Abstract
Knee osteoarthritis (OA) is one of the most common and disabling medical conditions. In the case of moderate to severe pain, a single intervention may not be sufficient to allay symptoms and improve quality of life. Examples include first-line, background therapy with symptomatic slow-acting drugs for OA (SYSADOAs) or non-steroidal anti-inflammatory drugs (NSAIDs). Therefore, the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) performed a review of a multimodal/multicomponent approach for knee OA therapy. This strategy is a particularly appropriate solution for the management of patients affected by knee OA, including those with pain and dysfunction reaching various thresholds at the different joints. The multimodal/multicomponent approach should be based, firstly, on different combinations of non-pharmacological and pharmacological interventions. Potential pharmacological combinations include SYSADOAs and NSAIDs, NSAIDs and weak opioids, and intra-articular treatments with SYSADOAs/NSAIDs. Based on the available evidence, most combined treatments provide benefit beyond single agents for the improvement of pain and other symptoms typical of knee OA, although further high-quality studies are required. In this work, we have therefore provided new, patient-centered perspectives for the management of knee OA, based on the concept that a multimodal, multicomponent, multidisciplinary approach, applied not only to non-pharmacological treatments but also to a combination of the currently available pharmacological options, will better meet the needs and expectations of patients with knee OA, who may present with various phenotypes and trajectories.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Knee osteoarthritis is a common and disabling condition in older people. The guidelines suggest the use of non-pharmacological and pharmacological approaches, but the use of multidisciplinary and multimodal approaches is still underexplored. |
The European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) performed a review of a multimodal/multicomponent approach for knee OA therapy, finding that it is a particularly appropriate solution for the management of patients affected by knee OA, including those with pain and dysfunction reaching various thresholds at the different joints. |
In this work, we provided patient-centered perspectives for the management of knee OA, based on the concept that a multimodal, multicomponent, multidisciplinary approach, applied not only to non-pharmacological treatments but also to a combination of the currently available pharmacological options, is probably the best option available. |
1 Introduction
Osteoarthritis (OA) is one of the most common rheumatological diseases, being characterized by pain and stiffness that can lead to disability reduced social participation and decreased quality of life [1]. The knee is the most commonly affected joint, and symptomatic knee OA is highly prevalent in older people, affecting more than 250 million people across the globe [2]. Knee OA is a progressive condition, with different degrees of severity, requiring long-term management with various treatment options over the course of the disease [3]. The main aims of the treatment for knee OA are to reduce symptoms and slow disease progression, which might reduce the impact of knee OA on the patient's functional capacities as well as quality of life [3]. Guidelines recommend a combination of a pharmacological and non-pharmacological approach for treating knee OA symptoms [4,5,6,7]. In particular, the use of non-pharmacological approaches, such as healthy diet, weight loss, physical exercise, and education, is strongly recommended by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO), based on a solid literature, in the management of knee OA with different degrees of severity [3]. However, some issues remain unaddressed. First, the management of flares of knee OA (a common manifestation of the disease) vary across the guidelines available [8]. Second, the patients’ expectations are often not considered, despite increasing research highlighting the importance of a patient-centered approach to clinical practice [9,10,11]. Finally, the multimodal approach (i.e., the combination of two or more interventions), despite being common in daily clinical practice, is not adequately addressed in the current guidelines for knee OA [4,5,6,7].
The ESCEO algorithm for the management of knee OA was originally designed to offer a stepwise approach that could be considered by the prescribing physician as a guideline for the management of patients with mild to moderate knee pain [3]. The indications present in the algorithm have been widely endorsed and approved (for example in South-East Asia [12], China [13], Russia [14], and Central Europe [15]). Nevertheless, patient partners from around the world have suggested that common practices center on single agents that are not sufficient. This is particularly the case for patients with moderate to severe pain, patients initiated on symptomatic slow-acting drugs for OA (SYSADOAs) for first-line background therapy but who require rapidly acting pain relief, and patients treated with non-steroidal anti-inflammatory drugs (NSAIDs) for analgesia but who desire a therapy that may result in long-term structural modification. In none of the above examples is a single intervention approach sufficient or appropriate.
For this reason, ESCEO decided to review the literature surrounding a multimodal and multicomponent approach to the management of knee OA, using an experts’ consensus involving a working group including patients, clinicians and researchers in which the participants discussed the role of a multimodal, multicomponent, multidisciplinary management of patients with moderate to severe pain in knee OA to better meet patients’ expectancies. The current manuscript reports the consensus view emerging from this Working Group and reports the key areas that were discussed.
2 Methods
Several clinicians involved in this work (PGC, EMD, CC, NV, NRF, AM) and a patient expert in the field (MdW) prepared some presentations for all the members of the Working Group, searching in several databases (Pubmed, Scopus, Web of Science) from database inception to 21 January 2022, the date in which the Working Group was held. The works included were based on inclusion/exclusion criteria, specific for each paragraph detailed in this manuscript. All members of the Working Group decided on the concepts and the articles to report through discussion led by two expert clinicians (J-YR and AM).
3 Safety and Efficacy of Paracetamol and Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
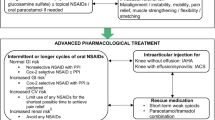
ESCEO has published recommendations for the clinical management of knee OA, providing a practical treatment algorithm that prioritizes interventions based not only on the efficacy of a given intervention but also the safety profile, and provides recommendations for treatment using progressive and logical steps [3]. It is important to emphasize that the management of knee OA is not purely pharmacological but that effective non-pharmacological treatments should be used as part of a holistic treatment strategy, as such an approach may reduce the dosage and frequency of the use of analgesic medications, including NSAIDs or opioids [3].
In the treatment of knee OA, paracetamol (acetaminophen) has been widely recommended in past years as a first-line step for rescue analgesia, even if its effects on pain are minimal and no effect on stiffness and physical function has been observed in several systematic reviews and meta-analyses [3, 16, 17]. However, increasing concerns over the safety profile of paracetamol have been raised due to the evidence of the high rate of gastrointestinal, cardiovascular, hepatic, and renal adverse events [18]. Therefore, in the ESCEO algorithm, it is recommended that paracetamol should be used at doses no greater than 3 g/day and only as short-term rescue analgesia and when NSAIDs are contraindicated [3, 4, 7]. Based on this literature, the efficacy of paracetamol is conditionally recommended against use both in the short and long term in the Osteoarthritis Research Society International (OARSI) guidelines, and only in the short term in the ESCEO indications [4,5,6,7].
NSAIDs are included in the first step (in topical formulations) and second step (in oral form) of the ESCEO algorithm, for patients with persisting symptoms despite appropriate background therapy [3].
Regarding topical NSAIDs, a recent network meta-analysis has reported that topical diclofenac appears to be effective and generally well tolerated and should be considered as a first-line pharmacological treatment for knee OA [17], and other topical NSAIDs can be considered similarly [3, 19]. These medications are supported in the OARSI guidelines as first pharmacological interventions, and in the ESCEO algorithm after background therapy with SYSADOAs [4,5,6,7].
With regard to oral NSAIDS, the effect on pain is similar to SYSADOAs, but they are probably more appropriate in patients with more severe pain or when SYSADOAs fail [3]. Oral NSAIDs may give better symptom relief than paracetamol and are usually preferred by patients [20]. The first indication given in the ESCEO algorithm is to carefully assess the cardiovascular, hepatic, renal, and gastrointestinal profile of a patient before starting the use of this class of medications [3]. Therefore, in 2019, the ESCEO afforded a strong recommendation to the use of oral NSAIDs (selective or non-selective) as a second-step therapy, but only if used intermittently or for longer cycles and based on the patient risk profile [3]. The OARSI guidelines fully supported this indication [4]. It must be remembered that continuous NSAID use should be never ‘chronic’, a recommendation that is supported by safety concerns, a lack of long-term trials, and the recent finding that a large number of patients are taking NSAIDs inappropriately (including those with multimorbidity) [21]. Finally, despite no significant difference in efficacy between cyclooxygenase-2 (COX-2) selective, partially selective, or non-selective NSAIDs, the most recent literature regarding NSAIDs suggests that celecoxib may reduce pain more effectively than other medications in knee OA [22], with the potential added benefit of the suppression of inflammation [23, 24]. Overall, these findings suggest that oral NSAIDs are useful but that their safety profile must be adequately considered on a patient-by-patient basis.
4 Synergistic Effect of NSAIDs and Symptomatic Slow-Acting Drugs for Osteoarthritis (SYSADOAs) in the Management of Knee Osteoarthritis
The possible synergistic effect of the combination between NSAIDs and SYSADOAs is of clinical interest when considering a multimodal approach to therapy with the SYSADOA as a maintenance, background therapy and the oral NSAID to acutely manage symptoms related to knee OA (and potentially delay the progression of the pathology). The use of SYSADOAs is supported by a high certainty of evidence in the ESCEO algorithm, only in the case of pharmaceutical grade products, while OARSI was more prudent in this sense indicating the need for more literature [4,5,6,7].
A recent in vitro study reported the anti-inflammatory and chondroprotective effects of a combination of celecoxib and prescription-grade crystalline glucosamine sulfate (pCGS) in human OA chondrocyte cultures stimulated with interleukin (IL)-1β. The cells were treated with concentrations of celecoxib and pCGS, which reflect the mean plasma concentration of the medications when reaching the systemic circulation [25]. This study demonstrated that both medications, especially when used in combination, significantly reversed the unfavorable effect of IL-1β, reducing inflammation, apoptosis, oxidative stress, and cartilage degradation, and increasing matrix synthesis [25]. The authors highlighted the synergistic effect of celecoxib and pCGS on chondrocyte metabolism, inflammation, apoptosis, and oxidative stress through the modulation of the nuclear factor (NF)-κB pathway, supporting the combined use of SYSADOAs and NSAIDS for the treatment of OA [25].
The possible rationale for the use of these medications is justified by additional literature showing that the main mechanism of action of pCGS in knee OA is the inhibition of the IL-1 pathway at the NF-κB level, with subsequent inhibition of molecules that increase inflammation and cartilage degradation [26]. Indeed, it has been reported that pCGS has anabolic effects, promoting chondrocyte proliferation and stimulating matrix extracellular synthesis [26, 27]. Among all the effects of pCGS, the primary mechanisms of action are the anti-inflammatory and anti-catabolic effects.
On the contrary, celecoxib may have other important mechanisms of action. Beyond the well-known, anti-inflammatory action (primarily via an anti-IL-1 mechanism of action [28]), celecoxib seems to have a direct effect on cartilage metabolism, influencing cartilage, bone, and synovium [29]. It has been reported that celecoxib may prevent the deleterious effects of prostaglandins and nitric oxide on cartilage destruction by inhibiting both COX-2 and NF-κB/JNK [29]. Overall, these findings suggest that pCGS and celecoxib may have not only symptomatic effects but also lead to a structure modification in the context of OA.
Overall, studies in humans support the above in vitro findings. In a case-control study, 60 women took a combination of celecoxib (200 mg/day) and pCGS (a total of 1500 mg/day) versus celecoxib alone (200 mg/day). This study concluded that the concomitant use of pCGS and celecoxib was more effective than celecoxib alone in reducing pain, morning stiffness and function in women affected by early knee OA [30]. Another case-control study evaluated the therapeutic effects of the combination of meloxicam and pCGS in knee OA patients, concluding that the combination of meloxicam and pCGS is more effective than meloxicam alone in reducing several serum markers of inflammation as well as clinical symptoms of knee OA [31]. In an observational study, the effectiveness of the combination of pCGS and conventional NSAIDs (ibuprofen or piroxicam) compared with pCGS alone in mild to moderate OA was evaluated in a group of 100 patients [32]. The study concluded that the combination of pCGS and NSAIDs showed superior improvement in visual analog scale (VAS) pain scores, reduced stiffness, and increased physical function [32].
Two randomized controlled trials (RCTs) were designed to evaluate the reparative effects of combination therapy with pCGS and NSAIDs in patients with knee OA. In the study by Gang and colleagues, 120 patients with knee OA were randomized to two groups, with the intervention group treated with a combination of pCGS (a total of 1500 mg/day) and celecoxib (200 mg/day), while the control group received celecoxib alone (200 mg/day) [33]. This study showed that the combination of pCGS and celecoxib significantly reduced the levels of inflammatory and oxidative stress parameters and markedly lowered pain scores. This supports a possible synergistic role in inhibiting the progression of OA and improving joint function [33]. An RCT conducted by Sun and colleagues compared the use of pCGS and etoricoxib versus etoricoxib alone. The authors found that the combination improved clinical parameters, reduced pain, and suppressed inflammatory markers compared with the single-agent group [34]. Moreover, the combination led to reduced expression of markers involved in the degradation of cartilage matrix [34]. The rate of adverse effects in these two RCTs was similar between the intervention and control groups; however, it should be acknowledged that these RCTs have not been conducted using the highest methodological standards.
In summary, studies in humans overall reported that combining SYSADOAs (particularly pCGS) and NSAIDs (particularly celecoxib) seem to provide a synergistic benefit and could be a primary option for patients with moderate to severe pain or during flares of OA. Moreover, in patients with a high gastrointestinal risk and moderate to severe knee pain, celecoxib in combination with pCGS is a verified combination (but the use of a proton pump inhibitor should be considered) [35,36,37,38]. Similarly, the use of topical NSAIDs in people with a high risk of adverse effects could be considered, if pCGS is not effective [5]. However, further RCTs at low risk of bias are necessary to confirm that the combination of SYSADOAs and NSAIDs has a beneficial effect on the long-term evolution of the disease.
5 Combined Effect of NSAIDs and Weak Opioids
The OARSI guidelines strongly recommended against the use of any opioids in the treatment of knee OA, while the ESCEO indicated the use of weak opioids only [4,5,6,7].
Another common combination therapy in the management of knee OA symptoms is weak opioids (e.g., codeine) and NSAIDs. While NSAIDs reduce prostaglandin production through the inhibition of COX enzymes and their anti-inflammatory effect is largely peripheral [39], weak opioids manifest their therapeutic action act through Mu receptors (present in the dorsal horn of the spinal cord and the brain); however, their adverse effects are due to activation of receptors in the gastrointestinal system (leading to constipation) [40]. The combination of oral NSAIDs and weak opioids has the potential to be beneficial, as demonstrated in systematic reviews and meta-analyses. Overall, in pain due to cancer, a Cochrane review reported that several studies demonstrated superiority of combination therapy, including weak opioids with oral NSAIDs, in reducing pain compared with opioids alone, without a significant increase in the incidence of adverse effects [41]. Moreover, there is evidence that the combination of weak opioids and oral NSAIDs can decrease opioid use in patients with cancer [41]. Similar findings have been shown when combining oral NSAIDs and weak opioids in the context of postoperative pain, with a significant decrease in morphine consumption (between 30% and 50%), and a concomitant reduction in some common adverse effects such as nausea, vomiting, and sedation was observed [42]. Unfortunately, our literature review yielded only one study, with a short follow-up, regarding the combination of weak opioids and oral NSAIDs in the context of OA in which the concomitant use of ibuprofen and codeine was superior to ibuprofen alone in improving pain [43]. Thus, further high-quality research in patients with knee OA is urgently required.
6 Combined Effect of Intra-Articular Treatments, SYSADOAs and Oral NSAIDs
Intra-articular treatments are commonly employed in those with knee OA, particularly if affected by advanced forms of the disease, and supported by both ESCEO and OARSI guidelines by an important certainty of evidence [3,4,5,6,7]. In this regard, the combination of intra-articular injections and oral NSAIDS is an intriguing option, particularly if elevated levels of pain, synovitis or effusions are present or to facilitate early rehabilitation [44]. The rational use of oral NSAIDs and intra-articular injections could be justified by the fact that, from a pharmacokinetic point of view, NSAIDs have a fast onset of action (2–3 h), with a plateau lasting 7–10 days, and a residual analgesic effect for 30 days [45]. The effect of the most common intra-articular products (such as hyaluronic acid [HA] [46] and glucocorticoids [47]) starts after a few days and usually lasts up to months.
The combination of intra-articular injections and SYSADOAs is supported by increasing evidence. In particular, recent studies suggest this approach for polyarticular OA, in the case of a low degree of OA in the other joints, and to maintain analgesic effects [48]. Additionally, this combination may prolong inter-injection intervals and delay joint replacement [49].
To date, there is no consensus regarding the respective value of using low-molecular-weight, intermediate-molecular-weight, or high-molecular-weight HA for the management of OA [48]. One study comparing Western Ontario and McMaster University (WOMAC) pain subscale score changes for intermediate-molecular-weight HA versus low-molecular-weight HA came to the conclusion that intermediate-molecular-weight HA provided a greater benefit in the setting of knee OA [50]. It will be interesting to see if this finding is replicated in further studies. In the perspective of a multicomponent approach, a recent study suggested that a single intra-articular injection of a novel high- and low-molecular-weight HA formulation provides a rapid-onset yet sustained reduction in pain and improvement in function, with an appropriate safety profile [51]. This may be an interesting avenue for future therapeutic development in order to reduce the number of injections and to achieve an onset of action similar to that obtained with intra-articular injections of corticosteroids. Indeed, using intra-articular HA may have the benefit of reducing corticosteroid injections and steroid-related adverse events [51].
From a clinical point of view, the injection of HA associated with oral NSAIDs has potential as a very interesting approach that may provide rapid symptomatic relief due to the NSAID combined with a long-term symptomatic benefit afforded by intra-articular HA [48].
7 All Patients are Different: The Need to Titrate and Adapt the Treatment to All Clinical Situations
Using a multimodal approach to treating knee OA opens the important question of how to titrate and adapt the clinical strategy to different situations. This practically means that patients’ preferences are comprehensively sought and followed to maximize compliance to therapy. This holistic approach should be based on the premise that ‘one size does not fit all’. All patients are different and therefore require personalized management of their knee OA [11]. In this regard, the patient’s knowledge of their disease, experiences in daily life, and personal circumstances should guide management strategies [11]. For example, data from the Osteoarthritis Initiative clearly showed that pain trajectory significantly differs (across 6 years of follow-up) [52], emphasizing that different approaches are required.
The key attributes of patient centered, multidisciplinary team care in clinical practice are based on several aspects. First, patients’ values and experiences must be incorporated into daily clinical management of knee OA [53]. Core outcomes for OA should be used to support value-based healthcare provision and patient-oriented research. Second, shared decision making is another important aspect of the management of patients with knee OA. As a long-term condition, patients must be actively involved. This is particularly true in the presence of less evidence-based treatment recommendations when the values and experiences of the patients should steer the shared decision-making process [54]. Self-management of knee OA is another crucial aspect since it encourages patients to take control of their condition, and empowers them to be the coordinators of their own care. This important aspect should be reflected in research where patients should be involved on a collective level in the selection of outcomes, development of guidelines, and implementation of health innovations [55]. Knee OA often requires multidisciplinary care, particularly for non-pharmacological treatment that is often associated with low patient compliance [3]. In this context, patient education is a crucial facet. Previous high-quality research indicates that the education of patients with knee OA is an essential non-pharmacological approach [3], and from a research point of view, a public summary of treatment recommendations for patients is strongly encouraged. Finally, coordination of the care is paramount. Suboptimal organization of care is one of the principle barriers to the effective implementation of non-pharmacological interventions. To overcome this issue, co-creation of a care plan (i.e., the involvement of patients throughout the development process) is recommended. Through the genuine involvement of patients in research, knowledge translation, and innovation of health service delivery, the adherence of patients can be increased. In guidelines, for example, working towards value-based health care and using health indicators that are important to patients is strongly recommended, as well measuring the efficacy of therapy using a balanced, robust collection of clinical and patient-reported outcomes.
All these aspects underline the fact that instead of a ‘one size fits all’ approach, patients prefer a tailor-made approach, and patient involvement is crucial in this regard. In particular, in patients with moderate-to-severely painful knee OA, clinicians should prescribe a combination of pharmacological and non-pharmacological interventions, taking into account some important factors such as multimorbidity, polypharmacy, and contraindications in patients often reluctant to take additional medications.
8 Conclusions
As described by the ESCEO and patient partners, the ESCEO algorithm for the management of knee OA, published in 2014 and revised in 2016 and 2019, remains an appropriate tool for the management of patients with mild to moderate symptoms of knee OA. However, it is clear that a ‘one size fits all’ approach to the treatment of OA does not meet the needs and expectations of the OA patient population. Several situations, including acute phases (flares) of pain exacerbation, moderate to severe symptoms, patients who need rapid relief from symptoms concurrently to the initiation of long-term, background treatment, or patients who expect a strategy allowing for structure modification, concomitant to rapid symptom relief, justify the use of a multimodal, multicomponent approach.
From the very beginning, the ESCEO guidelines have recommended, as a basic principle, that a combination of treatment modalities including non-pharmacological and pharmacological therapies is required for the management of knee OA. However, since core non-pharmacological therapies are usually insufficient to control symptoms, and since compliance is usually low, a similar multicomponent, multimodal approach is necessitated when using pharmacological modalities.
There is a rationale to suggest that a combination of pCGS and celecoxib may be beneficial, both for symptom relief and structure modification. This biological plausibility is supported by preclinical studies in human osteoarthritic chondrocytes that such a combination may reduce the expression of mediators of inflammation and cartilage degradation. These non-clinical data were further supported by clinical experiments showing a synergistic effect of SYSADOAs and NSAIDs on pain in OA, but further research is needed.
Although few studies were conducted specifically in knee OA, results from other therapeutic areas (e.g., cancer or postoperative pain) suggest that the combination of NSAIDs and (weak) opioids could lead to a reduction of opioid consumption, hence significantly reducing the risk of adverse events related to opioid use, including nausea, vomiting, and sedation.
The combination of oral NSAIDs and HA injections may provide concomitant short- and long-term symptomatic relief, although further trials are needed. A combination of low- and high-molecular-weight HAs in the same administration device is another multicomponent approach that, if the current results are replicated, might provide rapid-onset symptomatic relief and sustained symptomatic benefit, resulting in a potential reduction in the number of injections needed to achieve clinically relevant outcomes. Combinations of SYSADOAs and HA are also a potentially fruitful approach that requires further investigation.
In conclusion, this paper provides new perspectives for the management of knee OA, based on the concept that a multimodal, multicomponent, multidisciplinary approach, including pharmacological and pharmacological approaches, also in combination with the currently available pharmacological options, will better meet the needs and expectations of the OA patient population presenting with a broad range of phenotypes and disease trajectories. However, future studies are needed to further confirm these findings.
References
Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet. 2012;380(9859):2197–223.
Cui A, Li H, Wang D, Zhong J, Chen Y, Lu H. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine. 2020;29: 100587.
Bruyère O, Honvo G, Veronese N, Arden NK, Branco J, Curtis EM, et al. An updated algorithm recommendation for the management of knee osteoarthritis from the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Semin Arthritis Rheum. 2019;49(3):337–50.
Bannuru RR, Osani M, Vaysbrot E, Arden N, Bennell K, Bierma-Zeinstra S, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr Cartil. 2019;27(11):1578–89.
Arden NK, Perry TA, Bannuru RR, Bruyère O, Cooper C, Haugen IK, et al. Non-surgical management of knee osteoarthritis: comparison of ESCEO and OARSI 2019 guidelines. Nat Rev Rheumatol. 2021;17(1):59–66.
Hochberg MC, Altman RD, Brandt KD, Clark BM, Dieppe PA, Griffin MR, et al. Guidelines for the medical management of osteoarthritis. Part II. Osteoarthritis of the knee American College of Rheumatology. Arthritis Rheum. 1995;38(11):1541–6.
Kolasinski SL, Neogi T, Hochberg MC, Oatis C, Guyatt G, Block J, et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol. 2020;72(2):220–33.
Parry EL, Thomas MJ, Peat G. Defining acute flares in knee osteoarthritis: a systematic review. BMJ Open. 2018;8(7): e019804.
Hiligsmann M, Pinto D, Dennison E, Al-Daghri N, Beaudart C, Branco J, et al. Patients’ preferences for osteoarthritis treatment: the value of stated-preference studies. Springer; 2019. p. 1–3.
de Wit M, Cooper C, Reginster J-Y. Practical guidance for patient-centred health research. The Lancet. 2019;393(10176):1095–6.
de Wit M, Cooper C, Tugwell P, Bere N, Kirwan J, Conaghan PG, et al. Practical guidance for engaging patients in health research, treatment guidelines and regulatory processes: results of an expert group meeting organized by the World Health Organization (WHO) and the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Aging Clin Exp Res. 2019;31(7):905–15.
Yeap SS, Tanavalee A, Perez EC, Tan MP, Reyes BHM, Lee JK, et al. 2019 revised algorithm for the management of knee osteoarthritis: the Southeast Asian viewpoint. Aging Clin Exp Res. 2021;33(5):1149–56.
Zhang Z, Huang C, Cao Y, Mu R, Zhang MC, Xing D, et al. 2021 revised algorithm for the management of knee osteoarthritis—the Chinese viewpoint. Aging Clin Exp Res. 2021;33(8):2141–7.
Denisov L, Tsvetkova E, Golubev G. Algoritm lecheniya osteoartrita kolennogo sustava Evropeiskogo obschestva po klinicheskim I ekonomicheskim aspektam osteoporoza I osteoartrita (ESCEO) primenim v rossiiskoi klinicheskoi praktike: sovmestnoe zaklyuchenie veduschikh rossiiskikh spetsialistov i ekspertov ESCEO po osteoartritu [The European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) algorithm for the management of knee osteoarthritis is applicable to Russian clinical practice: a consensus statement of leading russian and ESCEO osteoarthritis experts]. Nauchno-prakticheskaya revmatologiya (Rheumatology Science and Practice). 2016;54(6):641–53.
Kucharz EJ, Szántó S, Ivanova Goycheva M, Petronijević M, Šimnovec K, Domżalski M, et al. Endorsement by Central European experts of the revised ESCEO algorithm for the management of knee osteoarthritis. Rheumatol Int. 2019;39(7):1117–23.
Zeng C, Doherty M, Persson MS, Yang Z, Sarmanova A, Zhang Y, et al. Comparative efficacy and safety of acetaminophen, topical and oral non-steroidal anti-inflammatory drugs for knee osteoarthritis: evidence from a network meta-analysis of randomized controlled trials and real-world data. Osteoarthr Cartil. 2021;29(9):1242–51.
da Costa BR, Pereira TV, Saadat P, Rudnicki M, Iskander SM, Bodmer NS, et al. Effectiveness and safety of non-steroidal anti-inflammatory drugs and opioid treatment for knee and hip osteoarthritis: network meta-analysis. BMJ. 2021;375:n2321.
Conaghan PG, Arden N, Avouac B, Migliore A, Rizzoli R. Safety of paracetamol in osteoarthritis: what does the literature say? Drugs Aging. 2019;36(1):7–14.
Wolff DG, Christophersen C, Brown SM, Mulcahey MK. Topical nonsteroidal anti-inflammatory drugs in the treatment of knee osteoarthritis: a systematic review and meta-analysis. Phys Sportsmed. 2021;49(4):381–91.
Pincus T, Koch G, Lei H, Mangal B, Sokka T, Moskowitz R, et al. Patient Preference for Placebo, Acetaminophen (paracetamol) or Celecoxib Efficacy Studies (PACES): two randomised, double blind, placebo controlled, crossover clinical trials in patients with knee or hip osteoarthritis. Ann Rheum Dis. 2004;63(8):931–9.
Patel J, Ladani A, Sambamoorthi N, LeMasters T, Dwibedi N, Sambamoorthi U. A machine learning approach to identify predictors of potentially inappropriate non-steroidal anti-inflammatory drugs (NSAIDs) use in older adults with osteoarthritis. Int J Environ Res Public Health. 2021;18(1):155.
Álvarez-Soria MA, Largo R, Santillana J, Sánchez-Pernaute O, Calvo E, Hernández M, et al. Long term NSAID treatment inhibits COX-2 synthesis in the knee synovial membrane of patients with osteoarthritis: differential proinflammatory cytokine profile between celecoxib and aceclofenac. Ann Rheum Dis. 2006;65(8):998–1005.
Huang H, Luo M, Liang H, Pan J, Yang W, Zeng L, et al. Meta-analysis comparing celecoxib with diclofenac sodium in patients with knee osteoarthritis. Pain Med. 2021;22(2):352–62.
Alvarez-Soria M, Herrero-Beaumont G, Moreno-Rubio J, Calvo E, Santillana J, Egido J, et al. Long-term NSAID treatment directly decreases COX-2 and mPGES-1 production in the articular cartilage of patients with osteoarthritis. Osteoarthritis Cartilage. 2008;16(12):1484–93.
Cheleschi S, Tenti S, Giannotti S, Veronese N, Reginster J-Y, Fioravanti A. A combination of celecoxib and glucosamine sulfate has anti-inflammatory and chondroprotective effects: results from an in vitro study on human osteoarthritic chondrocytes. Int J Mol Sci. 2021;22(16):8980.
Rovati LC, Girolami F, Persiani S. Crystalline glucosamine sulfate in the management of knee osteoarthritis: efficacy, safety, and pharmacokinetic properties. Ther Adv Musculoskelet Dis. 2012;4(3):167–80.
Noyszewski EA, Wroblewski K, Dodge GR, Kudchodkar S, Beers J, Sarma A, et al. Preferential incorporation of glucosamine into the galactosamine moieties of chondroitin sulfates in articular cartilage explants. Arthritis Rheum. 2001;44(5):1089–95.
Crofford LJ, Lipsky PE, Brooks P, Abramson SB, Simon LS, Van De Putte L. Basic biology and clinical application of specific cyclooxygenase-2 inhibitors. Arthritis Rheum. 2000;43(1):4–13.
Zweers MC, de Boer TN, van Roon J, Bijlsma JW, Lafeber FP, Mastbergen SC. Celecoxib: considerations regarding its potential disease-modifying properties in osteoarthritis. Arthritis Res Ther. 2011;13(5):1–11.
Amuzadeh F, Kazemian G, Rasi AM, Khazanchin A, Khazanchin A, Kazemi P. Comparison of the efficacy of combination of glucosamine sulfate and celecoxib versus celecoxib alone for the pain, morning stiffness, function relief of females with osteoarthritis grade 1&2 of the knee (a comparative study). Indian J Fundam Appl Life Sci. 2015;5:129–36.
Zhijun L, Rongchun C, Feixiang L, Yaohong W, Ning L, Shufang Z, et al. Therapeutic effects of combined meloxicam and glucosamine sulfate treatment on patients with osteoarthritis, and its effect on serum CTX-I, CTX-II, COMP and MMP-3. Trop J Pharm Res. 2019;18(7):1553–7.
Selvan T, Rajiah K, Nainar M, Mathew EM. A clinical study on glucosamine sulfate versus combination of glucosamine sulfate and NSAIDs in mild to moderate knee osteoarthritis. Scientific World J. 2012;2012: 902676.
Gang D, Xiaguang C, Kanghua Y, Aiping W, Guangxuan Z. Combined effect of celecoxib and glucosamine sulfate on inflammatory factors and oxidative stress indicators in patients with knee osteoarthritis. Trop J Pharm Res. 2019;18(2):397–402.
Sun Y, Wang C, Gong C. Repairing effects of glucosamine sulfate in combination with etoricoxib on articular cartilages of patients with knee osteoarthritis. J Orthop Surg Res. 2020;15(1):1–9.
Chan FK, Lanas A, Scheiman J, Berger MF, Nguyen H, Goldstein JL. Celecoxib versus omeprazole and diclofenac in patients with osteoarthritis and rheumatoid arthritis (CONDOR): a randomised trial. The Lancet. 2010;376(9736):173–9.
Chan FK, Ching JY, Tse YK, Lam K, Wong GL, Ng SC, et al. Gastrointestinal safety of celecoxib versus naproxen in patients with cardiothrombotic diseases and arthritis after upper gastrointestinal bleeding (CONCERN): an industry-independent, double-blind, double-dummy, randomised trial. The Lancet. 2017;389(10087):2375–82.
Chan FK, Hung LC, Suen BY, Wu JC, Lee KC, Leung VK, et al. Celecoxib versus diclofenac and omeprazole in reducing the risk of recurrent ulcer bleeding in patients with arthritis. N Engl J Med. 2002;347(26):2104–10.
Cryer B, Li C, Simon LS, Singh G, Stillman MJ, Berger MF. GI-REASONS: a novel 6-month, prospective, randomized, open-label, blinded endpoint (PROBE) trial. Am J Gastroenterol. 2013;108(3):392.
Green GA. Understanding NSAIDs: from aspirin to COX-2. Clin Cornerstone. 2001;3(5):50–9.
Amaechi O, Huffman MM, Featherstone K. Pharmacologic therapy for acute pain. Am Fam Physician. 2021;104(1):63–72.
McNicol ED, Strassels S, Goudas L, Lau J, Carr DB. NSAIDS or paracetamol, alone or combined with opioids, for cancer pain. Cochrane Database of Syst Rev. 2005;1:CD005180.
Marret E, Kurdi O, Zufferey P, Bonnet F, Warltier DC. Effects of nonsteroidal antiinflammatory drugs on patient-controlled analgesia morphine side effects: meta-analysis of randomized controlled trials. J Am Soc Anesthesiolog. 2005;102(6):1249–60.
Quiding H, Grimstad J, Rusten K, Stubhaug A, Bremnes J, Breivik H. Ibuprofen plus codeine, ibuprofen, and placebo in a single- and multidose cross-over comparison for coxarthrosis pain. Pain. 1992;50(3):303–7.
Mobasheri A, Saarakkala S, Finnilä M, Karsdal MA, Bay-Jensen A-C, van Spil WE. Recent advances in understanding the phenotypes of osteoarthritis. F1000Research. 2019;8:2091.
Oertel B, Lötsch J. NSAIDs, pharmacokinetics. In: Schmidt RF, Willis WD, editors. Encyclopedia of pain. Berlin: Springer, Berlin Heidelberg; 2007. p. 1479–87.
Gupta RC, Lall R, Srivastava A, Sinha A. Hyaluronic acid: Molecular mechanisms and therapeutic trajectory. Frontiers in veterinary science. 2019;6:192.
Derendorf H, Möllmann H, Grüner A, Haack D, Gyselby G. Pharmacokinetics and pharmacodynamics of glucocorticoid suspensions after intra-articular administration. Clin Pharmacol Ther. 1986;39(3):313–7.
Cooper C, Rannou F, Richette P, Bruyère O, Al-Daghri N, Altman RD, et al. Use of intraarticular hyaluronic acid in the management of knee osteoarthritis in clinical practice. Arthritis Care Res. 2017;69(9):1287–96.
Saengnipanthkul S, Waikakul S, Rojanasthien S, Totemchokchyakarn K, Srinkapaibulaya A, Cheh Chin T, et al. Differentiation of patented crystalline glucosamine sulfate from other glucosamine preparations will optimize osteoarthritis treatment. Int J Rheum Dis. 2019;22(3):376–85.
Maheu E, Rannou F, Reginster J-Y. Efficacy and safety of hyaluronic acid in the management of osteoarthritis: evidence from real-life setting trials and surveys. Semin Arthritis Rheum. 2016;45(4 Suppl):S28–33.
Migliore A, Blicharski T, Plebanski R, Zegota Z, Gyula G, Rannou F, et al. Knee Osteoarthritis pain management with an innovative high and low molecular weight hyaluronic acid formulation (HA-HL): a randomized clinical trial. Rheumatol Ther. 2021;8(4):1617–36.
Nicholls E, Thomas E, Van der Windt D, Croft P, Peat G. Pain trajectory groups in persons with, or at high risk of, knee osteoarthritis: findings from the Knee Clinical assessment Study and the Osteoarthritis Initiative. Osteoarthr Cartil. 2014;22(12):2041–50.
de Wit M. The patient perspective. In: Hochberg G, Silman S, Weinblatt W, editors. Rheumatology. Elsevier; 2017. p. 409–12.
Schoemaker CG, van der Heijden GJ. Does GRADE gently close the door on sharing decisions with patients? J Clin Epidemiol. 2018;102:146–7.
Armstrong MJ, Mullins CD, Gronseth GS, Gagliardi AR. Impact of patient involvement on clinical practice guideline development: a parallel group study. Implement Sci. 2018;13(1):1–13.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open access funding provided by Università degli Studi di Palermo within the CRUI-CARE Agreement.
Conflicts of interest
Nicola Veronese reports personal fees from IBSA, Mylan, and Fidia outside of the submitted work. Cyrus Cooper reports personal fees from Alliance for Better Bone Health, Amgen, Eli Lilly, GSK, Medtronic, Merck, Novartis, Pfizer, Roche, Servier, Takeda, and UCB outside of the submitted work. Jean-Yves Reginster reports grants from IBSA-Genevrier, Mylan, CNIEL, and Radius Health (through his institution); consulting fees from IBSA-Genevrier, Mylan, CNIEL, Radius Health, and Pierre Fabre; fees for participation in review activities from IBSA-Genevrier, Mylan, CNIEL, Radius Health, and Teva; and payment for lectures from Ag-Novos, CERIN, CNIEL, Dairy Research Council (DRC), Echolight, IBSA-Genevrier, Mylan, Pfizer Consumer Health, Teva, and Theramex outside of the submitted work. Olivier Bruyère reports grants or lecture fees from Amgen, Aptissen, Biophytis, IBSA, MEDA, Mylan, Novartis, Sanofi, Servier, SMB, TRB Chemedica, UCB, and Viatris outside of the submitted work. Ali Mobasheri declares personal fees from Abbott, Abbvie, Achē Laboratórios Farmacêuticos, Galapagos, GSK Consumer Healthcare, Kolon TissueGene, Laboratoires Expansciences, Merck, Pacira Biosciences, Pfizer, Sanofi, and Servier. François Rannou reports grants or lecture fees from Pierre Fabre, Mylan, MSD, Thuasne, IBSA, Pfizer, Genévrier, Expanscience, Scarcell, Skindermic, and Peptinov. Ida K. Haugen reports grants from Pfizer and is a consultant for Novartis outside of the submitted work. Elaine M. Dennison declares grants/fees from Pfizer, Lilly, UCB, and Viatris. Philip G. Conaghan is supported in part by the National Institute for Health and Care Research (NIHR) Leeds Biomedical Research Centre (the views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health), and reports consultancies or lecture fees from AbbVie, Amgen, AstraZeneca, Eli Lilly, Galapagos, GSK, Grunenthal, Pfizer, Novartis, and UCB. Nasser M. Al-Daaghri, Antonella Fioravanti, Sara Cheleschi, Jean-Pierre Pelletier, Maarten de Wit, Etienne Cavalier, Radmila Matijevic, Germain Honvo, Régis Pierre Radermecker, René Rizzoli, Jaime Branco, Andrea Laslop, María Concepción Prieto Yerro, Alberto Migliore, Gabriel Herrero-Beaumont, and Nicholas R. Fuggle declare that they have no conflicts of interest.
Ethics approval
Not required for this article type.
Data and code availability statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author contributions
NV, NMA, EC, SC, MCdSR, EMD, MdW, GH-B, GH, AL, J-P, and RPR wrote the draft of the manuscript. JYR, CC, OB, JB, PGC, AF, NRF, IKH, RM, AM, AM, MCPY, FR, and RR critically revised the work. All authors revised and approved the version submitted to the journal.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Veronese, N., Cooper, C., Bruyère, O. et al. Multimodal Multidisciplinary Management of Patients with Moderate to Severe Pain in Knee Osteoarthritis: A Need to Meet Patient Expectations. Drugs 82, 1347–1355 (2022). https://doi.org/10.1007/s40265-022-01773-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-022-01773-5