Abstract
Elacestrant (ORSERDU™) is an orally available selective estrogen receptor degrader (SERD) being developed by Stemline Therapeutics, a subsidiary of Menarini Group, for the treatment of estrogen receptor (ER)-positive, human epidermal growth factor receptor 2 (HER2)-negative breast cancer. In January 2023, elacestrant received its first approval for the treatment of postmenopausal women or adult men with ER-positive, HER2-negative, estrogen receptor 1 (ESR1)-mutated (as determined by a US FDA-approved test) advanced or metastatic breast cancer with disease progression following ≥ 1 line of endocrine therapy in the USA. A regulatory assessment of elacestrant for the treatment of ER-positive, HER2-negative advanced or metastatic breast cancer is currently underway in the EU. Development of elacestrant for the treatment of vasomotor symptoms has been discontinued. This article summarizes the milestones in the development of elacestrant leading to this first approval for this indication.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Digital Features for this AdisInsight Report can be found at https://doi.org/10.6084/m9.figshare.22273063. |
An orally available SERD being developed by Stemline Therapeutics, a subsidiary of Menarini Group, for the treatment of ER-positive, HER2-negative breast cancer |
Received its first approval on 27 January 2023 in the USA |
Approved for use in postmenopausal women or adult men with ER-positive, HER2-negative, ESR1-mutated (as determined by a US FDA-approved test) advanced or metastatic breast cancer with disease progression following ≥ 1 line of endocrine therapy |
1 Introduction
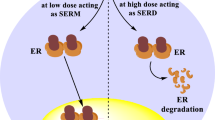
Endocrine therapy plays a pivotal role in the management of early and advanced estrogen receptor (ER)-positive breast cancer [1]. These tumours (which express ERα) depend upon estrogen-mediated growth signalling, with endocrine therapy targeting the ER directly [by competitively binding to the ER and either mediating a tissue-dependent anti-estrogen effect (selective ER modulators; SERMs) or antagonizing ER transcriptional activity and promoting ER degradation (selective ER degraders; SERDs)] and/or reducing the levels of circulating estrogens available to bind to the ER [by blocking the aromatization of androgens to estrogen (aromatase inhibitors)]. Disease progression in patients with ER-positive metastatic breast cancer receiving endocrine therapy occurs as a result of acquired or intrinsic resistance mechanisms; resistance is driven by ER-mediated signalling (that promotes tumour proliferation in the absence of the hormone) and ER-independent signalling (that circumvents endocrine regulated transcription pathways). Mutations in the gene (ESR1) encoding ERα induce ER activity and upregulate ER-dependent gene transcription, which provokes resistance to estrogen deprivation and aromatase inhibitor therapy [1].
Among the multiple oral SERDs in development is elacestrant (ORSERDU™), which is being developed by Stemline Therapeutics, a subsidiary of Menarini Group, for the treatment of ER-positive, human epidermal growth factor receptor 2 (HER2)-negative breast cancer [2]. On 27 January 2023, elacestrant received its first approval for the treatment of postmenopausal women or adult men with ER-positive, HER2-negative, ESR1-mutated (as determined by a US FDA-approved test) advanced or metastatic breast cancer with disease progression following ≥ 1 line of endocrine therapy in the USA [2,3,4].

Elacestrant is available as 345 mg (equivalent to 400 mg elacestrant dihydrochloride) and 86 mg (equivalent to 100 mg elacestrant dihydrochloride) tablets [3]. The recommended dosage is 345 mg, administered orally, once daily until disease progression or unacceptable toxicity. Elacestrant should be swallowed whole and taken with food (to reduce nausea and vomiting) and at approximately the same time each day. Local prescribing information should be consulted for information regarding dose modification recommendations in the event of adverse reactions and for patients with moderate hepatic impairment. The use of elacestrant should be avoided in patients receiving strong or moderate cytochrome P450 (CYP)3A4 inducers and inhibitors and in those with severe hepatic impairment (Child-Pugh C) [3]. A regulatory assessment of elacestrant for the treatment of ER-positive, HER2-negative advanced or metastatic breast cancer is currently underway in the EU [5]. Development of elacestrant for the treatment of vasomotor symptoms has been discontinued [6].
1.1 Company Agreements
Elacestrant was originally discovered by Eisai before being licensed to Radius Health in June 2006 [7]. Under the terms of the agreement, Radius Health acquired exclusive worldwide rights, excluding Japan [7]. In March 2015, Radius Health obtained a licence from Eisai to develop and market elacestrant in Japan, meaning that it had acquired the full global rights to develop and commercialize the drug [8]. Menarini Group and Radius Health entered into an exclusive global licence agreement in July 2020 for the development and commercialization of elacestrant [9]. Under the terms of the agreement, Menarini Group will be responsible for the worldwide commercialization of elacestrant, following completion of the EMERALD phase III study and, assuming positive results, the successful registration of elacestrant [9]. Stemline Therapeutics will commercialize elacestrant in the USA [2].

2 Scientific Summary
2.1 Pharmacodynamics
Elacestrant is an ER antagonist that selectively binds to ERα over ERβ (half maximal inhibitory concentration 48 vs 870 nmol/L) [3, 10]. In ER-positive breast cancer cell lines, elacestrant inhibited ER expression through degradation, and estradiol-mediated cell proliferation [10,11,12]. Moreover, in a phase I study (NCT02650817) in heavily pretreated postmenopausal women with ER-positive, HER2-negative advanced breast cancer (n = 16), it reduced the availability of ER (for binding 17β-estradiol) in tumour metastases by a median of 89% from baseline (primary endpoint); 75% of patients displayed a reduction of ≥ 75% (a value that is thought to be clinically relevant) [13]. In mouse xenograft models of breast cancer [10,11,12] and in patient-derived ER-positive tumour xenograft models [11], elacestrant inhibited estradiol-activated tumour growth. It has also displayed antitumour activity in patient-derived ER-positive tumour xenograft models resistant to cyclin-dependent kinase 4/6 (CDK4/6) inhibitors [14] and those harboring ESR1 mutations [11, 15].
In a phase I study [ELIPSE (NCT04797728)] [16], elacestrant 345 mg once daily for 4 weeks was associated with complete cell cycle arrest (defined as Ki67 ≤ 2.7%) in 6 (27%) of 22 postmenopausal women with ER-positive, HER2-negative early breast cancer amenable to surgery. A Ki67 ≤ 10% was achieved in 64% of patients [16].
At the recommended dose, elacestrant is not associated with a mean > 20 msec increase in corrected QT interval [3].
2.2 Pharmacokinetics
Elacestrant maximum concentration (Cmax) and area under the concentration–time curve (AUC) values increase more than dose proportionally over a 43–862 mg once daily (i.e. 0.125–2.5-fold the recommended dosage) dosage range [3]. The oral bioavailability of elacestrant is ≈ 10% and the time to Cmax is 1–4 h. Elacestrant steady state is reached by day 6 and its mean accumulation ratio (based on AUC0–24 h) is twofold. Elacestrant is > 99% bound to plasma proteins, with binding independent of concentration. It is predominately metabolized by CYP3A4 and, to a lesser extent, by CYP2A6 and CYP2C9. Following the administration of a single oral 345 mg dose of radiolabelled elacestrant, 82% (34% unchanged) and 7.5% (< 1% unchanged) of the dose was recovered in the faeces and urine. Elacestrant has an elimination half-life of 30–50 h [3].
The pharmacokinetics of elacestrant are not affected to a clinically relevant extent by age (24–89 years), sex and body weight (41–143 kg) [3]. While no clinically relevant differences in elacestrant Cmax and AUC values were seen in patients with mild hepatic impairment (Child-Pugh A), AUC was increased by 83% in those with moderate hepatic impairment (Child-Pugh B) [Sect. 1]. Elacestrant has not been studied in patients with severe hepatic impairment (Child-Pugh C) [Sect. 1] [3].
As elacestrant is a CYP3A4 substrate, its coadministration with a moderate or strong CYP3A4 inhibitor increases elacestrant exposure (which may increase the risk of elacestrant adverse reactions) while the concomitant use of elacestrant with a moderate or strong CYP3A4 inducer decreases elacestrant exposure (potentially reducing the effectiveness of elacestrant) [3]. Consequently, the concomitant use of elacestrant with a moderate or strong CYP3A4 inhibitor or a moderate or strong CYP3A4 inducer should be avoided. Elacestrant is an inhibitor of P-gp and BRCP; thus, its concomitant use with P-gp substrates and BRCP substrates increases the concentration of these substrates, potentially increasing the risk of adverse reactions related to the substrates. Consequently, the dosage of P-gp substrates and BRCP substrates should be reduced when minimal changes to the substrates’ concentrations may lead to serious or life-threatening adverse reactions [3].
Features and properties of elacestrant
Alternative names | ORSERDU; RAD 1901 |
|---|---|
Class | Amines; Antineoplastics; Ethers; Small molecules; Tetrahydronaphthalenes |
Mechanism of action | Selective estrogen receptor degrader |
Route of administration | Oral |
Pharmacodynamics | Selectively binds to ERα over ERβ (half maximal inhibitory concentration 48 vs 870 nmol/L); displayed antitumour activity in patient-derived ER-positive tumour xenograft models, including those resistant to cyclin-dependent kinase 4/6 inhibitors and those harboring estrogen receptor 1 mutations |
Pharmacokinetics | Cmax and AUC values increase more than dose proportionally over a 43–862 mg once daily (i.e. 0.125–2.5-fold the recommended dosage) dosage range; oral bioavailability of ≈ 10%; time to Cmax of 1–4 h; elimination half-life of 30–50 h |
Most frequent adverse events | Nausea, fatigue, vomiting and decreased appetite |
ATC codes | |
WHO ATC code | L02B-A04 (Elacestrant) |
EphMRA ATC code | G3X (Other Sex Hormones and Similar Products); L1 (Antineoplastics) |
Chemical name | (6R)-6-[2-[ethyl-[[4-[2-(ethylamino)ethyl]phenyl]methyl]amino]-4-methoxyphenyl]-5,6,7,8-tetrahydronaphthalen-2-ol |
2.3 Therapeutic Trials
Monotherapy with oral elacestrant significantly reduced the risk of disease progression or death relative to standard of care (SOC) monotherapy in postmenopausal women and adult men with ER-positive, HER2-negative advanced or metastatic breast cancer participating in a randomized, open-label, multinational phase III study [EMERALD (NCT03778931)] [17]. Patients eligible for this study had histologically or cytologically proven disease that had progressed during or within 28 days of treatment with one or two prior lines of endocrine therapy (one of which contained a CDK4/6 inhibitor in combination with either fulvestrant or an aromatase inhibitor). One prior line of chemotherapy in the advanced or metastatic setting was permitted [17]. Patients received elacestrant 345 mg once daily or SOC (anastrozole, exemestane, fulvestrant or letrozole; all dosed according to their labelling) until disease progression or unacceptable toxicity [3, 17].
At a median follow-up duration of 15.1 months (data cut-off 6 September 2021), median blinded independent central review (BICR)-assessed progression-free survival (PFS) [primary endpoint] was 2.8 months with elacestrant (n = 239) and 1.9 months with SOC (n = 238) [hazard ratio (HR) 0.70 (95% CI 0.55–0.88); p = 0.002] in the overall population and 3.8 months with elacestrant (n = 115) and 1.9 months with SOC (n = 113) [HR 0.55 (95% CI 0.39–0.77); p = 0.0005] in the ESR1 mutation population [17, 18]. In landmark analyses, BICR PFS rates were 34.3% in the elacestrant group versus 20.4% in the SOC group at 6 months, 22.3% versus 9.4% at 12 months and 16.8% versus 0% at 18 months in the overall population, and 40.8% versus 19.1% at 6 months, 26.8% versus 8.2% at 12 months and 24.3% versus 0% at 18 months in the ESR1 mutation population [17, 18]. HRs for BICR-assessed PFS numerically favored elacestrant over SOC across the prespecified subgroups, with interaction p-values suggesting that the benefit of elacestrant on this endpoint was subgroup independent [17]. At the time of the PFS analysis a prespecified interim overall survival (OS; key secondary endpoint) analysis was conducted. At this timepoint, 149 events had occurred in all patients [HR 0.75 (95% CI 0.54–1.04)] and 68 events in patients with ESR1 mutations [HR 0.59 (95% CI 0.36–0.96); p = 0.03 (nonsignificant)]. A final OS analysis will occur when ≈ 50% of patients have died [17].
In an updated analysis of overall population data, median PFS was 2.8 months with elacestrant (n = 202) and 1.9 months with SOC (n = 205) [HR 0.69 (95% CI 0.54–0.88)] in patients who received ≥ 6 months of prior endocrine plus CDK4/6 inhibitor therapy, 3.8 months with elacestrant (n = 150) and 1.9 months with SOC (n = 160) [HR 0.61 (95% CI 0.45–0.83)] in those who received ≥ 12 months of prior endocrine plus CDK4/6 inhibitor therapy, and 5.5 months with elacestrant (n = 98) and 3.3 months with SOC (n = 119) [HR 0.70 (95% CI 0.48–1.02)] in those who received ≥ 18 months of prior endocrine plus CDK4/6 inhibitor therap [19]. In an updated analysis of ESR1 mutation population data, median PFS was 4.1 months with elacestrant (n = 103) and 1.9 months with SOC (n = 102) [HR 0.52 (95% CI 0.36–0.74)] in patients who received ≥ 6 months of prior endocrine plus CDK4/6 inhibitor therapy, 8.6 months with elacestrant (n = 78) and 1.9 months with SOC (n = 81) [HR 0.41 (95% CI 0.26–0.63)] in those who received ≥ 12 months of prior endocrine plus CDK4/6 inhibitor therapy, and 8.6 months with elacestrant (n = 55) and 2.1 months with SOC (n = 56) [HR 0.47 (95% CI 0.20–0.79)] in those who received ≥ 18 months of prior endocrine plus CDK4/6 inhibitor therap [19].
Patients with symptomatic metastatic visceral disease, and those who experienced various cardiovascular events [including coagulopathy (thrombosis), coronary/peripheral artery bypass graft, myocardial infarction, New York Heart Association Class II or greater heart failure, ongoing grade ≥ 2 cardiac dysrhythmias, severe/unstable angina, and uncontrolled atrial fibrillation] within 6 months of enrollment were among those excluded from EMERALD [17]. Randomization to treatment arms (elacestrant or SOC) was stratified by ESR1 mutational status (detected vs not detected), presence of visceral metastases (yes vs no) and previous treatment with fulvestrant (yes vs no) [3, 17]. At baseline, median patient age was 63 years; 47.8% of 477 patients had a detectable ESR1 mutation and 43.4% had received two prior lines of endocrine therapy [17].
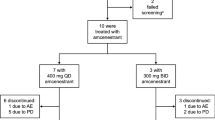
The findings of EMERALD are supported by efficacy data from heavily pretreated postmenopausal women with ER-positive, HER2-negative locally advanced and/or metastatic breast cancer participating in an open-label, multicentre, phase I dose-escalation study (NCT02338349) [20]. In the response-evaluable population (n = 31 receiving elacestrant 345 mg once daily), a partial response was seen in 6 (19.4%) patients [including those who had previously received CDK4/6 inhibitor (16.7%) and prior SERD (15.0%) therapy] and stable disease in 12 (38.7%) patients. The median time to and the median duration of response was 1.9 and 5.8 months. In the clinical benefit-evaluable population (n = 47 receiving elacestrant 345 mg once daily), the clinical benefit rate (defined as a partial response plus stable disease for ≥ 24 weeks) was 42.6% and median PFS was 4.5 months. Patients in NCT02338349 received elacestrant until disease progression or intolerability. At baseline (n = 50), median patient age was 63 years and 50% of patients had a detectable ESR1 mutation. Patients had previously received a median of three prior antitumour therapies [including CDK4/6 inhibitors (52% of patients) and SERDs (52%)] [20].
Key clinical trials of elacestrant
Drug(s) | Indication | Phase | Status | Location | Identifier | Sponsor (Collaborator) |
|---|---|---|---|---|---|---|
Elacestrant, SOC | ER-positive, HER2-negative advanced breast cancer | III | Active, no longer recruiting | Multinational | NCT03778931 (EMERALD) | Stemline Therapeutics |
Anastrozole, elacestrant, exemestane, letrozole, tamoxifen | ER-positive, HER2-negative breast cancer | III | Not yet recruiting | Multinational | NCT05512364 | EORTC (Breast International Group, Menarini Group) |
Elacestrant | ER-positive, HER2-negative advanced/metastatic breast cancer | II | Not yet recruiting | NCT05596409 (ELCIN) | Stemline Therapeutics | |
Elacestrant, onapristone | ER-positive, PR-positive, HER2-negative advanced/metastatic breast cancer | I/II | Recruiting | USA | NCT05618613 (ELONA) | Context Therapeutics |
Abemaciclib, elacestrant | ER-positive, HER2-negative breast cancer with or without brain metastases | I/II | Recruiting | Multinational | NCT05386108 (ELECTRA) | Stemline Therapeutics |
Abemaciclib, elacestrant | HR-positive/HER2-negative breast cancer with brain metastases | I/II | Recruiting | USA | NCT04791384 | Criterium |
Alpelisib, elacestrant, everolimus, palbociclib, ribociclib | ER-positive, HER2-negative advanced/metastatic breast cancer | I/II | Recruiting | NCT05563220 (ELEVATE) | Stemline Therapeutics | |
Elacestrant | ER-positive, HER2-negative early breast cancer | 1 | Completed | Spain | NCT04797728 (ELIPSE) | SOLTI Breast Cancer Research Group (Radius Health) |
Elacestrant | ER-positive, HER2-negative advanced breast cancer | I | Completed | USA | NCT02338349 | Stemline Therapeutics |
2.4 Adverse Events
Monotherapy with oral elacestrant exhibited a manageable safety profile consistent with other endocrine treatments in postmenopausal women and adult men with ER-positive, HER2-negative advanced or metastatic breast cancer participating in EMERALD [17]. Elacestrant also demonstrated an acceptable safety profile in the phase I NCT02338349 study [20].
Although almost all (92.0%) of 237 elacestrant recipients (vs 86.0% of 229 SOC monotherapy recipients) in EMERALD experienced adverse events (AEs) of any grade, few experienced an AE that resulted in dose reduction (3.0% vs 0% with fulvestrant; not applicable for patients receiving an aromatase inhibitor) or treatment discontinuation (6.3% vs 4.4%) [median follow-up duration 15.1 months (data cut-off 6 September 2021)] [17]. The most frequently reported AEs in the elacestrant and SOC groups included nausea (35.0% vs 18.8% of patients), fatigue (19.0% vs 18.8%), vomiting (19.0% vs 8.3%) and decreased appetite (14.8% vs 9.2%). Most AEs were grade 1 or 2 in severity. Grade 3 or 4 AEs occurred in 27.0% of elacestrant recipients and 20.5% of SOC recipients, with nausea (2.5% of patients), back pain (2.5%) and increased alanine aminotransferase levels (2.1%) the most common grade 3 or 4 AEs in the elacestrant group and nausea, fatigue, diarrhoea and increased aspartate aminotransferase levels (each occurring in 0.9% of patients) the most frequently reported in the SOC group. AEs considered by the investigator to be related to treatment occurred in 63.3% and 43.7% of patients receiving elacestrant and SOC, with treatment-related grade 3 or 4 AEs occurring in 7.2% and 3.1% of patients. No treatment-related deaths were reported [17]. At a data cut-off of 2 September 2022, safety data were consistent with previously reported results [19]. No haematological safety signal was seen and sinus bradycardia was not reported in either treatment group [19].
2.5 Companion Diagnostic
The US FDA has approved Guardant360® CDx, a blood based liquid biopsy companion diagnostic developed by Guardant Health, for the identification of ESR1 mutations in patients with advanced or metastatic breast cancer [21]. The test provides results in 7 days [21].
2.6 Ongoing Clinical Trials
In addition to the ongoing EMERALD study discussed previously, four phase I/II studies [ELONA (NCT05618613), ELECTRA (NCT05386108), NCT04791384 and ELEVATE (NCT05563220)] are currently recruiting patients, while a phase III study (NCT05512364) and a phase II study [ELCIN (NCT05596409)] are not yet recruiting patients.
3 Current Status
Elacestrant received its first approval on 27 January 2023 for the treatment of postmenopausal women or adult men with ER-positive, HER2-negative, ESR1-mutated (as determined by a US FDA-approved test) advanced or metastatic breast cancer with disease progression following ≥ 1 line of endocrine therapy in the USA [2,3,4].
Change history
25 November 2023
A Correction to this paper has been published: https://doi.org/10.1007/s40265-023-01978-2
References
Lloyd MR, Wander SA, Hamilton E, et al. Next-generation selective estrogen receptor degraders and other novel endocrine therapies for management of metastatic hormone receptor-positive breast cancer: current and emerging role. Ther Adv Med Oncol. 2022;14:1–25.
Menarini Group. Stemline Therapeutics, a subsidiary of Menarini Group, receives U.S. FDA approval for ORSERDU™ (elacestrant) as the first and only treatment specifically indicated for patients with ESR1 mutations in ER+, HER2- advanced or metastatic breast cancer [media release]. 30 Jan 2023. https://www.menarini.com/.
Stemline Therapeutics Inc. ORSERDU™ (elacestrant) tablets, for oral use: US prescribing information. 2023. https://www.fda.gov/. Accessed 31 Jan 2023.
US FDA. FDA approves elacestrant for ER-positive, HER2-negative, ESR1-mutated advanced or metastatic breast cancer [media release]. 27 Jan 2023. https://www.fda.gov/.
Menarini Group. Menarini Group's elacestrant Marketing Authorization Application accepted for review by the European Medicines Agency (EMA) for the treatment of ER+/HER2- advanced or metastatic breast cancer [media release]. 19 Aug 2022. http://www.menarini.com.
Radius Health. Radius investigational drug elacestrant (RAD1901) continues to show promise in advanced ER+ HER2- breast cancer at the 2017 San Antonio Breast Cancer Symposium [media release]. 12 Jul 2017. http://www.radiuspharm.com.
Radius Health. Radius announces licensing agreement to develop and commercialize Eisai SERMs [media release]. 29 June 2006. http://www.radiuspharm.com.
Radius Health. Radius Health announced today that it has acquired the license to develop and market RAD1901 in Japan, and has hired Dinesh Purandare, former Sanofi Oncology executive to the position of Senior Vice President (SVP) and Head of Global Oncology at Radius [media release]. 9 Mar 2015. http://www.radiuspharm.com.
Radius Health, Menarini Group. Menarini Group and Radius Health announce global license agreement for the development and commercialization of elacestrant [media release]. 23 Jul 2020. http://www.radiuspharm.com.
Garner F, Shomali M, Paquin D, et al. RAD1901: a novel, orally bioavailable selective estrogen receptor degrader that demonstrates antitumor activity in breast cancer xenograft models. Anticancer Drugs. 2015;26(9):948–56.
Bihani T, Patel HK, Arlt H, et al. Elacestrant (RAD1901), a selective estrogen receptor degrader (SERD), has antitumor activity in multiple ER(+) breast cancer patient-derived xenograft models. Clin Cancer Res. 2017;23(16):4793–804.
Wardell SE, Nelson ER, Chao CA, et al. Evaluation of the pharmacological activities of RAD1901, a selective estrogen receptor degrader. Endocr Relat Cancer. 2015;22(5):713–24.
Jager A, de Vries EGE, der Houven van Oordt CWM, et al. A phase 1b study evaluating the effect of elacestrant treatment on estrogen receptor availability and estradiol binding to the estrogen receptor in metastatic breast cancer lesions using (18)F-FES PET/CT imaging. Breast Cancer Res. 2020;22(97):1–11.
Patel HK, Tao N, Lee KM, et al. Elacestrant (RAD1901) exhibits anti-tumor activity in multiple ER+ breast cancer models resistant to CDK4/6 inhibitors. Breast Cancer Res. 2019;21(146):1–17.
Pancholi S, Simigdala N, Ribas R, et al. Elacestrant demonstrates strong anti-estrogenic activity in PDX models of estrogen-receptor positive endocrine-resistant and fulvestrant-resistant breast cancer. NPJ Breast Cancer. 2022;8(125):1–15.
Vidal M, Pascual T, Falato C, et al. Elacestrant in postmenopausal women with estrogen receptor positive and HER2-negative early breast cancer: primary efficacy and safety analysis of the preoperative, window of opportunity SOLTI-1905-ELIPSE trial [abstract no. PD13-01]. In: San Antonio Breast Cancer Symposium. 2022.
Bidard FC, Kaklamani VG, Neven P, et al. Elacestrant (oral selective estrogen receptor degrader) versus standard endocrine therapy for estrogen receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: results from the randomized phase III EMERALD trial. J Clin Oncol. 2022;40(28):3246–56.
US FDA Center for Drug Evaluation and Research. Elacestrant: multi-discipline review. 2021. https://www.fda.gov/. Accessed 17 Mar 2023.
Bardia A, Bidard FC, Neven P, et al. EMERALD phase 3 trial of elacestrant versus standard of care endocrine therapy in patients with ER+/HER2- metastatic breast cancer: updated results by duration of prior CDK4/6i in metastatic setting [abstract no. GS3-01 plus oral]. In: San Antonio Breast Cancer Symposium. 2022.
Bardia A, Kaklamani V, Wilks S, et al. Phase I study of elacestrant (RAD1901), a novel selective estrogen receptor degrader, in ER-positive, HER2-negative advanced breast cancer. J Clin Oncol. 2021;39(12):1360–70.
Guardant Health. Guardant Health receives FDA approval for Guardant360® CDx as companion diagnostic for Menarini Group’s ORSERDU™ for treatment of patients with ESR1 mutations in ER+, HER2- advanced or metastatic breast cancer [media release]. 30 Jan 2023. https://guardanthealth.com/.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Sheridan M. Hoy is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Open Access
This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
The original article has been revised due to retrospective open choice order.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit - http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Hoy, S.M. Elacestrant: First Approval. Drugs 83, 555–561 (2023). https://doi.org/10.1007/s40265-023-01861-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-023-01861-0