Abstract
Introduction
Darunavir is a human immunodeficiency virus type 1 (HIV-1) protease inhibitor boosted with ritonavir (DRV/r) or cobicistat.
Objective
This study provided continued access to DRV/r and assessed long-term safety in patients aged 3 to < 18 years.
Methods
Patients who had completed treatment in the DELPHI (TMC114-C212), DIONE (TMC114-TiDP29-C230), or ARIEL (TMC114-TiDP29-C228) studies were eligible to participate if they derived benefit from using DRV/r in countries where it was not available to them. DRV/r dosing was continued based on original study protocols. Only safety data were collected. Reportable adverse events (AEs) included AEs considered at least possibly related to treatment with DRV/r, AEs leading to discontinuation or treatment interruption, and serious AEs (SAEs).
Results
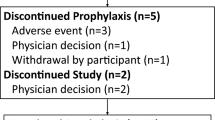
Forty-six patients rolled over to this study and received at least one dose of DRV/r. Median duration of DRV/r intake was 4.2 years. Overall, 15/46 patients experienced one or more reportable AEs, 10/46 patients experienced one or more grade 3 or 4 AEs, and 12/46 patients experienced one or more SAEs. The most common grade 3 or 4 and SAEs were pneumonia (3/46) and asthma (2/46). Only one AE (grade 1 lipoatrophy) was considered probably related to DRV/r (DIONE, n = 1). Overall, 3/46 patients experienced an HIV-related AE (grade 3 pneumonia SAE; grade 2 tuberculosis SAE, and grade 2 lipoatrophy AE), none of which were considered related to DRV/r or led to study discontinuation. Two AEs leading to discontinuation were pregnancies.
Conclusion
These long-term safety results continue to support DRV/r as a valuable therapeutic option for the treatment of HIV-1 infection in pediatric patients aged ≥ 3 years.
Trial Registration
ClinicalTrials.gov: NCT01138605/EudraCT number: 2009-017013-29; first submitted 8 April 2010.
Similar content being viewed by others

References
UNICEF. UNICEF: Global and regional trends. 2019. https://data.unicef.org/topic/hivaids/global-regional-trends/. Accessed 01 May 2020.
Panel on Antiretroviral Therapy and Medical Management of Children Living with HIV. Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection. 2020. https://aidsinfo.nih.gov/guidelines. Accessed 01 May 2020.
Phelps BR, Rakhmanina N. Antiretroviral drugs in pediatric HIV-infected patients: pharmacokinetic and practical challenges. Paediatr Drugs. 2011;13:175–92.
Menson EN, Walker AS, Sharland M, Wells C, Tudor-Williams G, Riordan FA, et al. Underdosing of antiretrovirals in UK and Irish children with HIV as an example of problems in prescribing medicines to children, 1997–2005: cohort study. BMJ. 2006;332:1183–7.
Cahn P, Fourie J, Grinsztejn B, Hodder S, Molina JM, Ruxrungtham K, et al. Week 48 analysis of once-daily vs. twice-daily darunavir/ritonavir in treatment-experienced HIV-1-infected patients. AIDS. 2011;25:929–39.
Lathouwers E, Wong EY, Luo D, Seyedkazemi S, De Meyer S, Brown K. HIV-1 resistance rarely observed in subjects using darunavir once-daily regimens across clinical studies. HIV Clin Trials. 2017;18:196–204.
Orkin C, DeJesus E, Khanlou H, Stoehr A, Supparatpinyo K, Lathouwers E, et al. Final 192-week efficacy and safety of once-daily darunavir/ritonavir compared with lopinavir/ritonavir in HIV-1-infected treatment-naïve patients in the ARTEMIS trial. HIV Med. 2013;14:49–59.
Orkin C, Molina JM, Negredo E, Arribas JR, Gathe J, Eron JJ, et al. Efficacy and safety of switching from boosted protease inhibitors plus emtricitabine/tenofovir disoproxil fumarate regimens to the once-daily complete HIV-1 regimen of darunavir/cobicistat/emtricitabine/tenofovir alafenamide (D/C/F/TAF) in virologically suppressed, HIV-1-infected adults through 48 weeks (EMERALD): a phase 3, randomised, non-inferiority trial. Lancet HIV. 2018;5:e23–34.
Orkin C, Eron JJ, Rockstroh J, Podzamczer D, Esser S, Vandekerckhove L, et al. Week 96 results of a phase 3 trial of darunavir/cobicistat/emtricitabine/tenofovir alafenamide in treatment-naïve HIV-1 patients. AIDS. 2020;34:707–18.
Eron JJ, Orkin C, Gallant J, Molina JM, Negredo E, Antinori A, et al. A week-48 randomized phase-3 trial of darunavir/cobicistat/emtricitabine/tenofovir alafenamide in treatment-naïve HIV-1 patients. AIDS. 2018;32:1431–42.
Eron JJ, Orkin C, Cunningham D, Pulido F, Post FA, De Wit S, et al. Week 96 efficacy and safety results of the phase 3, randomized EMERALD trial to evaluate switching from boosted-protease inhibitors plus emtricitabine/tenofovir disoproxil fumarate regimens to the once daily, single-tablet regimen of darunavir/cobicistat/emtricitabine/tenofovir alafenamide (D/C/F/TAF) in treatment-experienced, virologically-suppressed adults living with HIV-1. Antiviral Res. 2019;170:104543.
Flynn P, Komar S, Blanche S, Giaquinto C, Noguera-Julian A, Welch S, et al. Efficacy and safety of darunavir/ritonavir at 48 weeks in treatment-naïve, HIV-1-infected adolescents: results from a phase 2 open-label trial (DIONE). Pediatr Infect Dis J. 2014;33:940–5.
Violari A, Bologna R, Kumarasamy N, Pilotto JH, Hendrickx A, Kakuda TN, et al. Safety and efficacy of darunavir/ritonavir in treatment-experienced pediatric patients. Pediatr Infect Dis J. 2015;34:e132–7.
Blanche S, Bologna R, Cahn P, Rugina S, Flynn P, Fortuny C, et al. Pharmacokinetics, safety and efficacy of darunavir/ritonavir in treatment-experienced children and adolescents. AIDS. 2019;23:2005–13.
Brochot A, Kakuda TN, Van De Casteele T, Opsomer M, Tomaka FL, Vermeulen A, et al. Model-based once-daily darunavir/ritonavir dosing recommendations in pediatric HIV-1-infected patients aged ≥ 3 to < 12 years. CPT Pharmacometr Syst Pharmacol. 2015;4:406–14.
Janssen Pharmaceutical Companies. Prescribing information for PREZISTA® tablets and oral suspension. 2006. Revised 2019. http://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/PREZISTA-pi.pdf. Accessed 01 May 2020.
Janssen-Cilag International NV. PREZISTA® tablets and oral suspension. Summary of Product Characteristics. 2007. Revised 2019. https://www.medicines.org.uk/emc. Accessed 01 May 2020.
PENTA. PENTA HIV first and second line antiretroviral treatment guidelines. 2019. https://penta-id.org/hiv/treatment-guidelines/. Accessed 01 May 2020.
Kakuda TN, Sekar V, Lavreys L, De Paepe E, Stevens T, Vanstockem M, et al. Pharmacokinetics of darunavir after administration of an oral suspension with low-dose ritonavir and with or without food. Clin Pharmacol Drug Dev. 2014;3:346–52.
US Department of Health and Human Services, National Institutes of Health, National Institute of Allergy and Infectious Diseases, Division of AIDS. Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events, Version 1.0 [updated August 2009]. https://rsc.niaid.nih.gov/sites/default/files/table-for-grading-severity-of-adult-pediatric-adverse-events.pdf. Accessed 26 Nov 2020.
Schlatter AF, Deathe AR, Vreeman RC. The need for pediatric formulations to treat children with HIV. AIDS Res Treat. 2016;2016:1654938.
Beghin JC, Yombi JC, Ruelle J, Van der Linden D. Moving forward with treatment options for HIV-infected children. Expert Opin Pharmacother. 2018;19:27–37.
Ortiz R, Dejesus E, Khanlou H, Voronin E, van Lunzen J, Andrade-Villanueva J, et al. Efficacy and safety of once-daily darunavir/ritonavir versus lopinavir/ritonavir in treatment-naive HIV-1-infected patients at week 48. AIDS. 2008;22:1389–97.
Janssen-Cilag International NV. SYMTUZA® (darunavir, cobicistat, emtricitabine, and tenofovir alafenamide) tablets: Summary of Product Characteristics. 2017. Revised: 2019. https://www.medicines.org.uk/emc/product/8430. Accessed 01 May 2020.
Janssen Pharmaceutical Companies. Prescribing information for SYMTUZA® (darunavir, cobicistat, emtricitabine, and tenofovir alafenamide) tablets. 2018. Revised: 2019. http://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/SYMTUZA-pi.pdf. Accessed 01 May 2020.
Acknowledgements
The authors thank the patients and their families for their participation and support during the study, the central and local Janssen study teams, study center staff, and the principal investigators, in particular Patricia Flynn for her review of this manuscript. The authors also thank other Janssen staff members for their input into this manuscript, and acknowledge Ian Woolveridge (Zoetic Science, an Ashfield company, Macclesfield, UK) for assistance in drafting the manuscript and coordinating and collating author contributions, which was funded by Janssen.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was sponsored by Janssen.
Conflict of interest
Avy Violari is a Data and Safety Monitoring Board member for study TMC114IFD3001, but has no commercial interests with Janssen. Maysseb Masenya and Stephane Blanche have no conflicts of interest to declare in relation to the DIANA study. Simon Vanveggel, Veerle Hufkens, Polan Chetty and Magda Opsomer are all full-time employees of Janssen and potential stockholders of Johnson and Johnson.
Ethics approval
The study protocol and amendments were reviewed by an Independent Ethics Committee/Institutional Review Board (IEC/IRB). This study was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and that are consistent with Good Clinical Practices and applicable regulatory requirements.
Consent to participate
Patients (where appropriate, depending on age) and their parent(s) or legal representative(s) had to sign the informed consent form voluntarily.
Consent for publication
All authors made the decision to submit the manuscript for publication.
Availability of data and material
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through the Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
Code availability
Not applicable.
Author contributions
Avy Violari was an investigator in the trial and reported data for those patients. Simon Vanveggel, Veerle Hufkens, Polan Chetty, and Magda Opsomer were involved in the data analyses. All authors were involved in the development of the primary manuscript and interpretation of data, have read and approved the final version, and have met the criteria for authorship as established by the International Committee of Medical Journal Editors (ICMJE).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Violari, A., Masenya, M., Blanche, S. et al. The DIANA Study: Continued Access to Darunavir/Ritonavir (DRV/r) and Long-Term Safety Follow-Up in HIV-1-Infected Pediatric Patients Aged 3 to < 18 Years. Drug Saf 44, 439–446 (2021). https://doi.org/10.1007/s40264-020-01032-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-020-01032-0