Abstract
Ritonavir is a potent inhibitor of the cytochrome P450 3A4 enzyme and is commonly used as a pharmacokinetic (PK) enhancer in antiviral therapies because it increases bioavailability of concomitantly administered antivirals. Decades of experience with ritonavir-enhanced HIV therapies and, more recently, COVID-19 therapies demonstrate that boosting doses of ritonavir are well tolerated, with an established safety profile. The mechanisms of PK enhancement by ritonavir result in the potential for drug–drug interactions (DDIs) with several classes of drugs, thus making co-medication management an important consideration with enhanced antiviral therapies. However, rates of DDIs with contraindicated medications are low, suggesting these risks are manageable by infectious disease specialists who have experience with the use of PK enhancers. In this review, we provide an overview of ritonavir’s mechanisms of action and describe approaches and resources available to mitigate adverse events and manage concomitant medication in both chronic and short-term settings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Ritonavir is a protease inhibitor that is frequently used as a pharmacokinetic booster because it inhibits cytochrome P450 3A4 (CYP3A4). |
Ritonavir-enhanced nirmatrelvir (nirmatrelvir/ritonavir) has been approved for short-term treatment of COVID-19. |
Drug–drug interactions may occur when nirmatrelvir/ritonavir is concomitantly administered, primarily due to ritonavir. |
Straightforward strategies, such as dose modification and monitoring, may allow nirmatrelvir/ritonavir to be used and potential drug–drug interactions to be mitigated. |
Introduction
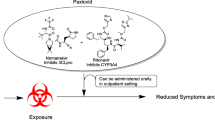
Ritonavir (Norvir®; AbbVie Inc., Chicago, IL, USA) was originally approved in 1996 as an antiretroviral therapy against human immunodeficiency virus (HIV)–1 [1]. Although it demonstrated powerful antiviral effects as a protease inhibitor [2], it is more commonly used as a pharmacokinetic (PK) enhancer in HIV and other therapies [3] because of its potent inhibition of the cytochrome P450 (CYP) 3A4 (CYP3A4) enzyme [4,5,6], which mediates oxidative metabolism of many drugs in vivo [7]. This inhibition increases the half-life of concomitantly administered drugs [8, 9], thereby increasing bioavailability and essentially lowering the required effective dose and dosing frequency of the coadministered drug [3, 10, 11]. In December 2021, nirmatrelvir coadministered with ritonavir as a PK enhancer (nirmatrelvir/ritonavir; Paxlovid™; Pfizer Inc, New York, NY, USA) [12] became the first oral antiviral treatment for COVID-19 granted emergency use authorization by the US Food and Drug Administration (FDA) [13]; full approval was granted in May 2023 [14].
When using ritonavir for PK enhancement in other treatment combinations, co-medication management to address potential drug–drug interactions (DDIs) is an important consideration with enhanced antiviral therapies because of ritonavir’s inhibition of CYP3A4 [15, 16] and because ritonavir is known to interact with a number of other enzymes via inhibition or induction [1, 15, 17, 18]. Nirmatrelvir/ritonavir is commonly prescribed by general practitioners and pharmacists, some of whom may not have had a great deal of experience managing DDIs associated with ritonavir, but more than two decades of experience by infectious disease specialists in the HIV therapeutic area support the capacity for safe coadministration of ritonavir as a PK enhancer. Here we provide an overview of ritonavir’s mechanisms of action along with approaches and resources available to mitigate adverse events (AEs) and manage concomitant medication. This article is based on previously conducted studies and does not contain any new studies with human participants or animals conducted by any of the authors.
Ritonavir Mechanisms of Action
Ritonavir exhibits three distinct mechanisms of action. Its initial intended use as a monotherapy HIV-1 protease inhibitor [19] occurs by binding to the active site of the HIV-1 protease, thus inhibiting its proteolytic activity and cleavage of Gag and Gag-Pol polyproteins into mature, functional proteins [1, 20]. Additionally, ritonavir inhibits several metabolic enzymes and transporters, including CYP3A4/5, P-glycoprotein, organic anion-transporting polypeptide 1B1 (OATP1B1), and, to a lesser extent and at higher concentrations (600 mg twice daily), CYP2D6 [1, 4,5,6, 15, 17, 18, 21, 22]. Most notable among these is the inhibition of CYP3A4/5 because 30% of clinically used drugs are primarily metabolized by this enzyme [7]. Because ritonavir results in irreversible inhibition of CYP3A4, new enzymes need to be synthesized to reverse its effects, thus causing a drug interaction by affecting the clearance and increasing the half-life and therapeutic efficacy of CYP3A4 substrates [6]. The maximum CYP3A inhibitory activity of ritonavir has been observed 48 h after treatment initiation with 300-mg twice-daily dosing [23]. CYP3A activity started to recover 2 days after treatment discontinuation, but was only 27% of baseline levels after 3 days [23]; however, recent modeling suggests that > 80% of CYP3A4 inhibition resolves within 3 days after stopping ritonavir treatment [24].
Several mechanisms have been proposed to describe the irreversible inhibition of CYP3A4 by ritonavir, including formation of a complex between a metabolic intermediate of ritonavir and the heme iron of the enzyme, tight binding between unchanged ritonavir and the heme iron, destruction of the heme iron, and covalent bonding between the CYP3A polypeptide and a reactive intermediate of ritonavir [6]. It is possible, particularly given the large size and flexibility of the CYP3A4 active site, that all of these mechanisms are involved [6]. Finally, ritonavir also acts as an inducer of many drug metabolizing enzymes, including CYP3A, CYP1A2, CYP2B6, CYP2C9, CYP2C19, and uridine 5'-diphospho-glucuronosyltransferase (UGT) [1, 3]. This is accomplished through the activation of pregnane X receptor (PXR), a nuclear receptor that promotes transcription of many genes, including those in the CYP and UGT families [8, 25], via a combination of hydrophobic interactions and hydrogen bonds between ritonavir and the PXR active site [26].
Ritonavir Clinical Profile: Initial Approval to Treat HIV and Subsequent Identification as a Pharmacokinetic Enhancer
Ritonavir was initially approved in 1996 by the FDA as a treatment for HIV-1 infection because it inhibits the viral protease [1]. Although ritonavir was effective, 33% of patients receiving 600 mg twice daily discontinued due to intolerance [27], resulting in it being relegated to a second-line therapy [10]. However, it was subsequently discovered that because ritonavir inhibited CYP3A-mediated oxidative metabolism, coadministration led to increased bioavailability and longer half-lives of antiretroviral (or concomitantly administered) drugs through their decreased metabolism, thus leading to ritonavir’s use as a PK enhancer in HIV treatments [8, 9, 28,29,30,31]. A benefit of using ritonavir-enhanced therapies rather than unenhanced therapies was that low doses of ritonavir (100–200 mg once or twice daily) could be used. This was associated with improved tolerability while increasing the bioavailability (and therefore lowering the required effective dose) of the coadministered drug [10, 12, 18] and still reducing HIV viral load and improving immunologic parameters [32,33,34]. Ritonavir-enhanced therapies are also associated with reduced costs because lower levels of the coadministered drug are required [3]. For example, lopinavir/ritonavir became the first coformulated combination [11], further reducing the pill burden.
Safety Experience with Long-Term Ritonavir-Enhanced Antiviral Therapy for HIV
Decades of experience with ritonavir-enhanced treatment regimens have established that it is well tolerated, with an established safety profile. Since being clinically established as a PK enhancer [29, 35, 36], ritonavir has consistently been included in combination therapies for individuals with HIV [37] and is still recommended as a PK enhancer in initial antiretroviral regimens [38]. Ritonavir’s most frequently reported AEs are gastrointestinal-related events (including nausea, vomiting, abdominal pain, or diarrhea), neurologic disturbances (including paresthesia, dysgeusia, and dizziness), rash, and fatigue [1, 30, 39,40,41]. Overall, however, the rates of AEs associated with treatment are low; real-world data reflect this in HIV combination therapies [39, 41,42,43].
The same mechanism that allows ritonavir to be successfully used as a PK enhancer also allows potential drug–drug interactions (DDIs) with many classes of drugs [8, 16]. However, use of contraindicated medications is low [44, 45], suggesting infectious disease specialists are able to navigate these risks. Assessing the effects of concomitant medications and standard AE/laboratory abnormality monitoring is routine among healthcare providers treating individuals with HIV [30]. This is especially important because multimorbidity and polypharmacy are more prevalent in this population compared with those without HIV [46]. Approaches taken to help mitigate AEs and DDIs include dose adjustment or titration of concomitant medications or changing medications [30, 38, 47]. Because hepatitis B and hepatitis C virus infections can frequently be found in individuals with HIV [48], healthcare providers may need to assess if pathways/proteins affected by coinfection are also affected by ritonavir to determine if an increased risk of AE exists.
Safety Experience with Short-Term Ritonavir-Enhanced Antiviral Therapy for COVID-19
Recently, a ritonavir PK-enhanced regimen was approved for short-term (5-day) antiviral treatment of COVID-19 [12]. Nirmatrelvir/ritonavir is approved for the treatment of individuals with mild to moderate COVID-19 who are at high risk of progression to severe disease and is strongly recommended by the World Health Organization in this population [49, 50]. Approval was based on results from the Evaluation of Protease Inhibition for COVID-19 in High-Risk Patients (EPIC-HR) phase 2/3 study (NCT04960202), which demonstrated an 89% relative risk reduction of COVID-19–associated hospitalization or death when twice-daily treatment for 5 days starting within 5 days of symptom onset [12, 51]. Supportive, but statistically nonsignificant, findings were observed in a subsequent phase 2/3, placebo-controlled study (NCT05011513) in nonhospitalized adults with COVID-19 at standard risk of developing severe disease that included a cohort of individuals vaccinated against COVID-19 who had ≥ 1 risk factor for progressing to severe illness [52]. In the high-risk vaccinated cohort of this study, clinical benefit was observed among those who received nirmatrelvir/ritonavir versus placebo, but the study was not designed to detect statistically significant differences in subpopulations. A pooled analysis of unvaccinated high-risk participants in EPIC-HR and the high-risk vaccinated participants in the subsequent phase 2/3 study showed a statistically significant 83% relative risk reduction associated with nirmatrelvir/ritonavir compared with placebo for COVID-19–related hospitalization or any-cause death within 28 days [53]; notably, COVID-19 cases reported in this study included confirmed Omicron infections [52]. To date, nirmatrelvir/ritonavir has not shown statistically significant clinical benefit in a standard risk population. Real-word studies support the tolerability of nirmatrelvir/ritonavir, and the safety profile is similar to that of ritonavir PK-enhanced HIV treatments [54,55,56,57,58,59,60]. The overall rates of AEs associated with nirmatrelvir/ritonavir use in real-world studies have been generally low (< 20%) [54, 56, 57], although one study reported rates of approximately 50% [55]. Among most of these studies, the most frequently reported AE was dysgeusia, accounting for 8–85% of all events [54,55,56, 58]. Additional care may be warranted when ritonavir is used in certain populations. For example, although ritonavir can be safely used without dose adjustment in those with mild to moderate hepatic impairment (Child–Pugh Classification Class B) or mild renal impairment (estimated glomerular filtration rate [eGFR] 60 to < 90 ml/min), it is not recommended for those with severe hepatic or renal impairment (eGFR < 30 ml/min) due to the lack of PK and safety data for this population [1]. However, several studies have demonstrated low rates of AEs even in special populations treated with nirmatrelvir/ritonavir, such as those with impaired kidney function, immunosuppressive disorders, or children with hematologic diseases (leukemia, aplastic anemia) [61,62,63].
Short-Term Co-Medication Management with Ritonavir-Enhanced Antiviral Therapy
Key differences for ritonavir PK-enhanced antiviral treatment in patients with COVID-19 versus treatment for people living with HIV are the short-term duration of treatment and broader target population, which is more likely to receive prescriptions from general practitioners or pharmacists. Individuals with mild to moderate COVID-19 who are considered to be at high risk for progression to severe disease, as defined by the Centers for Disease Control and Prevention, are eligible for treatment with nirmatrelvir/ritonavir [12, 64]. Approximately one in five individuals globally falls into this category [65], with older age and certain underlying conditions (e.g., cardiovascular disease, obesity, and diabetes) being associated with an increased risk of severe outcomes from COVID-19 [64, 66,67,68,69]. Individuals with multiple comorbidities are also associated with severe illness [64]. These individuals may be more likely to be on medications for their conditions, potentially putting them at risk for DDIs if they receive nirmatrelvir/ritonavir. Drug–drug interactions with nirmatrelvir/ritonavir are expected to primarily be due to ritonavir [70]. Proactive management of concomitant medications requires considering the contribution of ritonavir’s mechanisms of action to DDIs and adherence to labeled contraindications. Boxed warnings on the regulatory labels for both ritonavir and nirmatrelvir/ritonavir note the potential for serious or significant interactions [1, 12], and health bodies such as the WHO recommend that clinicians review all medications an individual is taking and nirmatrelvir/ritonavir should be avoided in those with possible dangerous DDIs [50]. For example, ritonavir is contraindicated with potent CYP3A inducers and drugs highly dependent on CYP3A for clearance, and dose reductions may be necessary when used with other protease inhibitors (i.e., atazanavir, darunavir, fosamprenavir, saquinavir, and tipranavir) [1, 12]. The nirmatrelvir/ritonavir prescribing label notes that individuals receiving a ritonavir- or cobicistat-containing HIV regimen could continue their treatment as indicated, but should be monitored for increased nirmatrelvir/ritonavir or protease inhibitor AEs [12]. Additionally, therapeutic concentration monitoring is recommended in patients receiving immunosuppressive drugs, such as tacrolimus; concomitant treatment should be avoided if monitoring is not possible, [1, 12] because coadministration is associated with a risk for decreased clearance that can lead to adverse outcomes [71,72,73,74,75,76,77,78]. However, strategies to manage these interactions have been described for both long-term and short-term treatments [75, 77, 79,80,81,82]. Because many commonly prescribed medications among individuals who received or could receive nirmatrelvir/ritonavir for COVID-19 are not likely to cause a DDI with ritonavir [83,84,85], most patients will not require complex co-medication management, especially for a short course of treatment for COVID-19.
Several real-world studies emphasize the importance of vigilance on the part of practitioners prescribing nirmatrelvir/ritonavir concurrently with other treatments; however, the overall rates and severity of DDIs among different populations imply a generous margin of safety. Among claims analyses and pharmacovigilance reports, 13–75% of individuals with COVID-19 who received nirmatrelvir/ritonavir were expected to be at risk for or experienced DDIs [60, 85,86,87,88]. The highest rate of DDIs was reported in a retrospective analysis of 60 patients (n = 55 with ≥ 2 risk factors for progression to severe disease) who received nirmatrelvir/ritonavir from clinical pharmacists, with 101 DDIs among 45 patients (75%), with 36 patients (60%) having ≥ 1 significant interaction [86]. In the available literature, it is notable that problematic DDIs generally arise in a limited subset of nirmatrelvir/ritonavir recipients. In a survey of the French pharmacovigilance database that included 12,179 individuals who received nirmatrelvir/ritonavir in 2022, only 30 (13%) reports of DDIs were identified from 228 selected reports. The most frequently reported DDIs were with immunosuppressants (tacrolimus and ciclosporin) and anticoagulants; only 20 reports (9%) were considered serious [60]. Methemoglobinemia related to a dapsone hydroxylamine and ritonavir DDI as well as bradycardia associated with anti-hypersensitive drugs were reported. A retrospective analysis of claims data from German statutory health insurance from 2018–2019 reported that approximately 44% of individuals were at risk for potential DDIs with nirmatrelvir/ritonavir, with 31–32% receiving contraindicated medications and 12–13% receiving medications that would require monitoring or dose adjustments [87]. A cross-sectional study using US electronic health records from the National COVID Cohort Collaborative Enclave reported that only about 16% of individuals taking nirmatrelvir/ritonavir were at risk for a potential moderate-to-severe DDI [89]. An analysis of pharmacy databases and electronic health records among individuals who tested positive for SARS-CoV-2 at the Chelsea and Westminster Hospital in London revealed that 11% received drugs with interactions that would have precluded coadministration [88]. Several of the real-world studies above report that 60% of interactions may be addressed through mitigation strategies, with only about one-third of nirmatrelvir/ritonavir recipients estimated to be at risk for potential exposure to contraindicated medications or major DDIs [60, 85, 86]. In fact, among the top 100 drugs most likely to be prescribed to US adults who are eligible for nirmatrelvir/ritonavir treatment due to high risk of progression to severe COVID-19, the vast majority (70%) are not expected to cause DDIs with nirmatrelvir/ritonavir [83].
Considerations and Tools for Effective Short-Term Co-Medication Management with Ritonavir-Enhanced Antiviral Therapy
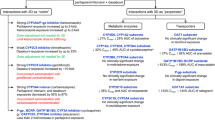
Approximately 39 million people globally were living with HIV in 2022, and an additional 30 million were accessing retroviral therapy [65]. In 2020, it was estimated that more than 1.7 billion people, or about 22% of the global population, had at least one underlying condition (e.g., age or underlying health condition) that put them at risk for developing severe disease due to COVID-19 [90]. Individuals eligible to receive nirmatrelvir/ritonavir to treat COVID-19 can obtain a prescription from a general practitioner or pharmacist. Infectious disease specialists in the HIV therapeutic area may be more familiar with and have more experience managing DDIs due to ritonavir, whereas general healthcare providers who prescribe nirmatrelvir/ritonavir to patients with COVID-19 at high risk of severe outcomes may not be as familiar assessing ritonavir-associated DDIs and may require guidance and education regarding co-medication management. Regulatory labels are the primary resource regarding potential interactions with nirmatrelvir/ritonavir and provide information on potential DDIs. Additional resources, such as the National Institutes of Health (NIH) COVID-19 Treatment Guidelines [91], are available to help general practitioners evaluate and manage concomitant medications when prescribing nirmatrelvir/ritonavir. Additional resources with specific guidance for nirmatrelvir/ritonavir DDIs include the NIH DDI list [92], the Ontario COVID-19 Science Advisory DDI table [93], and the University of Liverpool COVID-19 Drug Interactions Checker [94] (Table 1).
The NIH DDI list summarizes selected commonly prescribed outpatient medications grouped by the expectation of having no clinically relevant interactions with ritonavir-enhanced nirmatrelvir versus having clinically relevant interactions specific for a 5-day treatment course. For the latter, the medications are further classified as those that should be avoided (i.e., an alternate COVID-19 therapy should be prescribed), be temporarily withheld (if clinically appropriate), have the dose or dosing interval adjusted, or be continued with monitoring. The Ontario COVID-19 Science Advisory DDI table [93] provides recommendations for selected medications based on whether the drug is classified into one of the following severity categories: contraindicated, contraindicated (has been used within the past 14 days), should not be coadministered, should be used with caution, and drug interaction is not likely to be clinically relevant. General recommendations based on the potential interaction severity as well as specific recommendations for individual drugs are provided as well as information regarding potential effects of coadministration on drug levels and other PK parameters. The University of Liverpool COVID-19 Drug Interactions Checker [94,95,96] provides four caution levels: red, which means do not coadminister; amber, which indicates potential interaction that may be manageable with dose adjustment and/or requiring close monitoring; yellow, which indicates a potential interaction that is likely to be of weak intensity, with additional monitoring and/or dose adjustments not likely to be needed; and green, which indicates no clinically significant interaction is expected). It also provides an overview regarding the potential mechanism for interaction and potential adverse outcomes if coadministered. A notice as to whether the drugs can be concomitantly administered and whether any dose alterations are recommended is included.
Other publicly available prescriber-targeted tools include the Paxlovid Patient Eligibility Screening Checklist Tool for Prescribers [97] and the Drug Interaction resource on the Pfizer Medical Information portal [98]. The former supports clinical decision making through a patient checklist for the prescriber to follow that includes questions regarding risk factors associated with progression to severe COVID-19, history of renal or hepatic impairment, and concomitant medications and provides a selected list of drugs that are contraindicated or should be avoided with nirmatrelvir/ritonavir unless dose adjustments or monitoring is possible [97]. The Drug Interaction resource on the Pfizer Medical Information portal describes how coadministration may affect bioavailability and offers recommendations for dose adjustments or contraindications [98].
The published literature also reports on specific drug classes and commonly prescribed drugs with the potential for DDIs with ritonavir [16, 24, 99,100,101,102,103,104,105]. In the recent analysis by Gerhart et al. of the potential for DDIs with the top 100 drugs prescribed in the United States to adults who are eligible for nirmatrelvir/ritonavir treatment for COVID-19, the most common classes of drugs expected to interact with the antiviral regimen included corticosteroids, narcotic analgesics, anticoagulants, and statins; among the top 10 most commonly prescribed drugs, only four have potential for DDI, all of which can be managed through monitoring or dose modification of the coadministered drug [83]. If DDIs are not specifically identified within the regulator labels or available tools, the prescriber/healthcare professional may need to assess drug metabolism pathways for potentially relevant interactions with nirmatrelvir/ritonavir and determine the clinical relevance of the interaction (if expected) [83].
It should be noted, enhancer-free antiviral therapies for short-term treatment of COVID-19 are currently in clinical trials and include protease inhibitors (PF-07817883, ensitrelvir, RAY1216, pomotrelvir), deuterated remdesivir hydrobromide (VV116), a microtubule disruptor (sabizabulin), guanosine nucleotide analog double prodrug RNA polymerase inhibitor (bemnifosbuvir), and designed ankyrin repeat protein (ensovibep) [106,107,108,109,110,111,112,113,114,115,116,117]. Depending on demonstration of antiviral efficacy, safety, and tolerability of these new putative therapies, such enhancer-free drugs may eventually mitigate some of the challenges associated with co-medication management, presuming the new therapies do not themselves perpetrate major DDIs.
Conclusions
Ritonavir has been available as an HIV therapy or PK enhancer to other antiviral agents for decades and has a well-characterized safety profile. Ritonavir-enhanced treatments allow for lower doses of the concomitantly administered drugs due to increased bioavailability, improved dosing schedules, reduced pill burden, and lower costs. The same mechanism by which ritonavir acts as a PK enhancer to increase the bioavailability of antivirals for an effective and well-tolerated regimen is also responsible for potentially triggering DDIs. Because of the extensive experience with ritonavir, several tools and approaches exist to help healthcare providers identify and mitigate potential DDIs. Approaches to co-medication management vary depending on the coadministered therapy the patient is receiving and its associated risk for interaction. During short-term antiviral treatment in adults with mild to moderate COVID-19 at high risk of progression to severe diseases, many commonly prescribed drugs likely to be taken by antiviral-eligible patients having little to no potential interaction with ritonavir-enhanced nirmatrelvir, and those that do have potential for DDIs are mostly manageable with straightforward and routine approaches, such as increased monitoring or dose modification. When prescribed correctly, and with the proper consideration for concomitant medications, ritonavir-enhanced antiviral regimens are safe and manageable.
Data Availability
Data sharing is not applicable to this article because no datasets were generated or analyzed during the current study.
References
Norvir® (ritonavir). Full prescribing information. North Chicago, IL, USA: AbbVie Inc.; 2022.
Deeks SG, Smith M, Holodniy M, Kahn JO. HIV-1 protease inhibitors. A review for clinicians. JAMA. 1997;277:145–53.
Krauß J, Bracher F. Pharmacokinetic enhancers (boosters)-escort for drugs against degrading enzymes and beyond. Sci Pharm. 2018;86:E43.
Kumar GN, Rodrigues AD, Buko AM, Denissen JF. Cytochrome P450-mediated metabolism of the HIV-1 protease inhibitor ritonavir (ABT-538) in human liver microsomes. J Pharmacol Exp Ther. 1996;277:423–31.
Eagling VA, Back DJ, Barry MG. Differential inhibition of cytochrome P450 isoforms by the protease inhibitors, ritonavir, saquinavir and indinavir. Br J Clin Pharmacol. 1997;44:190–4.
Loos NHC, Beijnen JH, Schinkel AH. The mechanism-based inactivation of CYP3A4 by ritonavir: what mechanism? Int J Mol Sci. 2022;23:9866.
Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther. 2013;138:103–41.
Loos NHC, Beijnen JH, Schinkel AH. The inhibitory and inducing effects of ritonavir on hepatic and intestinal CYP3A and other drug-handling proteins. Biomed Pharmacother. 2023;162: 114636.
Majerová T, Konvalinka J. Viral proteases as therapeutic targets. Mol Aspects Med. 2022;88: 101159.
Cattaneo D, Cossu MV, Rizzardini G. Pharmacokinetic drug evaluation of ritonavir (versus cobicistat) as adjunctive therapy in the treatment of HIV. Expert Opin Drug Metab Toxicol. 2019;15:927–35.
Chandwani A, Shuter J. Lopinavir/ritonavir in the treatment of HIV-1 infection: a review. Ther Clin Risk Manage. 2008;4:1023–33.
PaxlovidTM (nirmatrelvir tablets; ritonavir tablets). Full prescribing information. New York, NY, USA: Pfizer Inc; 2023.
US Food & Drug Administration. Coronavirus (COVID-19) update: FDA authorizes first oral antiviral for treatment of COVID-19. Available at https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-oral-antiviral-treatment-covid-19. Accessed September 11, 2023.
US Food & Drug Administration. FDA approves first oral antiviral for treatment of COVID-19 in adults. Available at https://www.fda.gov/news-events/press-announcements/fda-approves-first-oral-antiviral-treatment-covid-19-adults. Accessed September 11, 2023.
Foisy MM, Yakiwchuk EM, Hughes CA. Induction effects of ritonavir: implications for drug interactions. Ann Pharmacother. 2008;42:1048–59.
Marzolini C, Kuritzkes DR, Marra F, et al. Prescribing nirmatrelvir–ritonavir: how to recognize and manage drug–drug interactions. Ann Intern Med. 2022;175:744–6.
Shitara Y, Takeuchi K, Horie T. Long-lasting inhibitory effects of saquinavir and ritonavir on OATP1B1-mediated uptake. J Pharm Sci. 2013;102:3427–35.
Kharasch ED, Bedynek PS, Walker A, Whittington D, Hoffer C. Mechanism of ritonavir changes in methadone pharmacokinetics and pharmacodynamics: II. Ritonavir effects on CYP3A and P-glycoprotein activities. Clin Pharmacol Ther. 2008;84:506–12.
Kempf DJ, Marsh KC, Denissen JF, et al. ABT-538 is a potent inhibitor of human immunodeficiency virus protease and has high oral bioavailability in humans. Proc Natl Acad Sci U S A. 1995;92:2484–8.
De Clercq E. Novel compounds in preclinical/early clinical development for the treatment of HIV infections. Rev Med Virol. 2000;10:255–77.
Hsu A, Granneman GR, Bertz RJ. Ritonavir. Clinical pharmacokinetics and interactions with other anti-HIV agents. Clin Pharmacokinet. 1998;35:275–91.
Aarnoutse RE, Kleinnijenhuis J, Koopmans PP, et al. Effect of low-dose ritonavir (100 mg twice daily) on the activity of cytochrome P450 2D6 in healthy volunteers. Clin Pharmacol Ther. 2005;78:664–74.
Katzenmaier S, Markert C, Riedel K-D, et al. Determining the time course of CYP3A inhibition by potent reversible and irreversible CYP3A inhibitors using a limited sampling strategy. Clin Pharmacol Ther. 2011;90:666–73.
Marzolini C, Kuritzkes DR, Marra F, et al. Recommendations for the management of drug–drug interactions between the COVID-19 antiviral nirmatrelvir/ritonavir (Paxlovid) and comedications. Clin Pharmacol Ther. 2022;112:1191–200.
Lv Y, Luo YY, Ren HW, et al. The role of pregnane X receptor (PXR) in substance metabolism. Front Endocrinol (Lausanne). 2022;13:959902.
Xiao L, Nickbarg E, Wang W, et al. Evaluation of in vitro PXR-based assays and in silico modeling approaches for understanding the binding of a structurally diverse set of drugs to PXR. Biochem Pharmacol. 2011;81:669–79.
Rublein JC, Eron JJ Jr, Butts JD, Raasch RH. Discontinuation rates for protease inhibitor regimens containing ritonavir 600 mg versus ritonavir 400 mg plus saquinavir 400 mg. Ann Pharmacother. 1999;33:899–905.
Rathbun RC, Rossi DR. Low-dose ritonavir for protease inhibitor pharmacokinetic enhancement. Ann Pharmacother. 2002;36:702–6.
Kempf DJ, Marsh KC, Kumar G, et al. Pharmacokinetic enhancement of inhibitors of the human immunodeficiency virus protease by coadministration with ritonavir. Antimicrob Agents Chemother. 1997;41:654–60.
Talha B, Dhamoon AS. Ritonavir. StatPearls Publishing. Available at https://www.ncbi.nlm.nih.gov/books/NBK544312/. Accessed November 14, 2023.
van Heeswijk RP, Veldkamp AI, Hoetelmans RM, et al. The steady-state plasma pharmacokinetics of indinavir alone and in combination with a low dose of ritonavir in twice daily dosing regimens in HIV-1-infected individuals. AIDS. 1999;13:F95–9.
Hull MW, Montaner JS. Ritonavir-boosted protease inhibitors in HIV therapy. Ann Med. 2011;43:375–88.
Riddler SA, Haubrich R, DiRienzo AG, et al. Class-sparing regimens for initial treatment of HIV-1 infection. N Engl J Med. 2008;358:2095–106.
Torres B, Rallón NI, Loncá M, et al. Immunological function restoration with lopinavir/ritonavir versus efavirenz containing regimens in HIV-infected patients: a randomized clinical trial. AIDS Res Hum Retroviruses. 2014;30:425–33.
Wensing AM, van Maarseveen NM, Nijhuis M. Fifteen years of HIV protease inhibitors: raising the barrier to resistance. Antiviral Res. 2010;85:59–74.
Salama E, Eke AC, Best BM, Mirochnick M, Momper JD. Pharmacokinetic enhancement of HIV antiretroviral therapy during pregnancy. J Clin Pharmacol. 2020;60:1537–50.
Tseng A, Seet J, Phillips EJ. The evolution of three decades of antiretroviral therapy: challenges, triumphs and the promise of the future. Br J Clin Pharmacol. 2015;79:182–94.
Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in adults and Adolescents With HIV. Department of Health and Human Services. Available at https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv. Accessed September 30, 2023.
Ding Y, Duan S, Ye R, et al. Effects of aging, baseline renal function and stage of HIV infection on post-treatment changes in renal function among HIV-infected patients: a retrospective cohort study. HIV Med. 2019;20:591–600.
Marin RC, Tiț DM, Săndulescu O, Streinu-Cercel A, Bungău SG. Comparison of tolerability and impact on metabolic profiles of antiretroviral regimens containing darunavir/ritonavir or darunavir/cobicistat in Romanian HIV infected patients. Biomedicines. 2021;9:987.
Ryom L, Mocroft A, Kirk O, et al. Association between antiretroviral exposure and renal impairment among HIV-positive persons with normal baseline renal function: the D:A:D study. J Infect Dis. 2013;207:1359–69.
Alenzi KA, Alanazi NS, Almalki M, Alomrani H, Alatawi FO. The evaluation of adverse drug reactions in Saudi Arabia: a retrospective observational study. Saudi Pharm J. 2022;30:735–41.
Huang X, Xu L, Sun L, et al. Six-year immunologic recovery and virological suppression of HIV patients on LPV/r-based second-line antiretroviral treatment: a multi-center real-world cohort study in China. Front Pharmacol. 2019;10:1455.
Priyanka P, Varma DM, Immadisetti K, et al. Recognition of possible risk factors for clinically significant drug–drug interactions among Indian people living with HIV receiving highly active antiretroviral therapy and concomitant medications. Int J Risk Saf Med. 2017;29:25–55.
Patel N, Borg P, Haubrich R, McNicholl I. Analysis of drug–drug interactions among patients receiving antiretroviral regimens using data from a large open-source prescription database. Am J Health Syst Pharm. 2018;75:1132–9.
Paudel M, Prajapati G, Buysman EK, et al. Comorbidity and comedication burden among people living with HIV in the United States. Curr Med Res Opin. 2022;38:1443–50.
World Health Organization. Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach. Available at https://www.who.int/publications/i/item/9789240031593. Accessed July 17, 2023.
Shrestha LB, Yadav GK, Pradhan S, et al. Co-infection of hepatitis B and hepatitis C among HIV-infected patients: a cross-sectional study from tertiary care hospital of eastern Nepal. PLoS ONE. 2022;17:e0264791.
World Health Organization. WHO updates guidelines on treatments for COVID-19. Available at https://www.who.int/news/item/10-11-2023-who-updates-guidelines-on-treatments-for-covid-19. Accessed January 29, 2024.
World Health Organization. Therapeutics and COVID-19. Living guideline. Available at https://files.magicapp.org/guideline/e4b3c18e-0850-4a59-895e-f201cf9b4df2/published_guideline_7789-14_1.pdf. Accessed January 29, 2024.
Hammond J, Leister-Tebbe H, Gardner A, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19. N Engl J Med. 2022;386:1397–408.
US Food and Drug Administration. FDA briefing document. Antimicrobial drugs advisory committee meeting March 16, 2023. Available at https://www.fda.gov/media/166197/download. Accessed April 16, 2023.
Leister-Tebbe H, Bao W, Fountaine R, et al. Nirmatrelvir/ritonavir versus placebo in unvaccinated and vaccinated high-risk patients. Presented at IDWeek, 2023; Boston, MA, USA.
Weng C, Xie R, Han G, et al. Safety and efficacy of Paxlovid against Omicron variants of coronavirus disease 2019 in elderly patients. Infect Dis Ther. 2023;12:649–62.
Tiseo G, Barbieri C, Galfo V, et al. Efficacy and safety of nirmatrelvir/ritonavir, molnupiravir, and remdesivir in a real-world cohort of outpatients with COVID-19 at high risk of progression: the PISA outpatient clinic experience. Infect Dis Ther. 2023;12:257–71.
Gentile I, Scotto R, Schiano Moriello N, et al. Nirmatrelvir/ritonavir and molnupiravir in the treatment of mild/moderate COVID-19: results of a real-life study. Vaccines (Basel). 2022;10:1731.
Qiu C, Wu Z, Liu X, et al. Efficacy and safety of nirmatrelvir/ritonavir for treating the Omicron variant of COVID-19. Front Med (Lausanne). 2023;10:1161193.
Zhuang W, Xu J, Wu Y, et al. Post-marketing safety concerns with nirmatrelvir: a disproportionality analysis of spontaneous reports submitted to the FDA Adverse Event Reporting System. Br J Clin Pharmacol. 2023;89:2830–42.
Pannu V, Udongwo N, Imburgio S, et al. Adverse events of SARS-CoV-2 therapy: a pharmacovigilance study of the FAERS database. Ann Pharmacother. 2023;58:105–9.
Bihan K, Lipszyc L, Lemaitre F, et al. Nirmatrelvir/ritonavir (Paxlovid®): French pharmacovigilance survey 2022. Therapie. 2023;78:531–47.
Yan J, Cai H, Wang J, et al. Nirmatrelvir/ritonavir for patients with SARS-CoV-2 infection and impaired kidney function during the Omicron surge. Front Pharmacol. 2023;14:1147980.
Caso JM, Fernández-Ruiz M, López-Medrano F, et al. Nirmatrelvir/ritonavir for the treatment of immunocompromised adult patients with early-stage symptomatic COVID-19: a real-life experience. J Med Virol. 2023;95:e29082.
Li Y, Liu Y, Wen L, et al. Clinical efficacy analysis of Paxlovid in children with hematological diseases infected with the omicron SARS-CoV-2 new variant. Front Pediatr. 2023;11:1160929.
Centers for Disease Control and Prevention. Underlying medical conditions associated with higher risk for severe COVID-19: information for healthcare professionals. Available at https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html#:~:text=Age%20is%20the%20strongest%20risk,are%20also%20at%20higher%20risk.&text=Additionally%2C%20being%20unvaccinated%20or%20not,of%20severe%20COVID%2D19%20outcomes. Accessed September 14, 2023.
UN AIDS. Global HIV & AIDS statistics—fact sheet. Available at https://www.unaids.org/en/resources/fact-sheet#:~:text=Global%20HIV%20statistics,AIDS%2Drelated%20illnesses%20in%202022. Accessed October 4, 2023.
Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–42.
Kim L, Garg S, O’Halloran A, et al. Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the US coronavirus disease 2019 (COVID-19)-associated hospitalization surveillance network (COVID-NET). Clin Infect Dis. 2021;72:e206–14.
Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985.
Thakur B, Dubey P, Benitez J, et al. A systematic review and meta-analysis of geographic differences in comorbidities and associated severity and mortality among individuals with COVID-19. Sci Rep. 2021;11:8562.
Cox DS, Rehman M, Khan T, et al. Effects of nirmatrelvir/ritonavir on midazolam and dabigatran pharmacokinetics in healthy participants. Br J Clin Pharmacol. 2023;89:3352–63.
Berar Yanay N, Bogner I, Saker K, Tannous E. Paxlovid-tacrolimus drug–drug interaction in a 23-year-old female kidney transplant patient with COVID-19. Clin Drug Investig. 2022;42:693–5.
Qin F, Wang H, Li M, Zhuo S, Liu W. Drug–drug interaction of nirmatrelvir/ritonavir and tacrolimus: a potential risk disproportionality analysis of nephrotoxicity from COVID-19 reports in FAERS. Expert Opin Drug Saf. 2023;22:1321–7.
Prikis M, Cameron A. Paxlovid (nirmatrelvir/ritonavir) and tacrolimus drug–drug interaction in a kidney transplant patient with SARS-2-CoV infection: a case report. Transplant Proc. 2022;54:1557–60.
Coyne M, Aye M. Tacrolimus toxicity in two renal transplant recipients treated with nirmatrelvir/ritonavir: a case series. Ann Intern Med Clin Cases. 2023;2:e221121.
Sindelar M, McCabe D, Carroll E. Tacrolimus drug–drug interaction with nirmatrelvir/ritonavir (Paxlovid™) managed with phenytoin. J Med Toxicol. 2023;19:45–8.
Lindauer KE, Hamel AG. Case report: nirmatrelvir/ritonavir and tacrolimus in a kidney transplant recipient with COVID-19. Am Fam Physician. 2022;105:569–70.
Rose DT, Gandhi SM, Bedard RA, et al. Supratherapeutic tacrolimus concentrations with nirmatrelvir/ritonavir in solid organ transplant recipients requiring hospitalization: a case series using rifampin for reversal. Open Forum Infect Dis. 2022;9:ofac238.
Cordero CG, de Vicente MS. Elevated tacrolimus blood concentration due to the interaction with nirmatrelvir/ritonavir during COVID-19 treatment: a case report. Transpl Proc. 2023;55:1826–8.
Waldman G, Rawlings SA, Kerr J, et al. Successful optimization of antiretroviral regimens in treatment-experienced people living with HIV undergoing liver transplantation. Transpl Infect Dis. 2019;21:e13174.
University of Liverpool. HIV drug interactions. Available at https://hiv-druginteractions.org/. Accessed July 17, 2023.
Fishbane S, Hirsch JS, Nair V. Special considerations for Paxlovid treatment among transplant recipients with SARS-CoV-2 infection. Am J Kidney Dis. 2022;79:480–2.
Lange NW, Salerno DM, Jennings DL, et al. Nirmatrelvir/ritonavir use: managing clinically significant drug–drug interactions with transplant immunosuppressants. Am J Transplant. 2022;22:1925–6.
Gerhart J, Draica F, Benigno M, et al. Real-world evidence of the top 100 prescribed drugs in the USA and their potential for drug interactions with nirmatrelvir; ritonavir. AAPS J. 2023;25:73.
Igho-Osagie E, Brzozowski K, Jin H, et al. Prevalence of potential drug–drug interactions with ritonavir-containing COVID-19 therapy in the United States: an analysis of the National Health and Nutrition Examination Survey. Clin Ther. 2023;45:390-9.e4.
Igho-Osagie E, Puenpatom A, Williams MG, et al. Prevalence of potential drug–drug interactions with ritonavir-containing COVID-19 therapy. J Manage Care Spec Pharm. 2023;29:509–18.
Kane AM, Keenan EM, Lee K, et al. Nirmatrelvir-ritonavir treatment of COVID-19 in a high-risk patient population: a retrospective observational study. J Am Coll Clin Pharm. 2023;6:29–33.
Lübbert C, Dykukha I, Pelz JP, et al. Individuals at risk for severe COVID-19 in whom ritonavir-containing therapies are contraindicated or may lead to interactions with concomitant medications: a retrospective analysis of German health insurance claims data. Drugs Context. 2023;12:1–12.
Bukhari S, Hughes S, Mughal N, et al. Nirmatrelvir/ritonavir real world drug–drug interaction management experience. Presented at: International Workshop on Clinical Pharmacology of HIV, Hepatitis, and Other Antiviral Drugs, September 11–13, 2023; Rome, Italy.
Xiao X, Mehta HB, Curran J, Garibaldi BT, Alexander GC. Potential drug–drug interactions among U.S. adults treated with nirmatrelvir/ritonavir: a cross-sectional study of the National Covid Cohort Collaborative (N3C). Pharmacotherapy. 2023;8:e1003–17.
Clark A, Jit M, Warren-Gash C, et al. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study. Lancet Glob Health. 2020;8:e1003–17.
National Institutes of Health. Coronavirus disease 2019 (COVID-19) Treatment guidelines. Available at https://files.covid19treatmentguidelines.nih.gov/guidelines/covid19treatmentguidelines.pdf. Accessed August 24, 2023.
National Institutes of Health. Drug–drug interactions between ritonavir-boosted nirmatrelvir (Paxlovid) and concomitant medications. Available at https://www.covid19treatmentguidelines.nih.gov/therapies/antiviral-therapy/ritonavir-boosted-nirmatrelvir--paxlovid-/paxlovid-drug-drug-interactions/. Accessed October 9, 2022.
Ontario COVID-19 Drugs and Biologics Clinical Practice Guidelines Working Group. Nirmatrelvir/ritonavir (Paxlovid): what prescribers and pharmacists need to know. Available at https://covid19-sciencetable.ca/sciencebrief/nirmatrelvir-ritonavir-paxlovid-what-prescribers-and-pharmacists-need-to-know-3-0/. Accessed August 8, 2022.
University of Liverpool. COVID-19 drug interaction. Available at https://www.covid19-druginteractions.org/checker. Accessed September 13, 2023.
University of Liverpool. Evaluating the drug–drug interaction risk of COVID-19 therapies (licensed or under clinical investigation). Available at www.covid19-druginteractions.org/prescribing_resources/methods-metabolism. Accessed November 1, 2023.
University of Liverpool. Interactions with outpatient medicines and nirmatrelvir/ritonavir (NMV/r). Available at: www.covid19-druginteractions.org/prescribing_resources/paxlovid-outpatient-medicines. Accessed November 1, 2023.
US Food and Drug Administration. PAXLOVID patient eligibility screening checklist tool for prescribers. Available at https://www.fda.gov/media/158165/download. Accessed September 13, 2023.
Pfizer Inc. Drug interaction checker. Available at https://www.pfizermedicalinformation.com/en-us/drug-interaction-checker?product=PAXLOVID%E2%84%A2+%7C+nirmatrelvir+tablets%3B+ritonavir+tablets. Accessed September 13, 2023.
Lemaitre F, Grégoire M, Monchaud C, et al. Management of drug–drug interactions with nirmatrelvir/ritonavir in patients treated for COVID-19: guidelines from the French Society of Pharmacology and Therapeutics (SFPT). Therapie. 2022;77:509–21.
Lemaitre F, Budde K, Van Gelder T, et al. Therapeutic drug monitoring and dosage adjustments of immunosuppressive drugs when combined with nirmatrelvir/ritonavir in patients with COVID-19. Ther Drug Monit. 2022;45:191–9.
Yalcin N, Allegaert K. COVID-19 and antiepileptic drugs: an approach to guide practices when nirmatrelvir/ritonavir is co-prescribed. Eur J Clin Pharmacol. 2022;78:1697–701.
Shini Rubina SK, Anuba PA, Swetha B, et al. Drug interaction risk between cardioprotective drugs and drugs used in treatment of COVID-19: an evidence-based review from six databases. Diabetes Metab Syndr. 2022;16:102451.
Larsen CS. Assessing the proportion of the Danish population at risk of clinically significant drug–drug interactions with new oral antivirals for early treatment of COVID-19. Int J Infect Dis. 2022;122:599–601.
Saravolatz LD, Depcinski S, Sharma M. Molnupiravir and nirmatrelvir-ritonavir: oral coronavirus disease 2019 antiviral drugs. Clin Infect Dis. 2023;76:165–71.
Mikus G, Foerster KI, Terstegen T, et al. Oral drugs against COVID-19. Dtsch Arztebl Int. 2022;119:263–9.
Barnette KG, Gordon MS, Rodriguez D, et al. Oral sabizabulin for high-risk, hospitalized adults with COVID-19: interim analysis. NEJM Evid. 2022. https://doi.org/10.1056/EVIDoa2200145.
ClinicalTrials.gov. A study to learn safety and blood levels of PF-07817883 in healthy people. Available at https://classic.clinicaltrials.gov/ct2/show/NCT05580003?term=NCT05580003&draw=2&rank=1. Accessed October 3, 2023.
Mukae H, Yotsuyanagi H, Ohmagari N, et al. A randomized phase 2/3 study of ensitrelvir, a novel oral SARS-CoV-2 3C-like protease inhibitor, in Japanese patients with mild-to-moderate COVID-19 or asymptomatic SARS-CoV-2 infection: results of the phase 2a part. Antimicrob Agents Chemother. 2022;66:e0069722.
Mukae H, Yotsuyanagi H, Ohmagari N, et al. Efficacy and safety of ensitrelvir in patients with mild-to-moderate coronavirus disease 2019: the phase 2b part of a randomized, placebo-controlled, phase 2/3 study. Clin Infect Dis. 2023;76:1403–11.
Yotsuyanagi H, Ohmagari N, Doi Y, et al. A phase 2/3 study of S-217622 in participants with SARS-CoV-2 infection (phase 3 part). Medicine (Baltimore). 2023;102:e33024.
Barkauskas C, Mylonakis E, Poulakou G, et al. Efficacy and safety of ensovibep for adults hospitalized with COVID-19: a randomized controlled trial. Ann Intern Med. 2022;175:1266–74.
Cao Z, Gao W, Bao H, et al. VV116 versus nirmatrelvir–ritonavir for oral treatment of COVID-19. N Engl J Med. 2023;388:406–17.
Wang B, Li HJ, Cai MM, et al. Antiviral efficacy of RAY1216 monotherapy and combination therapy with ritonavir in patients with COVID-19: a phase 2, single centre, randomised, double-blind, placebo-controlled trial. EClinicalMedicine. 2023;63: 102189.
Boffito M, Dolan E, Singh K, et al. A phase 2 randomized trial evaluating the antiviral activity and safety of the direct-acting antiviral bemnifosbuvir in ambulatory patients with mild or moderate COVID-19 (MOONSONG Study). Microbiol Spectr. 2023;11:e0007723.
Horga A, Saenz R, Yilmaz G, et al. Oral bemnifosbuvir (AT-527) vs placebo in patients with mild-to-moderate COVID-19 in an outpatient setting (MORNINGSKY). Future Virol. 2023;18:839–53.
Shannon A, Fattorini V, Sama B, et al. A dual mechanism of action of AT-527 against SARS-CoV-2 polymerase. Nat Commun. 2022;13:621.
Borroto-Esoda K, Wilfret D, Tong X, et al. SARS-CoV-2 viral dynamics in a placebo-controlled phase 2 study of patients infected with the SARS-CoV-2 Omicron variant and treated with pomotrelvir. Microbiol Spectr. 2024;12:e0298023.
Acknowledgements
Medical Writing, Editorial, and Other Assistance
Editorial/medical writing support was provided by Erin P. O’Keefe, PhD, of ICON (Blue Bell, PA, USA) and was funded by Pfizer Inc.
Authorship
All authors made substantial contributions to this work, approved the version to be published, and agreed to be accountable for all aspects of the work.
Funding
This work, including the journal’s Rapid Service Fee, was supported by Pfizer Inc.
Author information
Authors and Affiliations
Contributions
Carolina Pein and Jennifer Bodie contributed to conceptualization. Romina Quercia, Giovanni Di Perri, Carolina Pein, Jennifer Bodie, Ravi Shankar P. Singh, Victoria Hendrick, and Marta Booffito critically reviewed the manuscript and approved the final version for submission.
Corresponding author
Ethics declarations
Conflict of Interest
Romina Quercia, Carolina Pein, Jennifer Bodie, Ravi Shankar P. Singh, and Victoria Hendrick are employees of and may hold stock or stock options in Pfizer. Giovanni Di Perri has received fees for lectures and participation in advisory boards from AbbVie, GS, ViiV Healthcare, GSK, Pfizer, AZ, Roche, MSD, and Janssen. Marta Boffito has consulted for or received speaker fees from ViiV Healthcare, Gilead, GlaxoSmithKline, Pfizer, Cypla, Mylan, and Janssen and has received research grants from Gilead, ViiV Healthcare, Mylan, Novavax, Janssen, Valneva, Moderna, and Roche.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals conducted by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Quercia, R., Di Perri, G., Pein, C. et al. Ritonavir: 25 Years’ Experience of Concomitant Medication Management. A Narrative Review. Infect Dis Ther 13, 1005–1017 (2024). https://doi.org/10.1007/s40121-024-00959-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-024-00959-6