Abstract
Daridorexant (Quviviq™; Idorsia Pharmaceuticals Ltd.) is an orally administered dual orexin type 1 and type 2 (OX1 and OX2) receptor antagonist (DORA) being developed for the treatment of insomnia. It was selected from a pool of drug candidates on the basis of an expected effect duration of ≈ 8 h at a dose of 25 mg, with a half-life intended to minimize residual effects that might impair daytime functioning. Based on the results of two pivotal phase III trials, daridorexant was recently approved in the USA for the treatment of adult patients with insomnia characterized by difficulties with sleep onset and/or sleep maintenance. This article summarizes the milestones in the development of daridorexant leading to this first approval.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Digital Features for this AdisInsight Report can be found at https://doi.org/10.6084/m9.figshare.19304036. |
A dual orexin receptor antagonist is being developed by Idorsia Pharmaceuticals Ltd. for the treatment of insomnia. |
Received its first approval on January 7 2022 in the USA. |
Approved for use in adult patients with insomnia characterized by difficulties with sleep onset and/or sleep maintenance. |
1 Introduction
Daridorexant (Quviviq™) is an orally administered dual orexin type 1 and type 2 (OX1 and OX2) receptor antagonist (DORA) being developed by Idorsia Pharmaceuticals Ltd. for the treatment of insomnia. OX1 and OX2 are G protein-coupled receptors that are widely expressed in the brain. The endogenous ligands orexin A and orexin B (also known as hypocretin-1 and -2) are produced in the hypothalamus and promote wakefulness through interactions with OX1 and OX2 [1]. The findings that narcolepsy is associated with a loss of orexin-producing neurons, and that blockade of both OX1 and OX2 improves sleep variables, led to interest in the development of orexin antagonists as a potential treatment for insomnia [1, 2].
Daridorexant was selected from a pool of drug candidates on the basis of an expected effect duration of ≈ 8 h at a dose of 25 mg, with a half-life targeted to minimize residual effects likely to impair daytime functioning. The drug is approved in the USA [3] and has received a positive Committee for Medicinal Products for Human Use (CHMP) opinion in the EU [4], with regulatory review ongoing in Switzerland and Canada.
The recommended dosage of daridorexant in the USA is 25–50 mg once per night, taken within 30 min before going to bed, with at least 7 h remaining prior to planned awakening [5].

Key milestones in the development of daridorexant for the treatment of insomnia. EMA European Medicines Agency, FDA Food and Drug Administration, MAA Marketing Authorisation Application, NDA New Drug Application
US prescribing information contains warnings regarding potential for CNS-depressant effects and daytime impairment (which is increased when daridorexant is taken with other CNS depressants), potential worsening of depression or suicidal ideation, sleep paralysis, hypnagogic/hypnopompic hallucinations, and cataplexy-like symptoms. In addition, complex sleep behaviours including sleepwalking, sleepdriving and engaging in other activities while not fully awake may occur during treatment with daridorexant, and the drug should be discontinued immediately if complex sleep behaviour occurs. Potential depressant effects on respiratory function should also be considered [5].
1.1 Company Agreements
Daridorexant was originated by Actelion Pharmaceuticals Ltd. In June 2017, in connection with the acquisition of Actelion by Johnson & Johnson, Actelion spun off its drug discovery operations and early-stage clinical development assets into a newly created Swiss biopharmaceutical company, Idorsia Pharmaceuticals Ltd [6].
In December 2019, Idorsia entered into an exclusive licensing agreement with Mochida Pharmaceutical for the co-development, co-marketing and supply of daridorexant as treatment for insomnia and related disorders in Japan. Idorsia Pharmaceuticals will be responsible for the design and conduct of preclinical and clinical studies, and regulatory submission, under supervision from a Joint Development Committee. Mochida will have first right of refusal in the event that Idorsia Pharmaceuticals elects to license daridorexant for the treatment of conditions other than insomnia and related disorders in Japan [7].
In August 2020, Idorsia entered into a marketing agreement with Syneos Health for the commercialization of daridorexant in the USA [8]. In January 2022 this partnership was expanded to include Europe and Canada [9].
2 Scientific Summary
2.1 Pharmacodynamics
In in vitro studies, daridorexant had inhibition constants (Kb) for human OX1 and OX2 receptors of 0.52 nM and 0.78 nM, respectively, and receptor occupancy half-lives of ≈ 8 and ≈ 4 min, respectively [10].
In vivo in rats, oral administration of daridorexant 30 mg/kg was associated with a 22% reduction in time spent in active wake and 29 and 84% increases in time spent in non-REM and REM sleep, respectively, compared to vehicle, with the proportions of non-REM and REM sleep over the total sleep time maintained.

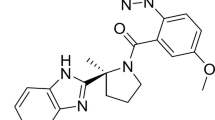
Chemical structure of daridorexant
The drug was also associated with significant decreases in the latency to persistent non-REM and REM sleep [10].
2.1.1 Human Studies
Administration of single ascending doses of daridorexant to male volunteers was associated with clear CNS effects (e.g. reduced vigilance, attention, visuomotor coordination, and postural stability) within 1 h. Maximal effects were observed ≈ 1.5 h after doses up to 100 mg, with the effects of 25 mg and 50 mg doses diminishing to baseline levels within 3–6 h and 6–8 h after administration, respectively [2]. Similar results were observed after administration of multiple 25 and 75 mg daily doses of daridorexant to volunteers, with reduced vigilance and attention, and visuomotor coordination and postural stability, observed on day 1 and day 5 compared to placebo. Maximal effects were seen ≈ 2 h post-dose and diminished to baseline levels within 4–10 h after administration [11].
Daridorexant was associated with dose-related drug-liking effects in a randomized, double-blind, placebo and active-controlled human abuse potential study (NCT03657355) conducted in recreational users of sedatives (n = 63). The drug-liking visual analogue scale (VAS) Emax for daridorexant was 73.2 at 50 mg, and 79.1 and 81.3 at supratherapeutic 100 mg 150 mg doses, respectively. Daridorexant 50 mg, but not 100 mg or 150 mg, was associated with significantly lower VAS Emax values compared to supratherapeutic doses of suvorexant (150 mg) and zolpidem (30 mg) [p ≤ 0.0007]. The drug-liking effects of all doses of daridorexant and both control drugs were distinguishable from placebo [12].
The effects of a 50 mg nightly dose of daridorexant on respiratory function have been investigated in placebo-controlled crossover studies in patients with mild-to-moderate obstructive sleep apnoea (OSA) [NCT03765294] and moderate chronic obstructive pulmonary disease (COPD) [NCT03646864]. Administration of daridorexant to patients (n = 25) with mild-to-moderate OSA (5–30 apnoea-hypopnoea events/h) not requiring continuous positive airway pressure (CPAP) for 5 days was associated with a 0.74 (90% CI – 1.43 to 2.92) events/h mean treatment difference in apnoea/hypopnoea index during total sleep time (TST) compared to placebo [13]. Administration of daridorexant to patients (n = 26) with moderate COPD was not associated with a significant change in peripheral oxygen saturation during TST compared to placebo (mean treatment difference 0.18% (90% CI − 0.21 to 0.57) [14]. However, because of study limitations including small patient numbers and short study durations, and because patients with severe OSA or COPD were not included, the possibility of clinically meaningful respiratory depressant effects occurring during treatment with daridorexant cannot be excluded [5].
The effects of night-time administration of single and multiple doses of daridorexant on the next-morning driving performance of elderly (n = 30; 65–79 years-of age [median 70]) and adult (n = 30; 50–64 years-of-age [median 58]). volunteers have been assessed in a placebo and active-comparator (zopiclone) -controlled double-blind trial. After the initial dose, daridorexant 50 mg and 100 mg were associated with a statistically significant impairment in next-morning driving performance compared to placebo. After four consecutive nights of treatment, the mean effect on driving performance was not statistically significant with either dose of daridorexant compared to placebo. However, driving ability was impaired in some daridorexant recipients and patients should be advised that driving performance may be impaired the morning after taking the drug [5].
Administration of therapeutic and supratherapeutic doses (200 mg) of daridorexant to volunteers was not associated with clinically relevant prolongation of the QT interval [15].
2.2 Pharmacokinetics
At doses of 25–50 mg, daridorexant exhibits dose proportional plasma exposure. The pharmacokinetic profile of the drug is similar after multiple-dose and single-dose administration and no accumulation is evident, with peak plasma concentrations occurring within 1–2 h (tmax). In volunteers, a high-fat/high-calorie meal delayed the tmax of daridorexant by 1.3 h and reduced Cmax by 16%, but did not affect total exposure (AUC). The drug has a volume of distribution of 31 L and is 99.7% bound to plasma proteins. Across studies, the terminal half-life (t½) of daridorexant was ≈ 8 h [5, 11].
Daridorexant is extensively metabolized, primarily by CYP3A4 (89%) and mostly excreted via faeces (≈ 57%) and urine (≈ 28%), primarily as metabolites [5, 16]
The pharmacokinetic properties of daridorexant are not clinically significantly affected by age, sex, race, body size [5], or mild to severe renal impairment (Cockcroft-Gault < 30 ml/min, not on dialysis) [17]. The presence of moderate (Child-Pugh B), but not mild (Child-Pugh A), liver impairment has been shown to prolong the t½ of daridorexant and thus the maximum dosage recommended in patients with Child-Pugh B impairment is 25 mg no more than once per night [5, 18]. The effects of severe hepatic impairment (Child-Pugh C) on the pharmacokinetics of the drug have not been studied and use of daridorexant in this patient population is not recommended [5].
Daridorexant at a dose of 50 mg can be coadministered with the histamine 2 receptor inhibitor famotidine without dosage adjustment [19]. Coadministration of daridorexant 50 mg with the SSRI citalopram was not associated with clinically relevant changes in pharmacokinetic parameters [20]. Coadministration of daridorexant with rosuvastatin did not affect the pharmacokinetics of the latter indicating that daridorexant can be safely coadministered with BCRP substrates without requiring dosage adjustments [21].
Coadministration of daridorexant 25 mg with the moderate CYP3A4 inhibitor diltiazem increased daridorexant AUC by 240%, and, based on physiologically-based pharmacokinetic modelling, coadministration with the strong CYP3A4 inhibitor itraconazole is expected to increase daridorexant AUC by > 400% [22]. Conversely, coadministration of daridorexant with the moderate CYP3A4 inducer efavirenz decreased daridorexant AUC by ≈ 35% [19] and coadministration with the strong CYP3A4 inducer rifampin is expected to decrease daridorexant AUC by > 50% [22]. On this basis, the maximum recommended dose of daridorexant is 25 mg when used concomitantly with a moderate CYP3A4 inhibitor, and concomitant use of daridorexant and a strong CYP3A4 inhibitor, or moderate or strong CYP3A4 inducer is not recommended [5].
Coadministration of daridorexant with alcohol prolonged daridorexant tmax and was associated with additive effects on psychomotor performance. On this basis patients are advised not to consume alcohol while taking daridorexant [5, 23]. Caution is advised when daridorexant is given concomitantly with other CNS depressants and dosage adjustments to either/both drugs should be considered [5].
Features and properties of daridorexant
Alternative names | ACT 541468, nemorexant |
Class | Benzimidazoles, chlorinated hydrocarbons, ketones, pyrrolidines, sleep disorder therapies, small molecules, triazoles |
Mechanism of action | Orexin receptor type 1 and 2 antagonist |
Route of administration | Oral |
Pharmacodynamics | Inhibition constants (Kb) of 0.52 nM and 0.78 nM for human OX1 and OX2 receptors, respectively |
Pharmacokinetics | tmax 1–2 h, t½ ≈ 8 h |
Adverse events | |
Most frequent | Nasopharyngitis, headache, fatigue, dizziness, nausea, somnolence |
Occasional | |
Rare | Sleep paralysis, hypnagogic and hypnopompic hallucinations, accidental overdose |
ATC codes | |
WHO ATC code | Not yet assigned |
EphMRA ATC code | N5B |
Chemical name | (S)-(2-(5-chloro-4-methyl-1H-benzo[d]imidazol-2-yl)-2-methylpyrrolidin-1-yl)(5methoxy-2-(2H-1,2,3-triazol-2-yl)phenyl)methanone hydrochloride |
2.3 Therapeutic Trials
2.3.1 Phase III
Daridorexant at a doses of 25 mg and 50 mg improved sleep outcomes, and at 50 mg improved daytime functioning, in patients with insomnia disorder in two pivotal phase III trials (trial one NCT03545191 and trial two NCT03575104) [24].
In trial one, adult patients with insomnia were randomized to daridorexant 25 mg (n = 310) or 50 mg (n = 310), or placebo (n = 310) once nightly for 3 months. The primary endpoints were change from baseline to month 1 and month 3 in wake time after sleep onset (WASO) and latency to persistent sleep (LPS), both measured by polysomnography in a sleep laboratory. Daridorexant 25 mg and 50 mg significantly (p < 0.0001) reduced WASO from baseline compared to placebo after both 1 month’ (18.4 and 29.0 min vs 6.2 min reduction) and 3 months’ (23.0 and 29.4 min vs 11.1 min reduction) treatment. LPS was also significantly (p ≤ 0.0005) reduced with both daridorexant doses at both time points (28.2 and 31.2 min vs 19.9 min reduction [1 month] and 30.7 and 34.8 min vs 23.1 min reduction [3 months]) [24].
The secondary endpoint of self-reported subjective total sleep time (sTST) was also significantly (p ≤ 0.05) improved with both daridorexant doses compared to placebo at 1 month and 3 months [24].
Daytime functioning (measured using the sleepiness domain of the validated Insomnia Daytime Symptoms and Impacts Questionnaire [IDSIQ]) was significantly (p ≤ 0.05) improved with daridorexant 50 mg, but not 25 mg, compared to placebo at 1 month and 3 months.
Comparable results were reported in a subgroup analysis of 364 patients (121 who received daridorexant 25 mg, 121 who received daridorexant 50 mg and 122 given placebo) who were aged ≥ 65 years [25].
In trial two, patients were randomized to daridorexant 25 mg (n = 309), daridorexant 10 mg (n = 307), or placebo (n = 308). Daridorexant 10 mg was not efficacious compared to placebo, is thus not an approved dose and is not discussed further. At the 25 mg dose, daridorexant significantly reduced WASO from baseline compared to placebo after 1 month’ (24.2 min vs 12.6 min reduction; p < 0.0001) and 3 months’ (24.3 min vs 14.0 min reduction; p = 0.003) treatment. LPS, however, was not significantly reduced with daridorexant 25 mg at either time points compared to placebo (26.5 vs 20.0 min at 1 month [p ≤ 0.03] and 28.9 min vs 19.9 min at 3 months [p ≤ 0.0053]). The secondary endpoint sTST was significantly (p ≤ 0.0001) improved with daridorexant 25 mg compared to placebo at 1 month and 3 months, however IDSIQ sleepiness domain scores were not significantly improved compared to placebo at either time point [24].
Key clinical trials of daridorexant (Idorsia Pharmaceuticals)
Drug(s) | Indication | Phase | Status | Location(s) | Identifier |
|---|---|---|---|---|---|
Daridorexant, Placebo | Insomnia disorder | III | Recruiting | Japan | jRCT2031200452 |
Daridorexant | Insomnia disorder | III | Recruiting | Japan | JapicCTI-205444 |
Daridorexant, famotidine, efavirenz | Drug-drug interactions in volunteers | I | Completed | Germany | NCT04390334 |
Daridorexant, moxifloxacin, placebo | Thorough QT study in volunteers | I | Completed | Czech Republic | NCT04250506 |
Daridorexant | Renal impairment | I | Completed | Germany | NCT04024332 |
Daridorexant, citalopram, placebo | Drug-drug interactions in volunteers | I | Completed | Germany | NCT03907215 |
Daridorexant, zopiclone, placebo | Driving performance in volunteers | I | Completed | Netherlands | NCT03892902 |
Daridorexant, placebo | Effects on respiration in patients with obstructive sleep apnoea | I | Completed | Germany | NCT03765294 |
Daridorexant | Effects of food on pharmacokinetics in volunteers | I | Completed | Czech Republic | NCT03799978 |
Daridorexant, placebo | Effects on respiration in patients with chronic obstructive pulmonary disease | I | Completed | Germany | NCT03646864 |
Daridorexant, placebo | Insomnia disorder | III | Completed | Multinational | NCT03679884 |
Daridorexant, suvorexant, zolpidem, placebo | Abuse potential in healthy recreational drug users | I | Completed | USA | NCT03657355 |
Daridorexant, ethanol, placebo | Drug-drug interactions in volunteers | I | Completed | Netherlands | NCT03609775 |
Daridorexant, placebo | Insomnia disorder | III | Completed | Multinational | NCT03545191 |
Daridorexant, placebo | Insomnia disorder | III | Completed | Multinational | NCT03575104 |
Daridorexant | Hepatic impairment | I | Completed | Switzerland | NCT03713242 |
Daridorexant, rosuvastatin | Drug-drug interactions in volunteers | I | Completed | Czech Republic | NCT03339752 |
Daridorexant, placebo | Ethnic sensitivity study in Japanese vs Caucasian volunteers | I | Completed | Netherlands | NCT03101189 |
Daridorexant, midazolam | Drug-drug interactions in volunteers | I | Completed | Germany | NCT03017495 |
Daridorexant, placebo | Insomnia disorder | II | Completed | USA, Germany | NCT02841709 |
Daridorexant, zolpidem, placebo | Insomnia disorder | II | Completed | Multinational | NCT02839200 |
Daridorexant, placebo | Multiple ascending dose study in volunteers | I | Completed | Netherlands | NCT02571855 |
Daridorexant, diltiazem | Drug-drug interactions in volunteers | I | Completed | Germany | NCT02526888 |
Daridorexant, 14C-labeled daridorexant microtracer, placebo microtracer | Single ascending dose study in volunteers | I | Completed | Netherlands | NCT02919319 |
2.3.2 Phase II
Daridorexant 5–50 mg was associated with a dose-dependent reduction in WASO in patients (18–64 years-of-age) with insomnia disorder in a randomized double-blind, phase II trial (NCT02839200) [26]. Similar results were observed in a further phase II study in elderly patients (median age 69 years) with insomnia disorder treated with daridorexant 5–50 mg (NCT02841709) [27]
2.4 Adverse Events
Adverse events occurring in ≥ 2% of patients treated with daridorexant in trial one (NCT03545191) included nasopharyngitis (6% with daridorexant 50 mg, 7% with daridorexant 25 mg and 6% with placebo), headache (6%, 5% and 4%), accidental overdose (3%, 1% and 2%), fatigue (2%, 2% and 1%), dizziness (2%, 2% and 1%), nausea (2%, < 1% and 1%) and somnolence (2%, 4% and 2%). Adverse events leading to treatment discontinuation occurred in 1% of daridorexant 50 mg recipients, 2% of daridorexant 25 mg recipients and 3% of placebo recipients. Serious adverse events (≥ 1) occurred in 1% of daridorexant 50 mg recipients, 1% of daridorexant 25 mg recipients and 2% of placebo recipients. The incidence of these adverse events was similar in patients who received daridorexant at a dose of 25 mg in trial two [24]. Other adverse events reported in trial one included sleep paralysis (0.5% with daridorexant 25 mg, 0.3% with daridorexant 50 mg, and 0% with placebo) and hypnagogic and hypnopompic hallucinations, which occurred in 0.6% of patients receiving daridorexant 25 mg, but in no patients receiving the higher daridorexant dose or placebo [5].
2.5 Ongoing Clinical Trials
Two phase III trials evaluating daridorexant as treatment for insomnia are currently recruiting patients in Japan; a randomized, double-blind, trial investigating efficacy and safety (jRCT2031200452), and a randomized, open-label study investigating long term safety (JapicCTI-205444).
3 Current Status
Daridorexant received its first approval on 7 January 2022 for the treatment of adult patients with insomnia characterized by difficulties with sleep onset and/or sleep maintenance in the USA [3].
Change history
27 April 2022
A Correction to this paper has been published: https://doi.org/10.1007/s40265-022-01719-x
References
Scammell TE, Winrow CJ. Orexin receptors: pharmacology and therapeutic opportunities. Annu Rev Pharmacol Toxicol. 2011;51:243–66.
Muehlan C, Heuberger J, Juif PE, et al. Accelerated development of the dual orexin receptor antagonist ACT-541468: integration of a microtracer in a first-in-human study. Clin Pharmacol Ther. 2018;104(5):1022–9.
GlobeNewswire. Idorsia receives US FDA approval of QUVIVIQ (daridorexant) 25 and 50 mg for the treatment of adults with insomnia [media release]. 10 Jan 2022. https://www.globenewswire.com/news-release/2022/01/10/2363527/0/en/Idorsia-receives-US-FDA-approval-of-QUVIVIQ-daridorexant-25-and-50-mg-for-the-treatment-of-adults-with-insomnia.html.
European Medicines Agency. Meeting highlights from the Committee for Medicinal Products for Human Use (CHMP) 21-24 February 2022 [media release]. 25 Feb 2022. https://www.ema.europa.eu/en/news/meeting-highlights-committee-medicinal-products-human-use-chmp-21-24-february-2022.
Idorsia Pharmaceuticals US Inc. QUVIVIQ (daridorexant) tablets, for oral use, [controlled substance schedule pending] US prescribing information. 2022. https://www.idorsia.us/documents/us/label/Quviviq_PI.pdf. Accessed 14 Feb 2022
Johnson & Johnson. Johnson & Johnson completes acquisition of Actelion [media release]. 2017.
Idorsia Pharmaceuticals. Idorsia and Mochida enter into a license agreement for the supply, co- development and co-marketing of daridorexant in Japan [media release]. 2019.
Idorsia Pharmaceuticals. Idorsia selects Syneos Health as commercialization partner to launch daridorexant in the United States [media release]. 2020.
Idorsia Pharmaceuticals. Idorsia expands its commercialization partnership with Syneos Health for daridorexant in Europe and Canada [media release]. 26 Jan 2022.
Treiber A, de Kanter R, Roch C, et al. The use of physiology-based pharmacokinetic and pharmacodynamic modeling in the discovery of the dual orexin receptor antagonist ACT-541468. J Pharmacol Exp Ther. 2017;362(3):489–503.
Muehlan C, Brooks S, Zuiker R, et al. Multiple-dose clinical pharmacology of ACT-541468, a novel dual orexin receptor antagonist, following repeated-dose morning and evening administration. Eur Neuropsychopharmacol. 2019;29(7):847–57.
Ufer M, Kelsh D, Schoedel KA, et al. Abuse potential assessment of the new dual orexin receptor antagonist daridorexant in recreational sedative drug users as compared to suvorexant and zolpidem. Sleep. 2021. https://doi.org/10.1093/sleep/zsab224
Boof ML, Dingemanse J, Lederer K, et al. Effect of the new dual orexin receptor antagonist daridorexant on nighttime respiratory function and sleep in patients with mild and moderate obstructive sleep apnea. Sleep. 2021;44(6):zsaa275.
Boof ML, Dingemanse J, Brunke M, et al. Effect of the novel dual orexin receptor antagonist daridorexant on night-time respiratory function and sleep in patients with moderate chronic obstructive pulmonary disease. J Sleep Res. 2021;30(4):e13248.
Schilling U, Henrich A, Muehlan C, et al. Impact of daridorexant, a dual orexin receptor antagonist, on cardiac repolarization following bedtime dosing: results from a thorough QT study using concentration-QT analysis. Clin Drug Investig. 2021;41(8):711–21.
Muehlan C, Fischer H, Zimmer D, et al. Metabolism of the dual orexin receptor antagonist ACT-541468, based on microtracer/ accelerator mass spectrometry. Curr Drug Metab. 2019;20(4):254–65.
Berger B, Muehlan C, Klein G, et al. Pharmacokinetics of daridorexant, a dual orexin receptor antagonist, in patients with renal impairment [abstract no. 23]. Eur J Clin Pharmacol. 2021;77(Suppl 1):S2–3.
Berger B, Dingemanse J, Sabattini G, et al. Effect of liver cirrhosis on the pharmacokinetics, metabolism, and tolerability of daridorexant, a novel dual orexin receptor antagonist. Clin Pharmacokinet. 2021;60(10):1349–60.
Gehin M, Wierdak J, Sabattini G, et al. Effect of gastric pH and of a moderate CYP3A4 inducer on the pharmacokinetics of daridorexant, a dual orexin receptor antagonist. Br J Clin Pharmacol. 2021;88(2):810–19.
Berger B, Kornberger R, Dingemanse J. Pharmacokinetic and pharmacodynamic interactions between daridorexant, a dual orexin receptor antagonist, and citalopram in healthy subjects. Eur Neuropsychopharmacol. 2021;51:90–104.
Zenklusen I, Muehlan C, Ulc I, et al. The dual orexin receptor antagonist daridorexant does not affect the pharmacokinetics of the BCRP substrate rosuvastatin. Clin Exp Pharmacol Physiol. 2020;47(11):1843–9.
US Food and Drug Administration. Daridorexant (Quviviq™) for Insomnia: integrated review. 2022. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2022/214985Orig1s000IntegratedR.pdf. Accessed 4 Mar 2022.
Berger B, Brooks S, Zuiker R, et al. Pharmacological interactions between the dual orexin receptor antagonist daridorexant and ethanol in a double-blind, randomized, placebo-controlled, double-dummy, four-way crossover phase I study in healthy subjects. CNS Drugs. 2020;34(12):1253–66.
Mignot E, Mayleben D, Fietze I, et al. Safety and efficacy of daridorexant in patients with insomnia disorder: results from two multicentre, randomised, double-blind, placebo-controlled, phase 3 trials. Lancet Neurol. 2022;21(2):125–39.
Fietze I, Bassetti C, Mayleben D, et al. Daridorexant is safe and improves both sleep and daytime functioning in elderly patients with insomnia [abstract no. 347]. Sleep. 2021;44(Suppl. 2):A138–9.
Dauvilliers Y, Zammit G, Fietze I, et al. Daridorexant, a new dual orexin receptor antagonist to treat insomnia disorder. Ann Neurol. 2020;87(3):347–56.
Zammit G, Dauvilliers Y, Pain S, et al. Daridorexant, a new dual orexin receptor antagonist, in elderly subjects with insomnia disorder. Neurology. 2020;94(21):e2222–32.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. A. Markham is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
The original article has been revised due to retrospective open choice order.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Markham, A. Daridorexant: First Approval. Drugs 82, 601–607 (2022). https://doi.org/10.1007/s40265-022-01699-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-022-01699-y