Abstract
Introduction
Montelukast is a medicine indicated for use in asthma. Psychiatric disorders including nightmares have not been described in clinical trials but during recent years have been included in the product information as having been reported post-marketing, without further description of the events. Previous descriptions in the scientific literature were based on limited numbers of reports or lacked detailed case information.
Objective
The study aim was to further characterise post-marketing adverse drug reactions for nightmares, suspected to be induced by montelukast, to facilitate safer use of the medicine by providing additional information to patients and healthcare professionals.
Methods
We clinically reviewed reports of nightmares with montelukast present in VigiBase, World Health Organization’s global database of suspected adverse reactions to medicinal products, developed and maintained by the Uppsala Monitoring Centre, until 3 May, 2020.
Results
There were 1118 reports of nightmares with montelukast in VigiBase, which provided valuable descriptions of the nightmares as well as information about the impact on the daily lives, with many cases describing a severe impact of the nightmares. About half of the reports were classified as serious. Two thirds concerned children, with the largest age group represented being children aged 5–10 years. In most cases, the nightmares disappeared upon discontinuation of the drug but for some patients it took a long time until the nightmares ceased.
Conclusions
The nature and potential severity of this adverse drug reaction, as described in these reports, present important knowledge for patients and healthcare providers that could help reduce drug-induced harm. This study highlights the value of post-marketing reports for further characterisation of known adverse drug reactions. The benefit–risk balance should be continuously monitored while patients are taking montelukast.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Global post-marketing reports of adverse drug reactions can provide valuable descriptive information about the reported events. |
The reports of nightmares and other psychiatric adverse events related to montelukast mainly concern children, whose daily life at home and at school was affected. |
The benefits and risks of montelukast should be considered before drug prescription, and regularly re-evaluated throughout the drug treatment. |
1 Introduction
Montelukast is a selective leukotriene receptor antagonist indicated for the treatment of asthma as an add-on therapy in adults and children with mild-to-moderate persistent asthma, and for the prophylaxis of asthma in which the predominant component is exercise-induced bronchoconstriction [1]. Montelukast was first approved in 1998, in the USA and in Europe [1, 2]. Dream abnormalities including nightmares, and other psychiatric disorders such as hallucinations, aggressive behaviour, depression, and suicidal ideation and behaviour have not been mentioned in clinical trials of montelukast [3,4,5,6]. Nightmares are listed as an uncommon adverse drug reaction (ADR) [i.e. occurring at a frequency ≥ 1/1000 to < 1/100], according to the product labelling [1].
A nightmare has been defined as an “extended, extremely dysphoric” dream that “usually involves efforts to avoid threats to survival, security, or physical integrity” [7]. The most common emotion connected to a nightmare is fear, but other emotions such as anger, shame and sadness may also occur [8,9,10,11]. Nightmares can be idiopathic, post-traumatic (as a part of post-traumatic stress reaction) or induced by medicines [12,13,14]. Nightmares may not be considered as unusual during childhood, but frequent nightmares should draw attention to associations with poor sleep quality and an increased risk of psychiatric disorders [15, 16].
Montelukast-induced ADRs, in particular neuropsychiatric events, have been studied previously, mostly in children [17,18,19], or on overall suicidal ideation and behaviour [20,21,22,23]. Nightmares set out in the scientific literature [24, 25], however, were either based on a limited number of reports [24] or lacked detailed information at a case level [25]. Based on post-marketing experience of montelukast, dream abnormalities, such as nightmares, as well as other psychiatric disorders, have been included in the product information as having been reported in post-marketing use [26,27,28]. More recently, the European Union Summary of Product Characteristics (SmPC) [1] of montelukast has been further updated to include a warning on neuropsychiatric events following the assessment of the Periodic Safety Update Report(s), according to the recommendation of the European Medicines Agency Pharmacovigilance Risk Assessment Committee [29]. In addition, the US Food and Drug Administration has strengthened existing labels by requiring a boxed warning about serious behaviour and mood-related changes with montelukast, following the re-evaluation of the benefits and risks of montelukast use, and advises restricted use for allergic rhinitis [30]. In order to quantify the neuropsychiatric risks, possible suicidal adverse events and behaviour-related adverse events that occurred during clinical trials have been examined following the regulatory actions on montelukast SmPC changes [3, 5, 6]. Patients treated with montelukast within the age groups of 12–17 years, and aged older than 65 years, were shown to be at a higher risk for serious behaviour-related events than those who received the placebo [3].
The Food and Drug Administration notes that the benefits of montelukast may not outweigh the risks in some patients, particularly when the symptoms of treated disease may be mild and adequately treated with other medicines [3]. Against this background, the aim of this study was to further characterise ADRs in the form of nightmares, as induced by montelukast according to post-marketing reports. The purpose is to help facilitate safer use of this medicine by providing complementary information to patients and healthcare professionals, for evaluation of benefits versus risks for patients. In particular, the focus was on patient characteristics (e.g. demographics) and the nature of the ADRs (temporal relationship with montelukast intake, seriousness, impact on patients or their families, and management of the ADRs).
2 Methods
The study used an explorative descriptive design with a quantitative inductive approach. Data were collected through VigiBase, the World Health Organization’s global database of suspected adverse reactions to medicinal products, developed and maintained by the Uppsala Monitoring Centre. It is the world’s largest of its type, with over 27 million suspected ADR reports, submitted by the World Health Organization member countries, from 1968 to 3 May, 2020. To identify reports of nightmares with montelukast, all those with the MedDRA® (Medical Dictionary for Regulatory Activities) Preferred Term (PT) nightmare for montelukast as the suspected or interacting drug were retrieved from VigiBase up until 3 May, 2020. Suspected duplicates were excluded using a statistical algorithm developed for this purpose [31]. The overall reporting rates of the different ADRs related to montelukast were compared with the rates for the same ADRs with other drugs in the database. This was achieved using the ‘Information Component’ (IC) [32], which gives a measure of the disproportionality between the expected and the reported rate of ADRs for a given drug. Positive values (IC > 0) represent combinations reported more frequently than expected in view of how often the drug and the ADR are each reported overall in the database. An IC025 with a positive lower 95% confidence limit indicates a statistically significant disproportionality between the expected and the reported rate for the drug and the ADR. A high IC denotes a strong association between the drug and the ADR in the database [33].
Available report variables were considered, such as: patient age, sex, montelukast treatment indications; co-reported medicines and co-reported ADRs; seriousness and outcome of the reports; temporal relationship between drug intake and the occurrence of the events; actions taken with the drug; and information on the type of reporter. The available informative narratives were clinically reviewed.
3 Results
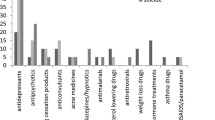
A total of 1118 unique reports with the term nightmare together with montelukast were reviewed. The number of reports for montelukast as suspected or interacting for any ADR in VigiBase from 1998 to 3 May, 2020, was 25,120. The World Health Organization Drug Dictionary preferred base was used to define the substance [34], and the MedDRA® PT (version 23.0) was used to define the ADR. Of all the reports for montelukast in the database, 32% noted a MedDRA® PT within the System Organ Class Psychiatric Disorders. This can be compared to the overall rate of 7.6% of reports for all drugs in the database with an ADR in this System Organ Class. The ten most commonly reported PTs within that System Organ Class are presented in Table 1 together with the IC025, showing the disproportionate reporting.
3.1 Review of the Reports of Nightmares with Montelukast
Montelukast was the single suspected drug in 97% of the 1118 reports and the only reported drug in 58% of them. In 30%, the reporter was a physician, and in 34%, the report came directly from the patient. The remainder came from other healthcare professionals, including pharmacists, or had a combination of reporters, for example, both a patient and a physician or other healthcare professional. The age of the patient was available in 972 reports, and of these, 669 (69%) concerned children aged below 18 years, of which most (550 reports, 57%) concerned children between the ages of 2 and 10 years. More male patients were aged below 11 years, and the opposite was true regarding the ages 11 years and older, see Table 2.
The prescription indications for montelukast were listed in 856 reports, of which 70% were for asthma or combinations of asthma and allergy, or hypersensitivity, 17% for allergies without asthma, and 13% for other indications. Concomitant medications were reported in 42% of the reports, most commonly anti-asthma or anti-allergy drugs. In only 3% of the reports was another drug co-suspected with montelukast. In 5.7% of the reports, Central Nervous System (CNS) drugs Anatomical Therapeutic Chemical (ATC) group N05 Psycholeptics (e.g. antipsychotics, anxiolytics, or hypnotics and sedatives) or ATC group N06 Psychoanaleptics (e.g. antidepressants, psychostimulants, agents used for attention-deficit/hyperactivity disorder and nootropics) were co-reported, and at least three of these were noted as suspected or interacting. Few patients below the age of 18 years (1.3%) were taking drugs belonging to the ATC groups N05 or N06; however, 15% of the adults aged between 18 and 64 years, and 21% aged 65 years and above were using them (see Table 2). In eight cases where information on timing regarding the use of concomitant CNS drugs was known, montelukast had been started after these were introduced, suggesting that these patients already experienced some CNS disorders when starting montelukast treatment.
In 171 reports, nightmare was the only reported term. The most commonly co-reported ADRs for children aged 2–10 years was aggression, while for teenagers and adults it was anxiety and depression. For patients aged 65 years or older, insomnia, headache and muscle spasms were the most commonly co-reported terms. Among the 146 co-reporting suicidal and self-injurious behaviour, patients aged 11–17 years had the highest proportion of these terms (35% of the reports).
When seriousness was known (n = 985), the reports were marked as serious in 52% of the cases, including five deaths (completed suicide). There were 42 reports marked as life threatening (including e.g. suicidal ideation), 45 were marked as requiring (prolonged) hospitalisation (including e.g. depression and suicidal ideation) and 60 reports were marked as disabling (incapacitating). The rest were marked as serious for other medically important conditions.
The median time to onset (TTO) for nightmares after the initial montelukast introduction was 3 days (with a range from the same day to 10 years) [based on information from 467 reports]. Most cases (62%) reported a TTO of 1 week or less; 86 (18%) had a TTO of longer than 1 week but within 1 month; 61 (13%) had a TTO of longer than 1 month but less than 1 year. Further, there were 29 reports with a reported TTO of 1 year or longer. The long TTO was confirmed by narratives in two cases but most reports (20) lacked narrative information, which makes it difficult to get a deeper understanding of how the ADR developed.
Available information about action taken and reaction outcome (n = 543) revealed that a positive dechallenge (that the reaction abated when the patient stopped taking the drug, or in three cases when the dose was reduced) was reported in 90% of the cases. In 44 of the 543 cases, a rechallenge (a reintroduction of the drug) was noted with a documented outcome, of which 32 reported that the nightmares reoccurred (positive rechallenge) and six reported no reoccurrence. For one child, montelukast was reintroduced three times, with nightmare reoccurring each time. In 40 cases where montelukast had been withdrawn, nightmares still remained (negative dechallenge). Follow-up time for several cases was 1–2 days, which is a short time for observing recovery. In three cases, the nightmares had remained unresolved when followed up 20 days to 6 months after montelukast withdrawal. In 15 cases, no action had been taken with the drug, and the ADR continued. For nine patients, the reaction abated although the montelukast dose remained unchanged, of which three patients changed montelukast dose timing from evening to morning intake (see Sect. 3.2). Three patients recovered a few days after switching to other brands for montelukast.
3.2 Review of Narratives for Descriptive Information
Among 1118 cases of nightmares associated with montelukast, 27% had available narratives that have been clinically reviewed, particularly regarding descriptions of the nightmares and other disorders, the time to recovery, potential risk factors, impact on the daily life of the patients or their families, and management of these events.
3.2.1 Description of Nightmares and Related Co-Reported Reactions
As mentioned, over two thirds of the cases of nightmares concerned children. These described varied sleep difficulties: for example, vivid nightmares resulting in extreme fear of sleeping and being left alone. Nightmares had appeared with sleep-talking, somnambulism, fighting or even attempts at self-harming during sleep. Terrifying nightmares woke children up, sometimes accompanying anxiety, crying and seeing strange things, as is illustrated by the following quote: “(A) A child, 1 month on montelukast … sees a cut off hand in the corridor … screaming, crying, seeing strange people in the room” (female child, age 6 years)
Other examples included a patient who had believed that a bomb would destroy the world or make it frozen. Many children got confused and panic-stricken after waking up, sometimes not recognising their parents. Severe nightmares could involve screaming for 45 min after awakening, with difficulties in getting back to reality or sleep. The nightmares could also occur repeatedly, for example, twice a week at night, when montelukast had been taken. The nightmares were in one patient also described as leading to being afraid to eat because of the risk of exploding. Some children also reported fearing death and being afraid of a body falling into pieces.
According to the narratives concerning children, they experienced irritability, mood swings and aggression, self-harming behaviour, hallucinations, vertigo attacks or extreme emotional breakdowns. A 10-year-old child had been prescribed anti-depressants for perceived hallucinations. Another boy threatened to slash his wrists with a knife, who comes from a broken home. Social withdrawal was noticed, and hospitalisation because of suspect acute psychosis or suicidal ideation was recorded in one report. Some narratives described fear of darkness, enuresis and panic attacks with the urge to die:
“2 weeks after starting montelukast, the school signals significant changes in the child's behaviour -moves itself, stops communicating with the environment, suicidal proclamation – “I do not want to live, I want to die” … [banging] head against the wall.” (female child aged 7 years).
All unfamiliar experiences made children frightened, not daring to close their eyes because they were afraid to experience the symptoms again, the following night.
For adults, montelukast-induced nightmares caused extremely unsettled dreams, which led to exhaustion and depressed feelings. The day after the nightmares could yield further feelings of panic, being chased and suicidal thoughts, especially when not being able to sleep, as is illustrated by the following quote:
”Symptoms appeared 1 h after taking tablet at night. On medicine for 2 years. Stopped drug himself due to severity of symptoms, depressed, suicidal thoughts: horrible feelings about self and what others think, self-critical and extremely unsettled dreams. After evening time came when drug was wearing off, I would feel better in myself, only to start feeling ill again on commencing to take another daily dose later in the evening" (male adult, age 35 years).
3.2.2 Impact on the Daily Life of the Patients and Their Families
Many reports described the negative impact on the daily life of the patients and their families. Children could change their behaviour, which for example could have influence on their siblings:
“Voice in head telling to do cruel things e.g. kill his sister” (male child aged 7 years).
This affected both the life at home and the children’s ability to manage pre-school and school. One child had not attended school for 3 years because of severe anxiety. Another child was prescribed anti-depressants, and several children in different reports were described to have undergone therapy to solve their behavioural problems. One mother strongly argued that everyone being prescribed montelukast should be regularly checked and asked about how they were feeling.
3.2.3 Awareness of the Neuropsychiatric ADR Risks of Montelukast
Some prescribers seemed unaware of the risks of neuropsychiatric ADRs in connection with montelukast. Further, an allergologist dismissed a suggested association between the psychiatric events and montelukast, according to the reporting parent, while some other prescribers or healthcare professionals had consulted colleagues for advice. The following is a statement from a parent of a patient:
“son was diagnosed with ADHD while on montelukast, which mother believe is wrong and consider to be related to montelukast.” (male adolescent aged 14 years).
Sometimes, the reporters did not think that the ADRs had potentially been caused by montelukast, until the drug was withdrawn, and improvement was confirmed. In some reports, patients or relatives/parents had tried on their own to quit montelukast after seeing media information, and noticed that the nightmares vanished, according to narratives. When patients and relatives were unaware of potential ADRs due to montelukast, they had understandably not contacted the prescriber for problems. This had led to a long suffering of nightmares, in one case for 5 years, for a child during the ages of 2–7 years. In five cases, the patients were found to have changed the dosage regime of montelukast from the evening to the morning. Three of these patients recovered from the nightmares, while two patients reported experiencing no difference when changing the dose in the morning.
3.3 Off-Label Use
In nine cases, the term off-label use was co-reported, for example, a 58-year-old man experienced suicidal ideation, nightmares and negative thoughts after starting with montelukast for allergy without asthma. A 3-year-old boy received montelukast for the treatment of obstructive bronchitis and pneumonia and experienced nightmares. After awakening, the patient was disoriented, restless and could not fall asleep again. A positive de-challenge was reported for both cases.
3.4 Time to Recovery
For the time to recovery, where the information was available, the nightmares usually diminished or disappeared within days to weeks after montelukast had been withdrawn. In 19 cases, the nightmares were documented to have resolved within 2 weeks. For 16 of the reports, the time to recovery was the same day to 7 days after montelukast had been stopped. However, there were also reports describing months to recovery. In one case, a mother had stopped giving her child montelukast after being alerted through the media of information about montelukast, potentially causing young children a variety of ADRs, for example, suicidal thoughts and vivid nightmares. The child’s physical ADRs were reported to have disappeared quickly, while the psychological ADRs initially worsened, but were completely recovered after 18 months.
4 Discussion
Among the 25,120 available post-marketing reports for montelukast reported as suspected or interacting in VigiBase up to 3 May, 2020, 32% reported psychiatric ADRs including 1118 reported nightmares. Other factors may have contributed to the occurrence of the ADR in some instances. There were examples of children from broken families and children with depression in the family. Additionally, it should be noted that uncontrolled asthma could in itself contribute to several comorbidities including psychological disorders and sleep apnoea [35]. Furthermore, nightmares could occur for a number of reasons that could not always be linked to a certain cause. For the adult cases, it was noted that 15–21% had co-reported CNS drugs including psycholeptics or psychoanaleptics. Two thirds of the reports concerned children below the age of 18 years, and a great majority of these were children aged 2–10 years with the largest group of reports from the age group of 5–10 years. This is consistent with studies that show that nightmares are most frequent between the ages of 6 and 10 years [36]. A previous study on neuropsychiatric events of montelukast, such as depression, aggression, suicidal ideation, abnormal behaviour and nightmares in VigiBase revealed that these events were more frequently reported in children compared with adults, consistent with the present results [17]. Although this was not confirmed in a study using national data in the Netherlands [25], an analysis of spontaneously reported psychiatric ADRs in children during a 10-year period in Sweden did disclose that montelukast was among the most frequently suspected drugs for psychiatric ADRs in children [37]. Furthermore, Benard and colleagues showed in a retrospective cohort study in children aged 1–17 years that the relative risk of adverse events related to montelukast versus inhaled corticosteroids was 12 (2–90) [38]. They also concluded that asthmatic children taking montelukast experienced a notable risk of neuropsychiatric adverse events related to the drug, which led to drug cessation to a larger extent than with inhaled corticosteroids [38].
Many of the reports for children described terrifying experiences from nightmares while taking montelukast. These ranged from screaming during sleep to violence and the wish to die. The consequences of sleep problems varied from sleepiness in the daytime to headaches, behavioural problems, poor school results and more [39]. The reports in VigiBase described the effect of the ADRs on the children’s lives as affecting their abilities to attend preschool and school as well as their lives at home. In such examples, there would presumably be several persons beyond the child that were affected, for example, the close family, school personnel and people involved in rehabilitation.
Although nothing could be said about the potential link between different reported ADRs, a sad finding is the severe co-reported ADRs present in 146 cases; of intention to inflict self-harm or accomplished self-injury, suicidal thoughts and behaviour, and even attempt to or completed suicide. The co-reporting of these terms often revealed a complex picture of for example depression, nightmares and suicidal behaviour in reports from both children and adults. Furthermore, 88 of the 146 reports with severe co-reported ADRs concerned children, 14 of whom were aged under 5 years. The fact that about one third (35%) of the reports with nightmares for adolescents aged 11–17 years reported suicidal or self-injurious behaviour calls for attention. “Depression and Suicidality in paediatric patients” were included in the Risk Management Plan as a safety concern in the European Union until 2018. The removal of this safety concern was based on the considerations that these ADRs are listed in the SmPC, and also there were neither additional pharmacovigilance activities nor additional risk minimisation measures proposed by the originator of the Marketing Authorisation Holder [40]. It was previously believed that montelukast hardly could pass across the blood–brain barrier, but recent updates of the US label indicate that this is possible. The label now recognises studies that have shown that the drug passes over to the brain in rats [3], opening up the possibility that this could apply also to humans, which would better explain the development of CNS ADRs.
In this study, the time from montelukast exposure to nightmare occurrence was within days for most of the patients. This is in line with the findings by Aldea Perona et al. in the study on montelukast-associated psychiatric disorders in children [17]. The TTO for the smaller number of reports of suicidal ideation or suicide attempt varied in the reports in VigiBase from being the same day to having a TTO of 12 months. This is less consistent with the findings of a TTO of months to years for depression and suicide-related behaviour according to the same study. The longer TTO of depression and suicidal behaviours for montelukast found by Aldea Perona et al. however, indicates that patients treated with montelukast should be monitored for the entire treatment period for psychiatric disorders [17].
Differences in the reporting rate of nightmares between male and female individuals were observed in this study: there were more male reports for children aged under 11 years, and the opposite for those aged over 11 years, in particular in adults aged 18–64 years. This may generally reflect the prevalence of asthma related to age and sex, i.e. there is a higher prevalence of asthma in male individuals before puberty and a higher prevalence in female individuals after puberty owing to an increased incidence and decreased remission of asthma in female individuals [41]. The shift occurs between the ages of 11 and 16 years [41]. It also seems like nightmares are more frequently reported for boys than for girls as it could be observed that in VigiBase, nightmares for all reported drugs in the database were more commonly reported for boys (8.2% of the reports for boys aged 2–17 years) than for girls (4.3% of the reports for girls aged 2–17 years).
For adults, the reports of nightmares were to a lesser extent co-reported with serious psychiatric ADRs such as suicidal behaviour. However, nightmares consisted of extremely unsettling dreams, leading to fatigue and depression. As adults were described to co-report the intake of CNS drugs to a higher extent than children, this may be something to be aware of when prescribing montelukast. A major difference for adults is that they can themselves stop taking the drug and follow-up the result. Aggression as the most commonly co-reported reaction together with nightmares for children aged 2–10 years, and anxiety and depression as the most co-reported for teenagers and adults, must be taken seriously for clinicians seeing patients with such behaviours and/or symptoms. In addition, school nurses could pay attention to, and be aware of, this issue.
A few of the reports were describing improvements from the nightmares after shifting dosage regimens and switching to take the dose in the morning instead of in the evening. The SmPC states that the posology should be one tablet each evening [1]; however, the dosage recommendations at the Mayo Clinic [42] gives the option of taking montelukast in the evening or morning to its patients, opening up the possibility to try another dosage regime for those experiencing nightmares and for which the benefits of the drug may still outweigh the risk. However, the Food and Drug Administration states that there should always be a communication between patients and treating healthcare professionals about the benefits and risks of the drug and that there are many other safe and effective allergy medicines available, including over-the counter medicines [43]. The overall high number of reported nightmares and other co-reported psychiatric events for children due to montelukast warrant increased caution as well as prescriber and consumer awareness together with further research to better understand the mechanisms causing nightmares and related psychiatric ADRs, something that has previously been highlighted in other studies [18].
4.1 Strengths and Limitations
For spontaneously reported ADRs, it must be remembered that often the causality between a specific drug (rather than, e.g. underlying illness or any concomitant drugs) and an ADR cannot be proven. Furthermore, the likelihood that the drug caused the ADR may vary between for example reporters and countries owing to differences in regulations or methods to assess the reports. However, as the under-reporting of ADRs is a major problem for post-marketing reports, there are reasons to believe that the cases presented in this study are only a small fraction of those experienced. The reports in this study were to a large extent contributed by patients. There are differences in opinions about the conclusions that can be drawn from reports where a healthcare professional has not been involved, as they cannot be medically confirmed. Nevertheless, causality is not the main issue in this study as the ADR was already recognised and listed in SmPCs. Furthermore, the importance of reports from patients within pharmacovigilance, which often gives a richer description of the experienced ADR, has previously been demonstrated [44,45,46].
The multidisciplinary research team participating in this study consisted of a physician, a registered nurse, a data scientist, and two pharmacists, which allowed for broader perspectives in the analysis of the reports. The authors had close collaboration during the analysis process and discussed the study in research meetings.
5 Conclusions
Although nightmares, together with other psychiatric disorders, are labelled ADRs for montelukast, reviewing the reports in this study revealed that the cases mainly concerned children and patients’ daily life at home and at school. The benefits and risks of montelukast should be considered before the drug prescription, and regularly re-evaluated throughout the drug treatment, in particular when any potential serious CNS ADRs, including nightmares occur. Post-marketing reports, such as in this study, could be used to further characterise ADRs.
References
Datapharm Limited. Montelukast 10 mg film coated tablets: summary of product characteristics (SmPC). https://www.medicines.org.uk/emc/product/1243/smpc. Accessed 2 Jun 2020.
US Food and Drug Administration. Drugs@FDA: FDA-approved drugs. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=020829. Accessed 13 Jul 2021.
Kelsay K. Assessing risk: data from montelukast clinical trials. J Allergy Clin Immunol. 2009;124(4):697–8. https://doi.org/10.1016/j.jaci.2009.09.001.
Holbrook JT, Harik-Khan R. Montelukast and emotional well-being as a marker for depression: results from 3 randomized, double-masked clinical trials. J Allergy Clin Immunol. 2008;122(4):828–9. https://doi.org/10.1016/j.jaci.2008.07.012.
Philip G, Hustad C, Noonan G, et al. Reports of suicidality in clinical trials of montelukast. J Allergy Clin Immunol. 2009;124(4):691-6.e6. https://doi.org/10.1016/j.jaci.2009.08.010.
Philip G, Hustad CM, Malice M-P, et al. Analysis of behavior-related adverse experiences in clinical trials of montelukast. J Allergy Clin Immunol. 2009;124(4):699-706.e8. https://doi.org/10.1016/j.jaci.2009.08.011.
America Psychiatric Association (APA). Diagnostic and statistical manual of mental disorders. 5th ed. Arlington: American Psychiatric Publishing; 2013.
Gieselmann A, Ait-Aoudia M, Carr M, et al. Aetiology and treatment of nightmare disorder: state of the art and future perspectives. J Sleep Res. 2019;28: e12820. https://doi.org/10.1111/jsr.12820.
Köthe M, Pietrowsky R. Behavioral effects of nightmares and their correlations to personality patterns. Dreaming. 2001;11(1):43–52. https://doi.org/10.1023/A:1009468517557.
Robert G, Zadra A. Thematic and content analysis of idiopathic nightmares and bad dreams. Sleep. 2014;37(2):409–17. https://doi.org/10.5665/sleep.3426.
Phelps AJ, Kanaan RAA, Worsnop C, Redston S, Ralph N, Forbes D. An ambulatory polysomnography study of the post-traumatic nightmares of post-traumatic stress disorder. Sleep. 2018;41(1):zsx188. https://doi.org/10.1093/sleep/zsx188.
Spoormaker V, Schredl M, Bout J. Nightmares: from anxiety to sleep disorder. Sleep Med Rev. 2006;10:19–31. https://doi.org/10.1016/j.smrv.2005.06.001.
Thompson DF, Pierce DR. Drug-induced nightmares. Ann Pharmacother. 1999;33(1):93–8. https://doi.org/10.1345/aph.18150.
Novak M, Shapiro CM. Drug-induced sleep disturbances: focus on nonpsychotropic medications. Drug Saf. 1997;16(2):133–49. https://doi.org/10.2165/00002018-199716020-00005.
Nielsen T, Levin R. Nightmares: a new neurocognitive model. Sleep Med Rev. 2007;11(4):295–310. https://doi.org/10.1016/j.smrv.2007.03.004.
Blagrove M, Farmer L, Williams E. The relationship of nightmare frequency and nightmare distress to well-being. J Sleep Res. 2004;13(2):129–36. https://doi.org/10.1111/j.1365-2869.2004.00394.x.
Aldea Perona A, García-Sáiz M, Sanz ÁE. Psychiatric disorders and montelukast in children: a disproportionality analysis of the VigiBase®. Drug Saf. 2016;39(1):69–78. https://doi.org/10.1007/s40264-015-0360-2.
Wallerstedt SM, Brunlöf G, Sundström A, Eriksson AL. Montelukast and psychiatric disorders in children. Pharmacoepidemiol Drug Saf. 2009;18(9):858–64. https://doi.org/10.1002/pds.1794.
Ernst P, Ernst G. Neuropsychiatric adverse effects of montelukast in children. Eur Respir J. 2017;50(2):1701020. https://doi.org/10.1183/13993003.01020-2017.
Schumock GT, Lee TA, Joo MJ, Valuck RJ, Stayner LT, Gibbons RD. Association between leukotriene-modifying agents and suicide: what is the evidence? Drug Saf. 2011;34(7):533–44. https://doi.org/10.2165/11587260-000000000-00000.
Jick H, Hagberg KW, Egger P. Rate of suicide in patients taking montelukast. Pharmacotherapy. 2009;29(2):165–6. https://doi.org/10.1592/phco.29.2.165.
Gorton HC, Webb RT, Kapur N, Ashcroft DM. Non-psychotropic medication and risk of suicide or attempted suicide: a systematic review. BMJ Open. 2016;6(1): e009074. https://doi.org/10.1136/bmjopen-2015-009074.
Gibbons RD, Mann JJ. Strategies for quantifying the relationship between medications and suicidal behaviour: what has been learned? Drug Saf. 2011;34(5):375–95. https://doi.org/10.2165/11589350-000000000-00000.
Cereza G, Garcia Doladé N, Laporte J-R. Nightmares induced by montelukast in children and adults. Eur Respir J. 2012;40(6):1574–5. https://doi.org/10.1183/09031936.00092812.
Haarman MG, van Hunsel F, de Vries TW. Adverse drug reactions of montelukast in children and adults. Pharmacol Res Perspect. 2017;5(5): e00341. https://doi.org/10.1002/prp2.341.
European Medicines Agency. Singulair. Article 30 referral: Annex I, II, III. August 5, 2008. https://www.ema.europa.eu/en/medicines/human/referrals/singulair. Accessed 2 Jun 2020.
US Food and Drug Administration. Drug safety information for healthcare professionals: early communication about an ongoing safety review of montelukast (Singulair). https://wayback.archive-it.org/7993/20170406045734/https:/www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm070618.htm. Accessed 28 Dec 2020.
US Food and Drug Administration. Drug safety information for healthcare professionals: updated information on leukotriene inhibitors: montelukast (marketed as Singulair), Zafirlukast (marketed as Accolate), and Zileuton (marketed as Zyflo and Zyflo CR). https://wayback.archive-it.org/7993/20170404172438/https:/www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm165489.htm. Accessed 28 Dec 2020.
European Medicines Agency. Pharmacovigilance Risk Assessment Committee (PRAC): 12–15 March 2019. https://www.ema.europa.eu/en/events/pharmacovigilance-risk-assessment-committee-prac-12-15-march-2019. Accessed 28 Dec 2020.
US Food and Drug Administration. FDA requires boxed warning about serious mental health side effects for asthma and allergy drug montelukast (Singulair); advises restricting use for allergic rhinitis. Drug Safety Communications. March 4, 2020. https://www.fda.gov/media/135840/download. Accessed 2 Jun 2020.
Norén GN, Orre R, Bate A, Edwards IR. Duplicate detection in adverse drug reaction surveillance. Data Min Knowl Discov. 2007;14(3):305–28. https://doi.org/10.1007/s10618-006-0052-8.
Bate A, Lindquist M, Edwards IR, Orre R. A data mining approach for signal detection and analysis. Drug Saf. 2002;25(6):393–7. https://doi.org/10.2165/00002018-200225060-00002.
Norén GN, Hopstadius J, Bate A. Shrinkage observed-to-expected ratios for robust and transparent large-scale pattern discovery. Stat Methods Med Res. 2013;22(1):57–69. https://doi.org/10.1177/0962280211403604.
Lagerlund O, Strese S, Fladvad M, Lindquist M. WHODrug: a global, validated and updated dictionary for medicinal information. Ther Innov Regul Sci. 2020;54(5):1116–22. https://doi.org/10.1007/s43441-020-00130-6.
Caminati M, Vaia R, Furci F, Guarnieri G, Senna G. Uncontrolled asthma: unmet needs in the management of patients. J Asthma Allergy. 2021;14:457–66. https://doi.org/10.2147/JAA.S260604.
Salzarulo P, Chevalier A. Sleep problems in children and their relationship with early disturbances of the waking-sleeping rhythms. Sleep. 1983;6(1):47–51. https://doi.org/10.1093/sleep/6.1.47.
Bygdell M, Brunlöf G, Wallerstedt SM, Kindblom JM. Psychiatric adverse drug reactions reported during a 10-year period in the Swedish pediatric population. Pharmacoepidemiol Drug Saf. 2012;21(1):79–86. https://doi.org/10.1002/pds.2265.
Benard B, Bastien V, Vinet B, Yang R, Krajinovic M, Ducharme FM. Neuropsychiatric adverse drug reactions in children initiated on montelukast in real-life practice. Eur Respir J. 2017;50(2):1700148. https://doi.org/10.1183/13993003.00148-2017.
Ophoff D, Slaats MA, Boudewyns A, Glazemakers I, Van Hoorenbeeck K, Verhulst SL. Sleep disorders during childhood: a practical review. Eur J Pediatr. 2018;177(5):641–8. https://doi.org/10.1007/s00431-018-3116-z.
Heads of Medicine Agencies (HMA). HaRP assessment report, montelukast. https://www.hma.eu/fileadmin/dateien/Human_Medicines/CMD_h_/Pharmacovigilance_Legislation/RMPs/HaRP_ARs/Montelukast_2019_06_HaRP_AR.pdf. Accessed 12 Jul 2021.
Vink NM, Postma DS, Schouten JP, Rosmalen JGM, Boezen HM. Gender differences in asthma development and remission during transition through puberty: the TRacking Adolescents’ Individual Lives Survey (TRAILS) study. J Allergy Clin Immunol. 2010;126(3):498-504.e6. https://doi.org/10.1016/j.jaci.2010.06.018.
Mayo Clinic. Montelukast, drug information. January 2021. https://www.mayoclinic.org/drugs-supplements/montelukast-oral-route/proper-use/drg-20064902. Accessed 12 Jul 2021.
US Food and Drug Administration. FDA requires boxed warning about serious mental health side effects for asthma and allergy drug montelukast (Singulair); advises restricting use for allergic rhinitis. March 2020. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-boxed-warning-about-serious-mental-health-side-effects-asthma-and-allergy-drug. Accessed 12 Jul 2021.
Rolfes L, van Hunsel F, Wilkes S, van Grootheest K, van Puijenbroek E. Adverse drug reaction reports of patients and healthcare professionals-differences in reported information: information reported by patients and healthcare professionals. Pharmacoepidemiol Drug Saf. 2015;24(2):152–8. https://doi.org/10.1002/pds.3687.
Härmark L, van Hunsel F, Grundmark B. ADR reporting by the general public: lessons learnt from the Dutch and Swedish systems. Drug Saf. 2015;38(4):337–47. https://doi.org/10.1007/s40264-015-0264-1.
Watson S, Chandler RE, Taavola H, et al. Safety concerns reported by patients identified in a collaborative signal detection workshop using VigiBase: results and reflections from Lareb and Uppsala Monitoring Centre. Drug Saf. 2018;41(2):203–12. https://doi.org/10.1007/s40264-017-0594-2.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to assist in the preparation of this study.
Conflict of interest
Sarah Watson, Elenor Kaminsky, Marian Attalla, Henric Taavola and Qun-Ying Yue have no conflicts of interest that are directly relevant to the content of this study. Marian Attalla is currently employed by PharmaRelations; however, her contributions to this work were made prior to the start of the employment at PharmaRelations and as part of her employment at the Uppsala Monitoring Centre. No changes to the article have been made based on her comments since the start of the employment at PharmaRelations.
Ethics approval
The reports shared within the World Health Organization Programme are deidentified, and research based on these data is not subject to ethics approval or informed consent.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The datasets generated and analysed during the current study are not publicly available because of agreements between contributors of data to the database used (VigiBase) and the custodian of this database. National centres (mainly national drug regulatory authorities) constituting the World Health Organization Programme for International Drug Monitoring contribute data to VigiBase and the Uppsala Monitoring Centre is the custodian in its capacity as the World Health Organization Collaborating Centre for International Drug Monitoring. Some subsets of the data may be available from the corresponding author on reasonable request.
Code availability
Not applicable.
Authors’ contributions
Sarah Watson wrote the draft and performed analyses. Henric Taavola, Marian Attalla and Elenor Kaminsky performed analyses and provided critical reviews and editing to the manuscript. Qun-Ying Yue contributed with conceptualisation, methodology, critical review and editing to the manuscript. All authors read and approved the final version.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Watson, S., Kaminsky, E., Taavola, H. et al. Montelukast and Nightmares: Further Characterisation Using Data from VigiBase. Drug Saf 45, 675–684 (2022). https://doi.org/10.1007/s40264-022-01183-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-022-01183-2