pharmacological aspects and side effect profiles. If a drug monograph reports a sedative or anticholinergic property, a DBI will be calculated using the DBI equation (B = D/(δ +D)), where D represents the daily dose taken by the patient and δ the minimum recommended daily dose approved by the US FDA. The total DBI for a drug regimen is calculated as the sum of exposure to each anticholinergic or sedative medication (sum of Bs from the equation). Some other limitations include that (1) all medications with clinically relevant sedative or anticholinergic properties are considered equivalent, (2) the DBI calculation should be based on a consensus list of medicines with anticholinergic and sedative properties that has not been recently updated, and (3) more recently, the authors of the DBI developed the DBI calculator, a software application used by pharmacists. Unfortunately, the DBI calculator website (drugburdenindex.com) is currently only open to Australian healthcare practitioners. Therefore, although the DBI is a scale developed to measure exposure to anticholinergic drugs and sedatives, it cannot be compared with other scales given the different way in which the score is calculated.
2.2.2 Chew’s List of Anticholinergic Drugs
Chew’s list is exclusively based on in vitro analysis of anticholinergic activity using radioreceptor assay [36]. However, we included in our analysis the Anticholinergic Activity Scale (AAS), which was developed based on Chew’s list of serum anticholinergic activity (SAA) for 107 drugs and on expert opinions as part of a longitudinal community cohort of patients with Parkinson’s disease [37].
2.2.3 The Clinician‐Rated Anticholinergic Score
The CrAS was developed in 2001 by Han et al. [38] as an alternative measure of anticholinergic medication exposure to Summer’s classification [39], a three-level ordinal scale published in 1978 that did not include several of the newer medications. Therefore, the CrAS was established to allow the addition of 340 medications that were used in the study population or reported in the literature as having anticholinergic effects. The anticholinergic effect of each medication was rated independently by three geriatric psychiatrists from 0 (none) to 3 (high) based on their clinical experience and knowledge of each medication’s properties. Then, the interrater reliability of the clinicians’ ratings of all medications was assessed and the agreement with Summer’s classification and laboratory data was evaluated. The concordance of the mean and median values was used to establish the CrAS [38]. A modified version of the original CrAS (CrAS-mod) was published by Carnahan et al. [40] in 2002 as part of a pilot validation analysis of the CrAS-mod through measuring SAA in a group of older long-term care residents and analyzing its association with CrAS-mod scores. Results of this pilot study supported the validity of the CrAS-mod and suggested opportunities for further improvement [40]. Therefore, in 2006, the same group further modified the CrAS-mod and changed the name to the ADS for brevity in a larger study that included 201 subjects who were not included in the pilot study [21]. The ADS was developed based on expert opinions and a literature search and was validated by measuring SAA and analyzing the association of the ADS with SAA [21]. Accordingly, we included the ADS and not the CrAS or CrAS-mod in our analysis.
2.3 Study Selection
Two clinical research scientists independently selected studies based on publication title and abstract and then reviewed the full texts of potential articles for final inclusion. The selected studies were further reviewed by a third clinical research scientist. Any discrepancies were resolved after a joint article review and discussion to reach a consensus with the help of the scientific committee supervisors and authors.
2.4 Data Extraction and Synthesis
The following information was extracted from eligible articles: country and year of development, classification method, scores for each category of drugs, number of drugs included, whether dose was considered in the final calculation, and the population used to validate the scale. This information was reviewed by an independent scientist and the data were compiled. Narratives of discussed strengths and limitations are provided in electronic supplementary material (ESM)-S1, which summarizes structured reviews of available methods to calculate drug burden and identifies potential research gaps.
2.5 Assessment of Study Quality
Three reviewers critically appraised each included study using the Hawker tool [41]. This tool was deemed more appropriate for this review as the included studies used different types of designs. The Hawker tool is designed to evaluate disparate data and studies covering a variety of research paradigms [41]. The tool comprises nine different items to assesses the reporting of a study in the following areas: (1) abstract and title, (2) introduction and aims, (3) method and data, (4) sampling, (5) data analysis, (6) ethics and bias, (7) results, (8) transferability and reliability, and (9) implications and usefulness. Each of the nine areas was given a score of either 1 (very poor), 2 (poor), 3 (fair), or 4 (good) for a maximum total score of 36 [41].
2.6 Scales Agreement
Two data scientists not involved in the data extraction and compilation analyzed the collected and systematized raw data about anticholinergic medications and their associated scores across selected scales. Data were reviewed and errors (e.g., incorrect spellings, multiple names for a single substance, etc.) and inconsistencies (e.g., multiple scores for a given substance in a single scale) were corrected through quality and integrity analyses. Cleaned data were manually reviewed by a clinical research scientist to ensure the correctness of performed analyses. This data set was further used to generate the descriptive summary: number of distinct and common medications between different scales, number of medications scored the same or differently among all available scales, etc. Microsoft SQL Server (v. 15; Microsoft Corporation, Albuquerque, NM, USA) was used for all analyses. The numbers and frequency of medications across all selected scales were reported using stacked bar charts plotted in Python (v.3.7.6; open-source software fiscally sponsored by NumFOCUS, Austin, TX, USA) using Matplotlib (v. 3.1.3; open-source comprehensive library sponsored by NumFOCUS), and seaborn (v. 0.10.0; a Python data visualization library created by Michael Waskom, New York, NY, USA) packages. The score agreement across selected scales was determined using Spearman’s correlation coefficient (using p=0.05 as the statistical significance threshold) and summarized in a corresponding correlation matrix using SciPy (v. 1.4.1; a Python-based ecosystem of open-source software and fiscally sponsored by NumFOCUS). According to Evan’s empirical classification to infer correlation strength, a score of 0–0.19 was interpreted as “very weak” correlation, 0.20–0.39 as “weak,” 0.40–0.59 as “moderate,” 0.60–0.79 as “strong,” and 0.80–1.0 as “very strong” [42].
2.7 The Comprehensive Review Table
Scales retained for our analysis (n = 11) ranked the anticholinergic activity of medications into three to five categories, ranging from no anticholinergic activity (=0) to definite/high anticholinergic activity (=3 or 4). The drugs described in the 11 rating scales and the two composite lists published by Durán et al. [27] and Salahudeen et al. [28] were collected into one cumulative anticholinergic review table. Similarly, the drugs listed in the sedative load model (SLM) and the Sloane model were merged into one cumulative sedative review table; the two cumulative review tables were merged into one cumulative table [29, 30].
Drugs with anticholinergic or sedative potency of 0 in different scales were labeled as “no activity,” drugs with a score of 1 were labeled as “low activity,” drugs with a score of 2 were labeled as “moderate activity,” and drugs with a score of 3 or 4 (representing high and very high in the different scales) were labeled as “high activity.” If we encountered discrepancies between multiple scales in classifying a drug’s anticholinergic or sedative potency, we categorized the drug based on the consensus of the majority of the evidence. For example, a drug categorized as low activity in five different scales and moderate activity in two scales would be classified as low activity in the cumulative review table.
If the distinction was unclear, i.e. two scales classified a drug as moderate and another two classified it as high, research scientists conducted additional literature searches to categorize the drug. Accordingly, the drug was classified as “high activity” if an anticholinergic or sedative effect was commonly seen in a majority of patients as reported in clinical studies or in the drug monograph. It was classified as “moderate activity” if the anticholinergic or sedative effect was seen in some patients or seemed to be dose dependent. A drug was classified as “low activity” if the anticholinergic or sedative effect was rare or only reported in sporadic cases and classified as “no activity” if no anticholinergic or sedative effects were reported in clinical studies.
The same strategy was used when a drug’s anticholinergic potencies were reported in the scales but its sedative potency was not described. Drugs were excluded only if they had no sedative activity—as reported by the SLM—and no information to report on activity in any of the anticholinergic scales analyzed or in the literature.
3 Results
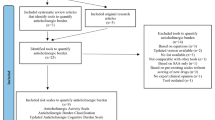
The initial literature assessment produced 3163 potentially relevant articles. The search focused on articles evaluating the anticholinergic burden and/or the sedative load of drugs used in patients aged ≥65 years. We identified 22 scales reporting drug anticholinergic activity and three scales reporting drug sedative activity. Following the elimination of duplicate articles, 3148 remained. The title and abstract of each publication was reviewed to determine eligibility for inclusion in subsequent analyses. The analysis of titles and abstracts identified 2944 papers that did not meet the inclusion criteria and were therefore excluded. Of the 204 studies remaining, 19 were deemed relevant and assessed for a classification of anticholinergic and/or sedative drugs into scales. Of those, 13 studies were included in the final step of our study (Fig. 1); a PRISMA flowchart of the systematic search describes the search strategy results and the process used to identify eligible studies for inclusion in this review.
3.1 Characteristics of the Included Studies
The 13 eligible studies included in this systematic review were published between 2003 and 2019, inclusive; 11 articles reported scales for the anticholinergic activity of drugs, and two articles reported scales for sedative activity. Clinical settings included hospitalized patients; community-based patients; and patients in nursing homes, long-term care facilities, residential homes, and dementia-specific care facilities [14, 21, 22, 26, 29, 30, 37, 38, 43]. Three studies were longitudinal cohort studies, three were prospective studies, one was a community-dwelling cohort study, one was an observational study, and five were literature reviews [14, 22,23,24,25,26, 29, 30, 37, 43,44,45]. The populations were all older adults, and the mean age, functionality, and disease diagnosis varied considerably across study participants. Table 1 provides details of the studies included in our analysis.
3.2 Quality of Evidence Assessment
A quality assessment analysis for the 13 selected studies and scales (see Table S1 in the ESM) was completed using Appendix D of Hawker et al. [41]. The average score among all studies was 32 out of 36 (range 29–35), indicating that most references were of good quality. The lowest scores were observed in the ethics and bias sections. Most publications that did not have an adequate section addressing ethics were merely systematic reviews or literature reviews of other published scales and did not involve a clinical trial.
3.3 Anticholinergic Burden Scales
The 11 anticholinergic burden scales identified by our search strategy were the ACB scale, the modified ACB (mACB) scale, the ADS, the ARS, the Anticholinergic Loading Scale (ALS/ACL), the Anticholinergic Burden Classification (ABC), the AAS, the KABS, the German ACB, the Brazilian AAS, and the Anticholinergic Effect on Cognition (AEC) [14, 21,22,23,24,25,26, 37, 43,44,45].
3.4 Anticholinergic Scales Development and Description
All 11 scales have a common goal: to categorize medications based on their ability to elicit anticholinergic effects and to provide an estimate of the total daily anticholinergic burden in older adults. In contrast, substantial differences were observed between the different scales regarding scale development settings and methods used to determine the anticholinergic activity. These differences were reflected in the variations of anticholinergic potency scores and the number of medications included in each scale. Drug anticholinergic activity is commonly divided into three or four categories, ranging from no activity (=0) to high activity (≥ 3). Scores in all 11 scales analyzed were decided based on the following methods, either directly or indirectly using previously validated scales:
-
1.
Expert-based classification of drugs into lists of drugs with varying anticholinergic activity based on clinical experience.
-
2.
Extensive literature review of medication properties, available studies associating drugs with their SAA through radioreceptor assays and in vitro measurement of drug binding affinity to muscarinic receptors, route of administration, potential drug interaction effects, and potential blood–brain barrier permeability.
Individual drug scores are then summated by either direct addition of scores or using an additional factor to account for drug dosage [14, 21,22,23, 26, 37]. A narrative detailed overview of the development and characteristics of the 11 selected scales is provided in ESM S1.
Most scales—such as the ADS, ACB, and ARS—are based on expert opinion and literature review and thus have been widely applied and validated [11, 21, 22, 26, 46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69]. These scales are considered clinically useful since the classification of drugs is based on outcomes observed by experts in the clinic or reported in the literature. The ARS scale, similar to the ADS scale, considers drug dose and score adjustments based on an individual's total daily dose received relative to the maximum recommended dose [21, 22]. The ARS was developed to predict both central and peripheral effects; the ACB scale only includes medications that are likely to have a negative effect on cognition and has been shown to predict central and peripheral adverse effects related to cumulative anticholinergic exposure [14, 22]. More recent scales, including the mACB, the German ACB, the KABS, and the Brazilian AAS, are based on expert opinion and systematic literature review and also include drugs not previously listed in earlier scales [23,24,25,26].
Figure 2 represents a stacked bar chart for the 11 anticholinergic scales, compiling the total number of drugs in each scale and the percentage of drugs in each anticholinergic potency group including no, low, moderate, and high anticholinergic activity. Interestingly, we found that at least 50% of all drugs listed in the ADS, German ACB, KABS, ACL/ALS, AEC, and AAS did not have any anticholinergic activity and were categorized as such [21, 23, 25, 37, 44, 45]. The ADS, German ACB, and KABS scales reported the highest number of drugs (at least 495), with 5–16% of these drugs having moderate to high anticholinergic activity [21, 23, 25].
Percentage of drugs listed in each anticholinergic potency group for each scale. Stacked bar chart for the 11 anticholinergic scales showing the percentage and number of drugs in each anticholinergic potency group, with 0 for no anticholinergic activity, 1 for low, 2 for moderate, and 3+ for high. The number in parentheses is the total number of drugs in each scale. Bars with no text are less than 6%. AAS Anticholinergic Activity Scale, ABC Anticholinergic Burden Classification, AC Anticholinergic activity, ACB Anticholinergic Cognitive Burden scale, ADS Anticholinergic Drug Scale, AEC Anticholinergic Effect on Cognition, ALS Anticholinergic Load Scale, ARS Anticholinergic Rating Scale, KABS Korean Anticholinergic Burden Scale, mACB modified Anticholinergic Cognitive Burden scale
3.5 Anticholinergic Scales Agreement
The 11 anticholinergic scales (with a total of 810 distinct drugs) were compared head to head to determine the number of drugs in common among all scales and the concordance in scoring of anticholinergic potency among scales. A list of the most classified drugs (N = 29), described in at least nine of the 11, with their anticholinergic potency score as described in each scale is provided in Table 2. Among a total of 810 drugs, 235 distinct drugs were exclusively found in one of the 11 scales: 103 and 59 drugs were solely included in the ADS and KABS scales, respectively. In addition, 404 drugs (~ 50%) scored the same in at least two scales, whereas 140 drugs scored differently in one or more scales. Only amitriptyline was classified as high anticholinergic activity with complete agreement in all 11 scales.
A heatmap showing Spearman’s rank correlation coefficients between scores for medications common to each pair of scales is presented in Fig. 3. With only 27 listed drugs, the ABC scale had the lowest number of correlations (n = 3), i.e. correlation with the Brazilian scale, AAS, and KABS, with coefficients of 0.8, 0.60, and 0.59, respectively. Conversely, the KABS, which includes a total of 494 medications, showed a significant correlation with all ten of the other anticholinergic scales, with a strong average correlation coefficient of 0.79. In addition, the ACB scale (which includes 88 medications) and the Brazilian scale (which includes 125 medications), both showed a significant correlation with nine other anticholinergic scales, with average correlation coefficients of 0.78 and 0.75, respectively. The AEC, the AAS, and the German scale had a significant correlation with eight other scales, the ALS and ADS had a correlation with seven scales, and the ARS and mACB had a correlation with six and five other scales, respectively.
Score agreement between the 11 anticholinergic scales. Heatmap showing Spearman’s rank correlation coefficient between scores for medications common to each pair of scales. Coefficients that were not statistically significant (p ≥ 0.05) are left blank. AAS Anticholinergic Activity Scale, ABC Anticholinergic Burden Classification, ACB Anticholinergic Cognitive Burden scale, ADS Anticholinergic Drug Scale, AEC Anticholinergic Effect on Cognition, ALS Anticholinergic Load Scale, ARS Anticholinergic Rating Scale, KABS Korean Anticholinergic Burden Scale, mACB modified Anticholinergic Cognitive Burden scale
Although the mACB scale, which includes 82 drugs, was developed based on modifying the ACB and the ARS scales, the Spearman’s correlation coefficient between the mACB and the ARS was 0.49 (considered moderate) and did not reach a statistically significant level (p = 0.109) [26]. This could be partly because of the low number of drugs common between the mACB and the ARS (N = 12). In general, correlation coefficients did not reach statistical significance among pairs of scales sharing fewer than 20 drugs in common. Figure S1 in the ESM gives an overview of the number of drugs in common between each pair of scales. This figure shows that the ADS–KABS pair had the highest number of medications in common, with 295 drugs in total, with a strong correlation coefficient of 0.72, followed by the ALS–KABS pair, with 183 drugs in common and a correlation coefficient of 0.64.
3.6 Sedative Burden Scales
The number of sedative burden scales is limited, and only two sedative burden scales met our inclusion criteria: the SLM and the Sloane model (derived from the SLM) [29, 30]. The SLM, which classifies drugs into no, low, moderate, or high sedative potency based on clinical expertise, is the most widely used scale [30].
The comprehensive list of over 900 medications in this publication, along with relevant anatomical therapeutic chemical (ATC) codes, made this model convenient to use in clinical settings [30]. Modifications to the SLM were introduced by Sloane et al. [29] in 2008 for drugs used in their clinical trial. The limitations of the Sloane model (modified SLM) are the small number of drugs included and that all drugs included in this model were also included in the SLM with minor differences in categorizing a few drugs between the two scales [29]. A detailed narrative overview of the development and characteristics of the SLM and Sloane’s scales is provided in ESM S1.
In the SLM, the potency scores for each drug were summated as a total sedative load [30]. A strength of this model is that the sedative load formula included both the measure of dose and the potency of the sedative medication. Even though dosage was not a factor in the original publications, literature from measurement of sedative loads seems to suggest that dosage and sometimes duration of action is considered an important factor in these calculations. The sedative load formula used by Sloane et al. included both to calculate sedative load, making the SLM more nuanced.
3.7 The Comprehensive Review Table
As mentioned in Sect. 3.5, there are inconsistencies and disagreements between scales in scoring anticholinergic drugs. In an attempt to streamline and collate all available evidence, we combined the 810 distinct considered anticholinergic drugs and the 900 considered sedative drugs into one table. After applying the conditions described in Sect. 2.1, we created a comprehensive review table of 642 drugs from the 13 scales (Table S2 in the ESM). In this newly created catalog (ACSBC), 44 drugs are classified as having high anticholinergic activity, 25 as moderate activity, 99 as low activity, and 474 as no activity. These medications have also been assigned a sedative potency. Among the 642 medications, 31 have high sedative activity, 126 have moderate activity, 225 have low sedative activity, and 260 have no sedative activity. Moreover, 618 of the 642 are approved by the FDA, 16 drugs are not approved, and eight drugs have been discontinued.
4 Discussion
To the best of our knowledge, this systematic review is the first to compare both the anticholinergic burden and the sedative load of several drugs regularly used in older adults. This literature review also offers an up-to-date evaluation of clinically relevant anticholinergic and sedative rating scales. As part of this literature search, 11 anticholinergic burden scales and two sedative load scales were selected. The comprehensive review table generated (ACSBC: Table S2 in the ESM) provides the largest catalog of drugs classified by their anticholinergic activity and the second largest repository of drugs classified by their sedative activity. Importantly, the ACSBC is one of the few databases to provide a combined anticholinergic and sedative potency classification of high, moderate, low, or no activity. Several anticholinergic medications have sedative properties. To avoid redundancy between scales and algorithms, drugs with both properties are merged in the ACSBC table. In addition, not all medications with anticholinergic effects necessarily have sedation burden, and vice versa. This table offers a complete summary of drugs with one or both properties (Table S2 in the ESM).
Medications with sedative and/or anticholinergic activity are commonly prescribed in older patients, and until now there has been no standardized consensus for which rating scale best measures drug anticholinergic or sedative activity [11, 70]. The citation analysis of the 13 scales showed that the ARS scale was the most cited of the anticholinergic scales (N=600), followed by the ABC (N=577) and the ADS scales (N = 481) [21, 22, 43]. A recent longitudinal study conducted from a large cohort of more than 500,000 patients (aged 37–73 years) derived anticholinergic burden using the ten most validated anticholinergic scales (including ADS, CrAS, ARS, ACB, Chew’s list, AAS, ALS, AEC, modified ARS, and the Anticholinergic Impregnation Scale [AIS]) [14, 17, 21, 36,37,38, 44, 45, 71]. The authors investigated the association between each scale and adverse outcomes linked to anticholinergic burden such as mortality, cardiovascular events, falls, and cognitive impairments (dementia, delirium). The four most validated scales were used for the assessment of overlap of anticholinergic scales (ARS, ADS, CrAS, ACB) for patients with a score ≥1 on these scales [17]. This study demonstrated that the anticholinergic burden was significantly associated with all-cause mortality and major adverse cardiovascular events (MACE) for each tested scale (in the adjusted model, the hazard ratios were greater for ACB and ADS) [17]. The degree of overlap in the cohort identified as at risk was reported at 23% of these participants scored on all of the four most validated scales. The results of this study showed that, depending on the scale evaluated, anticholinergic medication use varied from 8 to 17.6% [17]. Their results indicated that ARS and ACB showed the highest effect size for the primary outcomes (all-cause mortality/MACE), and effect sizes were greater for neurocognitive outcomes (dementia/delirium) with AEC and AIS scales. Moreover, irrespective of the scale used, there was an association between anticholinergic medication use and mortality, hospital admissions for falls/fractures, dementia/delirium, and cardiovascular events. However, the scale selected influenced the population identified as possibly requiring reduction of anticholinergic burden in clinical practice or intervention trials [17]. It should be noted that the mean age of this cohort was younger (average age 58 years) than previous populations used to validate these scales (mean ages generally > 70 years).
The SLM (N = 46) was the most validated sedative model [30]. A detailed year-to-date analysis is provided in Table S3 in the ESM. Consistent with these findings, our systematic review provides a comprehensive review table that combines all drugs in the ADS and most drugs in the SLM, in addition to the abovementioned scales.
The major strength of existing anticholinergic scales is that they are regularly updated. Published in 2019, the most recently developed scales are the mACB, the German ACB, the KABS, and the Brazilian anticholinergic activity drug scales [23,24,25,26]. These are based on systematic reviews that included previously validated scales, such as the ACB, ADS, and ARS scales, and expert opinion [14, 21, 22]. It is reassuring that the anticholinergic potency assigned to drugs is continually updated. However, these updates have mainly been applied in countries outside of the USA, and an updated version with medications approved by the FDA is lacking. In our comprehensive review table (Table S2 in the ESM), we identified which drugs are FDA approved.
The wide variability in classification of anticholinergic potency of drugs among different scales is considered a major limitation for clinicians. For example, olanzapine is classified as high potency in the ACB scale, moderate potency in the ARS and AAS scales, and low potency in the ADS scale [14, 21, 22]. This variability is because most of these scales are based on individual expert’s opinions. Newer scales such as the mACB, the German ACB, the KABS, and the Brazilian anticholinergic activity drug scale—which are based on systematic reviews—are attempting to reduce this variability by classifying drugs with anticholinergic activity and sedative activity based on specific categorization rules [23,24,25,26]. Expert opinion can also be used in the KABS or German scale as a part of decision making in their categorization rules [23, 25]. Interestingly, the mACB classifies drugs into the highest score among all the scales reviewed regardless of the evidence level [26]. Although it may be convenient, it may not be clinically accurate. To minimize the variability and help reach a consensus on classifying anticholinergic potency, our catalog intends to classify these drugs according to the greatest amount of evidence available, combines sedative and anticholinergic drug properties, and is not limited to drugs approved in a particular country. This is in contrast with some previously published systematic reviews that reported drugs with two or more different anticholinergic potency scores [11, 27, 28].
Other scale-specific limitations originate from the criteria used by different scales. For example, validated and frequently used scales such as the ADS, the ACB, the AAS, and the ALS are based on experts’ clinical experience and knowledge of the medications’ pharmacological properties extracted from literature and serum radioreceptor studies (to detect SAA, which may reflect the peripheral but not necessarily the central anticholinergic activity) [14, 21, 37]. Although this is a quantitative measure, it only measures the peripheral anticholinergic activity and may not clearly reflect central nervous system anticholinergic effects, especially if blood–brain barrier permeability is not considered. Additionally, SAA cannot differentiate between anticholinergic activity of endogenous substances and administered drugs [21]. Consequently, a correlation between SAA and anticholinergic adverse outcomes has not been conclusively confirmed [59, 72]. For example, the ARS scale considered the in vitro drug muscarinic receptor-binding affinity in addition to literature search and expert opinion as part of its development. Although this method can provide a better understanding of the anticholinergic potency of a particular drug, because of their costs, in vitro binding studies have not been conducted for a large proportion of medications on the market [22, 73, 74].
Our analyses also provide the sedative load potencies for each drug concomitantly with its anticholinergic activity score. Unlike drugs with anticholinergic properties, drugs with sedative properties do not have a mutually common pharmacology for sedation, making quantification of the overall effect of taking several drugs with sedative properties difficult [75]. Moreover, defining the term sedation is complex, as sedation is commonly described through both subjective feelings of sleepiness, lethargy/drowsiness, and reduced psychomotor processing [15]. Long-term use of sedative drugs is associated with dementia and not recommended in older adults [76]. However, a large percentage of older adults continue taking long-term sedative medications or drugs with off-target sedative side effects, such as antipsychotics, antidepressants, and antihistamines, which can contribute significantly to the total sedative burden in these patients [16, 77].
The sedative scales that met our inclusion criteria were only the SLM and its modified version published by Sloane et al. [29, 30]. The SLM provides drug classes that are presented with their ATC codes, potentially making it easy to update as the FDA approves newer medications [30]. Potency scores for each drug in a patient drug regimen is combined as a total sedative load, and significant correlation with higher age, female sex, poor basic education, poor health habits, depression, dementia, or impaired mobility was established in clinical studies [78]. At this point, the dosage of drugs was not considered, introducing some limitations to clinical applicability. The comprehensive scope of over 900 medications in this classification, along with relevant ATC codes, made it a convenient reference. The SLM has also been used to describe sedative drug use in patients with dementia [79, 80]. Other groups have used this method to show that increased sedative load increased the likelihood of caries and reduced grip strength [81]. The SLM has been widely applied to many other studies, and it has been suggested that using beta coefficient analysis associated with clinical outcomes may improve calibration of these scales [15].
Though multiple studies use these scales in retrospective medication reviews, few have used the SLM in prospective studies. A major limitation of all sedative scales is that they are based exclusively on clinical opinion. Additionally, these sedative scales have not been updated since they were first published. To our knowledge, with 642 drugs, our comprehensive table is the most updated catalog of FDA-approved anticholinergic and sedative medications (Table S2 in the ESM).
Given the variety of scales available in the literature, it is evident that the detrimental effects of anticholinergic and sedative drug use have been recognized, and an effort has been made by the scientific community to quantify anticholinergic burden and sedative load in older adults [9, 16, 82, 83]. Table S4 in the ESM provides a few examples, i.e. not a comprehensive list, of clinically relevant scale-validation studies that evaluated different anticholinergic and sedative rating scales with various clinical outcomes and results in the elderly. Studies aimed at the development and validation of a method that can longitudinally measure the burden of both anticholinergic and sedative drugs are needed. The establishment of the reliability and relevance of such a combined method will be helpful in improving healthcare, as long as it is always up to date to guide physicians and healthcare professionals in prescribing/deprescribing practices for potentially inappropriate medications in older populations. Further, a study using a validated and prospectively applied anticholinergic scale that accounts for anticholinergic drugs and clinical outcomes will lend greater strength to this effort.
Our systematic review had multiple strengths, including our inclusion criteria of studies that only used a standardized scale, followed by our data extraction methods and the quality of evidence obtained, as our systematic review was developed and revised by several clinical and nonclinical scientists. However, a possible limitation of our review is that it was not possible to conduct a quantitative meta-analysis because of the discrepancies between available anticholinergic scales and the very small number of available sedative load models. The consensus procedure used to create a new anticholinergic and sedative grading of potency is another potential limitation. We proposed four categories (i.e., no activity, low, moderate, and high) based on the highest level of evidence and consensus among different rating scales.
5 Conclusion
Our systematic review identified a high degree of variability and inconsistency among scales used to categorize drugs with anticholinergic or sedative burden. Several drugs possess both of these pharmacological properties, and the predictive value of the derived burden scores on outcomes could be difficult to establish. We have generated a comprehensive table that regroups a large number of drugs and, more importantly, proposes a new categorization of these drugs. Validation of a method that can longitudinally measure both anticholinergic and sedative drug burden in an accurate and evidence-based manner would be immensely useful for improving the healthcare and quality of life of older adults. Large clinical databases (e.g. UK BioBank) are currently being made available to researchers to perform simulation and validation studies using real-world data. This would allow testing of new models and algorithms without conducting long and expensive clinical trials. Furthermore, simulation studies with the virtual addition of drugs would also prevent exposure of patients to drugs and their side effects.
References
Halli-Tierney AD, Scarbrough C, Carroll D. Polypharmacy: evaluating risks and deprescribing. Am Fam Physician. 2019;100(1):32–8.
Hales CM SJ, Martin CB, Kohen D. Prescription drug use among adults aged 40–79 in the United States and Canada. NCHS Data Brief No 347. 2019. https://www.cdc.gov/nchs/products/databriefs/db347.htm#::text=Nearly%207%20in%2010%20adults,and%2018.8%25%20in%20Canada.
Hajjar ER, Cafiero AC, Hanlon JT. Polypharmacy in elderly patients. Am J Geriatr Pharmacother. 2007;5(4):345–51.
Barclay K, Frasetto A, Robb J, Mandel ED. Polypharmacy in the elderly: how to reduce adverse drug events. Clin Rev. 2018;28(2):38–44.
Turgeon J, Michaud V, Steffen L. The dangers of polypharmacy in elderly patients. JAMA Intern Med. 2017;177(10):1544.
Gadzhanova S, Roughead E, Robinson M. Use of medicines with anticholinergic and sedative effect before and after initiation of anti-dementia medications. Drugs Real World Outcomes. 2015;2(1):53–60.
Bell JS, Mezrani C, Blacker N, LeBlanc T, Frank O, Alderman CP, et al. Anticholinergic and sedative medicines—prescribing considerations for people with dementia. Aust Fam Physician. 2012;41(1–2):45–9.
Gnjidic D, Cumming RG, Le Couteur DG, Handelsman DJ, Naganathan V, Abernethy DR, et al. Drug Burden Index and physical function in older Australian men. Br J Clin Pharmacol. 2009;68(1):97–105.
Lim R, Kalisch Ellett LM, Widagdo IS, Pratt NL, Roughead EE. Analysis of anticholinergic and sedative medicine effects on physical function, cognitive function, appetite and frailty: a cross-sectional study in Australia. BMJ Open. 2019;9(9):e029221.
Taipale HT, Bell JS, Gnjidic D, Sulkava R, Hartikainen S. Sedative load among community-dwelling people aged 75 years or older: association with balance and mobility. J Clin Psychopharmacol. 2012;32(2):218–24.
Lozano-Ortega G, Johnston KM, Cheung A, Wagg A, Campbell NL, Dmochowski RR, et al. A review of published anticholinergic scales and measures and their applicability in database analyses. Arch Gerontol Geriatr. 2020;87:103885.
Risacher SL, McDonald BC, Tallman EF, West JD, Farlow MR, Unverzagt FW, et al. Association between anticholinergic medication use and cognition, brain metabolism, and brain atrophy in cognitively normal older adults. JAMA Neurol. 2016;73(6):721–32.
Mintzer J, Burns A. Anticholinergic side-effects of drugs in elderly people. J R Soc Med. 2000;93(9):457–62.
Boustani M, Campbell N, Munger S, Maidment I, Fox C. Impact of anticholinergics on the aging brain: a review and practical application. Aging Health. 2008;4(3):311–20.
Taipale HT, Hartikainen S, Bell JS. A comparison of four methods to quantify the cumulative effect of taking multiple drugs with sedative properties. Am J Geriatr Pharmacother. 2010;8(5):460–71.
Wouters H, Hilmer SN, Gnjidic D, Van Campen JP, Teichert M, Van Der Meer HG, et al. Long-term exposure to anticholinergic and sedative medications and cognitive and physical function in later life. J Gerontol A. 2019;75(2):357–65.
Hanlon P, Quinn TJ, Gallacher KI, Myint PK, Jani BD, Nicholl BI, et al. Assessing risks of polypharmacy involving medications with anticholinergic properties. Ann Fam Med. 2020;18(2):148–55.
Byrne CJ, Walsh C, Cahir C, Ryan C, Williams DJ, Bennett K. Anticholinergic and sedative drug burden in community-dwelling older people: a national database study. BMJ Open. 2018;8(7):e022500.
Hilmer SN, Mager DE, Simonsick EM, Cao Y, Ling SM, Windham BG, et al. A drug burden index to define the functional burden of medications in older people. Arch Intern Med. 2007;167(8):781–7.
O’Connell J, Burke É, Mulryan N, O’Dwyer C, Donegan C, McCallion P, et al. Drug burden index to define the burden of medicines in older adults with intellectual disabilities: an observational cross-sectional study. Br J Clin Pharmacol. 2018;84(3):553–67.
Carnahan RM, Lund BC, Perry PJ, Pollock BG, Culp KR. The Anticholinergic Drug Scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. J Clin Pharmacol. 2006;46(12):1481–6.
Rudolph JL, Salow MJ, Angelini MC, McGlinchey RE. The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch Intern Med. 2008;168(5):508–13.
Kiesel EK, Hopf YM, Drey M. An anticholinergic burden score for German prescribers: score development. BMC Geriatr. 2018;18(1):239.
Nery RT, Reis AMM. Development of a Brazilian anticholinergic activity drug scale. Einstein (Sao Paulo). 2019;17(2):eAO4435-eAO.
Jun K, Hwang S, Ah YM, Suh Y, Lee JY. Development of an Anticholinergic Burden Scale specific for Korean older adults. Geriatr Gerontol Int. 2019;19(7):628–34.
Kable A, Fullerton A, Fraser S, Palazzi K, Hullick C, Oldmeadow C, et al. Comparison of potentially inappropriate medications for people with dementia at admission and discharge during an unplanned admission to hospital: results from the SMS Dementia Study. Healthcare (Basel). 2019;7(1):8.
Durán CE, Azermai M, Vander Stichele RH. Systematic review of anticholinergic risk scales in older adults. Eur J Clin Pharmacol. 2013;69(7):1485–96.
Salahudeen MS, Duffull SB, Nishtala PS. Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review. BMC Geriatr. 2015;15(1):31.
Sloane P, Ivey J, Roth M, Roederer M, Williams CS. Accounting for the sedative and analgesic effects of medication changes during patient participation in clinical research studies: measurement development and application to a sample of institutionalized geriatric patients. Contemp Clin Trials. 2008;29(2):140–8.
Linjakumpu T, Hartikainen S, Klaukka T, Koponen H, Kivelä SL, Isoaho R. A model to classify the sedative load of drugs. Int J Geriatr Psychiatry. 2003;18(6):542–4.
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1.
Methley AM, Campbell S, Chew-Graham C, McNally R, Cheraghi-Sohi S. PICO, PICOS and SPIDER: a comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv Res. 2014;14:579.
Vogel F. Moderne Probleme der Humangenetik. In: Heilmeyer L, Schoen R, de Rudder B, editors. Ergebnisse der Inneren Medizin und Kinderheilkunde. Springer, Berlin Heidelberg; 1959. pp. 52–125.
Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JPT, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;2019:10.
Higgins J, Thomas, J, Chandler, J, Cumpston, M, Li, T, Page, MJ, Welch, VA (editors). Cochrane handbook for systematic reviews of interventions version 6.1. 2020. www.training.cochrane.org/handbook.
Chew ML, Mulsant BH, Pollock BG, Lehman ME, Greenspan A, Mahmoud RA, et al. Anticholinergic activity of 107 medications commonly used by older adults. J Am Geriatr Soc. 2008;56(7):1333–41.
Ehrt U, Broich K, Larsen JP, Ballard C, Aarsland D. Use of drugs with anticholinergic effect and impact on cognition in Parkinson’s disease: a cohort study. J Neurol Neurosurg Psychiatry. 2010;81(2):160–5.
Han L, McCusker J, Cole M, Abrahamowicz M, Primeau F, Elie M. Use of medications with anticholinergic effect predicts clinical severity of delirium symptoms in older medical inpatients. Arch Intern Med. 2001;161(8):1099–105.
Summers WK. A clinical method of estimating risk of drug induced delirium. Life Sci. 1978;22(17):1511–6.
Carnahan RM, Lund BC, Perry PJ, Culp KR, Pollock BG. The relationship of an anticholinergic rating scale with serum anticholinergic activity in elderly nursing home residents. Psychopharmacol Bull. 2002;36(4):14–9.
Hawker S, Payne S, Kerr C, Hardey M, Powell J. Appraising the evidence: reviewing disparate data systematically. Qual Health Res. 2002;12(9):1284–99.
Evans JD. Straightforward statistics for the behavioral sciences. Thomson Brooks/Cole Publishing Co; 1996.
Ancelin ML, Artero S, Portet F, Dupuy AM, Touchon J, Ritchie K. Non-degenerative mild cognitive impairment in elderly people and use of anticholinergic drugs: longitudinal cohort study. BMJ. 2006;332(7539):455–9.
Bishara D, Harwood D, Sauer J, Taylor DM. Anticholinergic effect on cognition (AEC) of drugs commonly used in older people. Int J Geriatr Psychiatry. 2017;32(6):650–6.
Sittironnarit G, Ames D, Bush AI, Faux N, Flicker L, Foster J, et al. Effects of anticholinergic drugs on cognitive function in older Australians: results from the AIBL study. Dement Geriatr Cogn Disord. 2011;31(3):173–8.
Kashyap M, Belleville S, Mulsant BH, Hilmer SN, Paquette A, Tu LM, et al. Methodological challenges in determining longitudinal associations between anticholinergic drug use and incident cognitive decline. J Am Geriatr Soc. 2014;62(2):336–41.
Koyama A, Steinman M, Ensrud K, Hillier TA, Yaffe K. Long-term cognitive and functional effects of potentially inappropriate medications in older women. J Gerontol A Biol Sci Med Sci. 2014;69(4):423–9.
Mangoni AA, van Munster BC, Woodman RJ, de Rooij SE. Measures of anticholinergic drug exposure, serum anticholinergic activity, and all-cause postdischarge mortality in older hospitalized patients with hip fractures. Am J Geriatr Psychiatry. 2013;21(8):785–93.
Pfistermeister B, Tümena T, Gaßmann K-G, Maas R, Fromm MF. Anticholinergic burden and cognitive function in a large German cohort of hospitalized geriatric patients. PLoS ONE. 2017;12(2):e0171353.
Hsu WH, Wen YW, Chen LK, Hsiao FY. Comparative associations between measures of anti-cholinergic burden and adverse clinical outcomes. Ann Fam Med. 2017;15(6):561–9.
Kersten H, Molden E, Tolo IK, Skovlund E, Engedal K, Wyller TB. Cognitive effects of reducing anticholinergic drug burden in a frail elderly population: a randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2013;68(3):271–8.
Aalto UL, Finne-Soveri H, Kautiainen H, Öhman H, Roitto HM, Pitkälä KH. Relationship between anticholinergic burden and health-related quality of life among residents in long-term care. J Nutr Health Aging. 2021;25(2):224–9.
Andersen F, Viitanen M, Halvorsen DS, Straume B, Engstad TA. Co-morbidity and drug treatment in Alzheimer’s disease. A cross sectional study of participants in the dementia study in northern Norway. BMC Geriatr. 2011;11:58.
Brombo G, Bianchi L, Maietti E, Malacarne F, Corsonello A, Cherubini A, et al. Association of anticholinergic drug burden with cognitive and functional decline over time in older inpatients: results from the CRIME project. Drugs Aging. 2018;35(10):917–24.
Cross AJ, George J, Woodward MC, Ames D, Brodaty H, Ilomäki J, et al. Potentially inappropriate medications and anticholinergic burden in older people attending memory clinics in Australia. Drugs Aging. 2016;33(1):37–44.
Herrero-Zazo M, Berry R, Bines E, Bhattacharya D, Myint PK, Keevil VL. Anticholinergic burden in older adult inpatients: patterns from admission to discharge and associations with hospital outcomes. Ther Adv Drug Saf. 2021;12:20420986211012590.
Hsu WH, Huang ST, Lu WH, Wen YW, Chen LK, Hsiao FY. Impact of Multiple Prescriptions With Anticholinergic Properties On Adverse Clinical Outcomes In The Elderly: A Longitudinal Cohort Study In Taiwan. Clin Pharmacol Ther. 2021.
Kose E, Hirai T, Seki T. Assessment of aspiration pneumonia using the Anticholinergic Risk Scale. Geriatr Gerontol Int. 2018;18(8):1230–5.
Lampela P, Lavikainen P, Garcia-Horsman JA, Bell JS, Huupponen R, Hartikainen S. Anticholinergic drug use, serum anticholinergic activity, and adverse drug events among older people: a population-based study. Drugs Aging. 2013;30(5):321–30.
Lowry E, Woodman RJ, Soiza RL, Mangoni AA. Associations between the anticholinergic risk scale score and physical function: potential implications for adverse outcomes in older hospitalized patients. J Am Med Dir Assoc. 2011;12(8):565–72.
Mate KE, Kerr KP, Pond D, Williams EJ, Marley J, Disler P, et al. Impact of multiple low-level anticholinergic medications on anticholinergic load of community-dwelling elderly with and without dementia. Drugs Aging. 2015;32(2):159–67.
Moga DC, Abner EL, Rigsby DN, Eckmann L, Huffmyer M, Murphy RR, et al. Optimizing medication appropriateness in older adults: a randomized clinical interventional trial to decrease anticholinergic burden. Alzheimer’s Res Ther. 2017;9(1):36.
Pond CD, Brodaty H, Stocks NP, Gunn J, Marley J, Disler P, et al. Ageing in general practice (AGP) trial: a cluster randomised trial to examine the effectiveness of peer education on GP diagnostic assessment and management of dementia. BMC Fam Pract. 2012;13(1):12.
Vetrano DL, La Carpia D, Grande G, Casucci P, Bacelli T, Bernabei R, et al. Anticholinergic medication burden and 5-year risk of hospitalization and death in nursing home elderly residents with coronary artery disease. J Am Med Dir Assoc. 2016;17(11):1056–9.
Richardson K, Fox C, Maidment I, Steel N, Loke YK, Arthur A, et al. Anticholinergic drugs and risk of dementia: case-control study. BMJ (Clin Res Ed). 2018;361:k1315-k.
Campbell NL, Boustani MA, Lane KA, Gao S, Hendrie H, Khan BA, et al. Use of anticholinergics and the risk of cognitive impairment in an African American population. Neurology. 2010;75(2):152–9.
Fox C, Richardson K, Maidment ID, Savva GM, Matthews FE, Smithard D, et al. Anticholinergic medication use and cognitive impairment in the older population: the medical research council cognitive function and ageing study. J Am Geriatr Soc. 2011;59(8):1477–83.
Pasina L, Colzani L, Cortesi L, Tettamanti M, Zambon A, Nobili A, et al. Relation between delirium and anticholinergic drug burden in a cohort of hospitalized older patients: an observational study. Drugs Aging. 2019;36(1):85–91.
Lockery JE, Broder JC, Ryan J, Stewart AC, Woods RL, Chong TT, et al. A cohort study of anticholinergic medication burden and incident dementia and stroke in older adults. J Gen Intern Med. 2021;36(6):1629–37.
Kersten H, Wyller TB. Anticholinergic drug burden in older people’s brain—how well is it measured? Basic Clin Pharmacol Toxicol. 2014;114(2):151–9.
Sumukadas D, McMurdo ME, Mangoni AA, Guthrie B. Temporal trends in anticholinergic medication prescription in older people: repeated cross-sectional analysis of population prescribing data. Age Ageing. 2014;43(4):515–21.
Salahudeen MS, Chyou TY, Nishtala PS. Serum anticholinergic activity and cognitive and functional adverse outcomes in older people: a systematic review and meta-analysis of the literature. PLoS ONE. 2016;11(3):e0151084.
Xu D, Anderson HD, Tao A, Hannah KL, Linnebur SA, Valuck RJ, et al. Assessing and predicting drug-induced anticholinergic risks: an integrated computational approach. Ther Adv Drug Saf. 2017;8(11):361–70.
Rudd KM, Raehl CL, Bond C, Abbruscato TJ, Stenhouse AC. Methods for assessing drug-related anticholinergic activity. Pharmacotherapy. 2005;25(11):1592–601.
Bourin M, Briley M. Sedation, an unpleasant, undesirable and potentially dangerous side-effect of many psychotropic drugs. Hum Psychopharmacol: Clin Exp. 2004;19(2):135–9.
Brandt J, Leong C. Benzodiazepines and Z-Drugs: an updated review of major adverse outcomes reported on in epidemiologic research. Drugs R D. 2017;17(4):493–507.
van der Meer HG, Taxis K, Teichert M, Griens F, Pont LG, Wouters H. Anticholinergic and sedative medication use in older community-dwelling people: a national population study in the Netherlands. Pharmacoepidemiol Drug Saf. 2019;28(3):315–21.
Linjakumpu TA, Hartikainen SA, Klaukka TJ, Koponen HJ, Hakko HH, Viilo KM, et al. Sedative drug use in the home-dwelling elderly. Ann Pharmacother. 2004;38(12):2017–22.
Bell JS, Taipale HT, Soini H, Pitkälä KH. Sedative load among long-term care facility residents with and without dementia. Clin Drug Investig. 2010;30(1):63–70.
Parsons C, Haydock J, Mathie E, Baron N, Machen I, Stevenson E, et al. Sedative load of medications prescribed for older people with dementia in care homes. BMC Geriatr. 2011;11(1):56.
Taipale HT, Bell JS, Gnjidic D, Sulkava R, Hartikainen S. Muscle strength and sedative load in community-dwelling people aged 75 years and older: a population-based study. J Gerontol A. 2011;66A(12):1384–92.
Marcum ZA, Perera S, Thorpe JM, Switzer GE, Gray SL, Castle NG, et al. Anticholinergic use and recurrent falls in community-dwelling older adults: findings from the health ABC study. Ann Pharmacother. 2015;49(11):1214–21.
López-Álvarez J, Sevilla-Llewellyn-Jones J, Agüera-Ortiz L. Anticholinergic drugs in geriatric psychopharmacology. Front Neurosci. 2019;13:1309.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research received no external funding. Tabula Rasa HealthCare (TRHC) sponsors TRHC Precision Pharmacotherapy Research and Development Institute research activities. The funder (TRHC board of directors) had no role in the study design or the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Conflicts of interest
Sweilem B Al Rihani, Malavika Deodhar, Lucy I Darakjian, Pamela Dow, Matt K Smith, Ravil Bikmetov, Jacques Turgeon, and Veronique Michaud are employees and shareholders of Tabula Rasa HealthCare.
Ethics approval
Not applicable.
Consent
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author Contributions
SBAR, MD, LID, PD, JT, and VM wrote the manuscript. PD, JT, and VM edited the manuscript. SBAR, MD, LID, PD, MKS, RB, JT, and VM provided graphics for the manuscript. SBAR, MD, LID, MKS, and RB performed research for the manuscript. JT and VM designed the research. JT and VM supervised the research. All authors read and approved the final version of the paper, and agree to be accountable for the work presented in the manuscript.
Acknowledgements
The authors thank Dana Filippoli, Communications Consultant, TRHC, for her comprehensive review and comments on the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Al Rihani, S.B., Deodhar, M., Darakjian, L.I. et al. Quantifying Anticholinergic Burden and Sedative Load in Older Adults with Polypharmacy: A Systematic Review of Risk Scales and Models. Drugs Aging 38, 977–994 (2021). https://doi.org/10.1007/s40266-021-00895-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40266-021-00895-x